Healing of Ischemic Colon Anastomosis in Rats Could Be Provided by Administering Dexpanthenol or Coenzyme Q10
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- -
- Sham-S group: A full layer colon resection was performed without creating ischemia to the rats, and end to tip anastomosis was applied without experimental drug administration. The rats were sacrificed at the end of 10 days.
- -
- Sham-I group: After ischemia was performed on the colon, a full layer colonic resection was performed and end to tip anastomosis was applied without experimental drug administration. The rats were sacrificed at the end of 10 days.
- -
- DXP group: After ischemia was performed on the colon, a full layer colonic resection was performed and end to tip anastomosis was applied. Dexpanthenol (Bepanthene 500 mg ampoule, Roche, Berlin, Germany) was administered at a dose of 50 mg/kg once a day for three days, intraperitoneally, and rats were sacrificed at the end of 10 days.
- -
- Q10 group: After ischemia was performed on the colon, a full layer colonic resection was performed and end to tip anastomosis was applied. CoQ10 (Sigma Chemical Co., St. Louis, MO, USA) was administered at a dose of 1 mg/100g once a day for three days, intraperitoneally, and rats were sacrificed at the end of 10 days.
2.2. Surgery
2.3. Burst Pressure Measurement
2.4. Biochemical Analysis
2.5. Histopathological Examination
2.6. Statistical Analysis
3. Results
3.1. Burst Pressure
3.2. Biochemical Analysis
3.3. Histopathological Evaluation
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tseng, J.; Loper, B.; Jain, M.; Lewis, A.V.; Margulies, D.R.; Alban, R.F. Predictive factors of mortality after colectomy in ischemic colitis: An ACS-NSQIP database study. Trauma Surg. Acute Care Open 2017, 2, e000126. [Google Scholar] [CrossRef] [PubMed]
- Beck, D.E.; de Aguilar-Nascimento, J.E. Surgical management and outcome in acute ischemic colitis. Ochsner J. 2011, 11, 282–285. [Google Scholar] [PubMed]
- Fielding, L.; Stewart-Brown, S.; Blesovsky, L.; Kearney, G. Anastomotic integrity after operations for large-bowel cancer: A multicentre study. Br. Med. J. 1980, 281, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Parra-Membrives, P.; Ruiz-Luque, V.; Escudero-Severín, C.; Aguilar-Luque, J.; Méndez-García, V. Effect of pentoxifylline on the healing of ischemic colorectal anastomoses. Dis. Colon Rectum 2007, 50, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Benacerraf, B.; Sebestyen, M.M. Effect of bacterial endotoxins on the reticuloendothelial system. Fed. Proc. 1957, 16, 860–867. [Google Scholar] [PubMed]
- Rosoky, R.M.A.; Wolosker, N.; Nasser, M.; Zerati, A.E.; Gidlund, M.; Puech-Leão, P. Oxidized low-density lipoprotein and ankle-brachial pressure index in patients with clinically evident peripheral arterial disease. Clinics 2010, 65, 383–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uysal, E.; Dokur, M. Comparison of effects of the tacrolimus and cyclosporine A on the colon anastomosis recovery of rats. Ann. Surg. Treat. Res. 2017, 92, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Karaca, G.; Pekcici, M.; Altunkaya, C.; Fidanci, V.; Kilinc, A.; Ozer, H. The effects of scalpel, harmonic scalpel and monopolar electrocautery on the healing of colonic anastomosis after colonic resection. Ann. Surg. Treat. Res. 2016, 90, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, T.; Mastboom, W.J. Healing of experimental intestinal anastomoses. Dis. Colon Rectum 1990, 33, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Biro, K.; ThaÇi, D.; Ochsendorf, F.R.; Kaufmann, R.; Boehncke, W.H. Efficacy of dexpanthenol in skin protection against irritation: A double-blind, placebo-controlled study. Contact Dermat. 2003, 49, 80–84. [Google Scholar] [CrossRef]
- Proksch, E.; Nissen, H. Dexapanthenol enhances skin barrier repair and reduces inflammation after sodium lauryl sulphate-induced irritation. J. Dermatol. Treat. 2002, 13, 173–178. [Google Scholar] [CrossRef]
- Yardimci, I.; Karakan, T.; Resorlu, B.; Doluoglu, O.G.; Ozcan, S.; Aydın, A. The effect of intraurethral dexpanthenol on healing and fibrosis in rats with experimentally induced urethral trauma. Urology 2015, 85, e279-13. [Google Scholar] [CrossRef] [PubMed]
- Oguz, A.; Uslukaya, O.; Alabalık, U.; Turkoglu, A.; Kapan, M.; Bozdag, Z. Topical N-acetylcysteine improves wound healing comparable to dexpanthenol: An experimental study. Int. Surg. 2015, 100, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Park, E.H.; Lim, C.J. Evaluation of anti-angiogenic, anti-inflammatory and antinociceptive activity of coenzyme Q10 in experimental animals. J. Pharm. Pharmacol. 2009, 61, 1391–1395. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Tomofuji, T.; Kawabata, Y.; Ekuni, D.; Azuma, T.; Kataoka, K. Application of coenzyme Q10 for accelerating soft tissue wound healing after tooth extraction in rats. Nutrients 2014, 6, 5756–5769. [Google Scholar] [CrossRef] [PubMed]
- Pepe, S.; Marasco, S.F.; Haas, S.J.; Sheeran, F.L.; Krum, H.; Rosenfeldt, F.L. Coenzyme Q10 in cardiovascular disease. Mitochondrion 2007, 7, S154–S167. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.P.; Tucker, G.T. An analysis of the applications of dissolution efficiency. J. Pharm. Pharmacol. 1976, 28, 932–933. [Google Scholar] [CrossRef] [PubMed]
- Posma, L.A.; Bleichrodt, R.P.; Lomme, R.M.; de Man, B.M.; van Goor, H.; Hendriks, T. Early anastomotic repair in the rat intestine is affected by transient preoperative mesenteric ischemia. J. Gastrointest. Surg. 2009, 13, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic, G.; Juttner, E.; zur Hausen, A.; Theodor Hopt, U.; Obermaier, R. Ischemic preconditioning improves stability of intestinal anastomoses in rats. Int. J. Colorectal Dis. 2009, 24, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.; Langfelt, S.; Laurberg, S. Bursting strength of experimental colonic anastomoses. A methodological study. Eur. Surg. Res. 1993, 25, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Kumashiro, R.; Oki, E.; Taketani, K.; Ando, K.; Aishima, S.; Akahoshi, T.; Morita, M.; Maehara, Y. Evaluation of techniques to prevent colorectal anastomotic leakage. J. Surg. Res. 2015, 194, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Braskén, P. Healing of experimental colon anastomosis. Eur. J. Surg. 1991, 566, 1–51. [Google Scholar]
- Sumer, A.; Altinli, E.; Senger, S.; Koksal, N.; Onur, E.; Eroglu, E. Effect of pentoxifylline and vinpocetine on the healing of ischemic colon anastomosis: An experimental study. Ulus Travma Acil Cerrahi Derg 2011, 17, 482–487. [Google Scholar] [PubMed]
- Rauchová, H.; Battino, M.; Fato, R.; Lenaz, G.; Drahota, Z. Coenzyme Q-pool function in glycerol-3-phosphate oxidation in hamster brown adipose tissue mitochondria. J. Bioenerg. Biomembr. 1992, 24, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Ogata, H.; Yazawa, M.; Watanabe, N.; Mori, T.; Nakajima, T. The effects of platelet-rich plasma on cutaneous incisional wound healing in rats. J. Dermatol. Sci. 2005, 40, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Ebner, F.; Heller, A.; Rippke, F.; Tausch, I. Topical use of dexpanthenol in skin disorders. Am. J. Clin. Dermatol. 2002, 3, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, N.; Korkmaz, S.; Öztürk, Y. Wound-healing activity of St. John’s Wort (Hypericum perforatum L.) on chicken embryonic fibroblasts. J. Ethnopharmacol. 2007, 111, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.S.; Song, H.S.; Kim, H.R.; Park, T.W.; Kim, T.D.; Cho, B.J. Effect of coenzyme Q10 on cutaneous healing in skin-incised mice. Arch. Pharm. Res. 2009, 32, 907–913. [Google Scholar] [CrossRef] [PubMed]


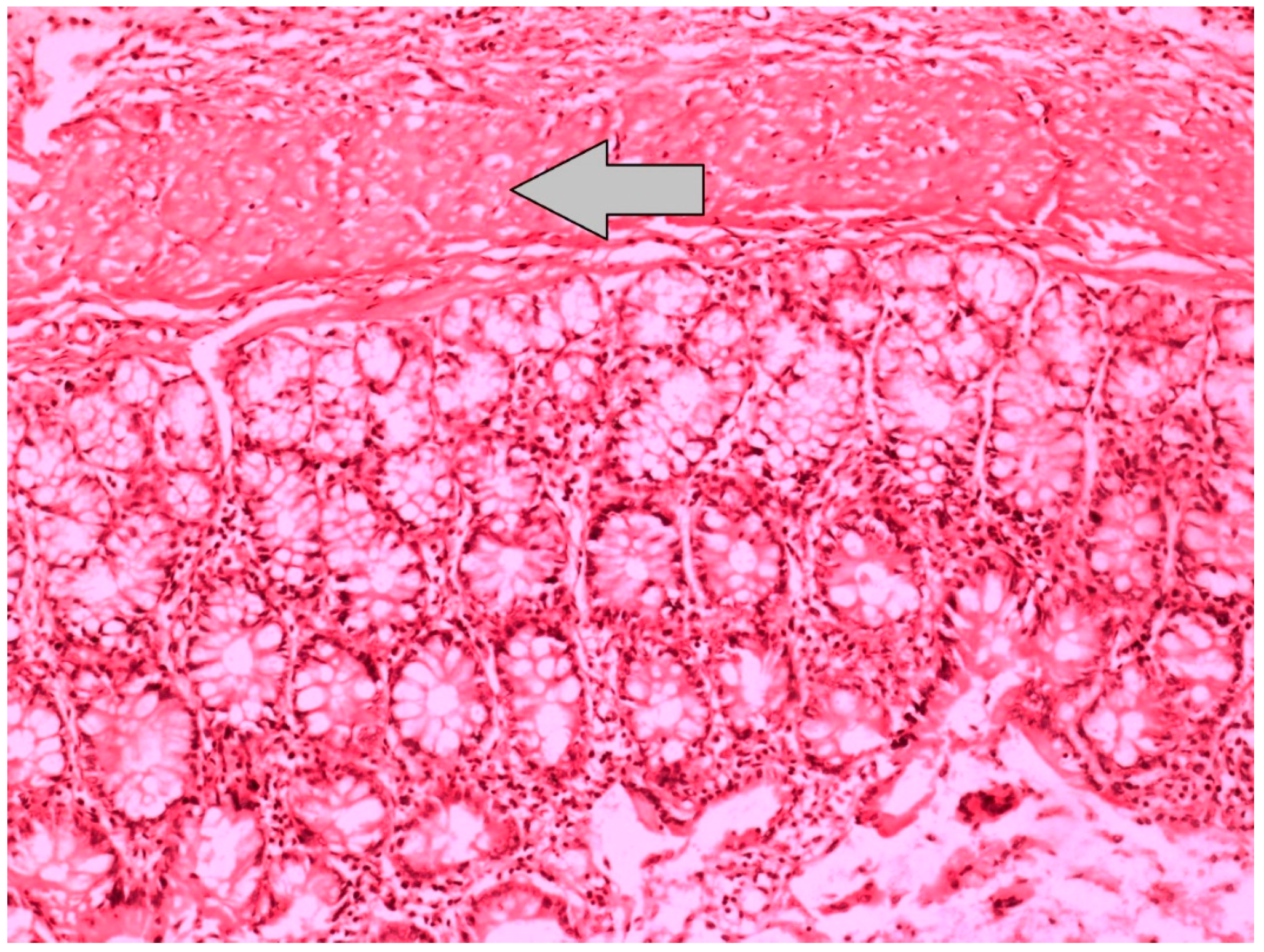
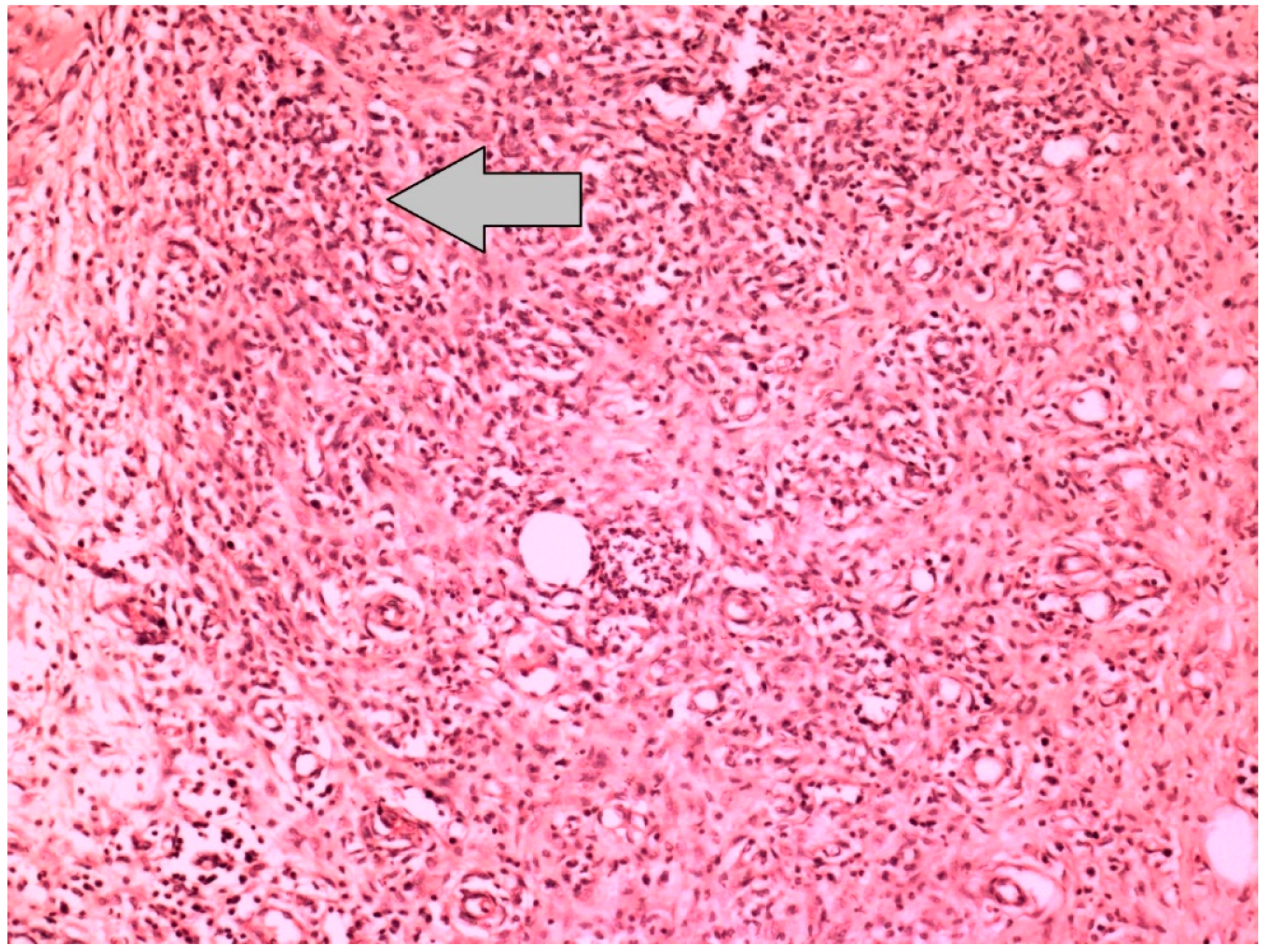



| Reepithelization of Anastomotic Mucosa (Components) | |
| Grade 0 | No epithelization at anastomotic line |
| Grade 1 | Incomplete coverage of the anastomotic line with a single cell layer |
| Grade 2 | Complete coverage of the anastomotic line with a single cell layer |
| Grade 3 | Complete reepithelization with glandular epithelium |
| Neovascularization | |
| Grade 1 | None |
| Grade 2 | Minimal |
| Grade 3 | Moderate |
| Grade 4 | Severe |
| Inflammation at Anastomotic Line (Polymorphonuclear Leukocyte or Lymphocyte) | |
| Grade 1 | None |
| Grade 2 | Minimal |
| Grade 3 | Moderate |
| Grade 4 | Severe |
| Completeness of Muscular Layer | |
| Grade 1 | Complete disruption |
| Grade 2 | Incomplete disruption |
| Grade 3 | Complete healing |
| Group | Variable | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|---|
| Sham-I | Burst pressure | 131.00 | 260.00 | 221.25 | 55.65 |
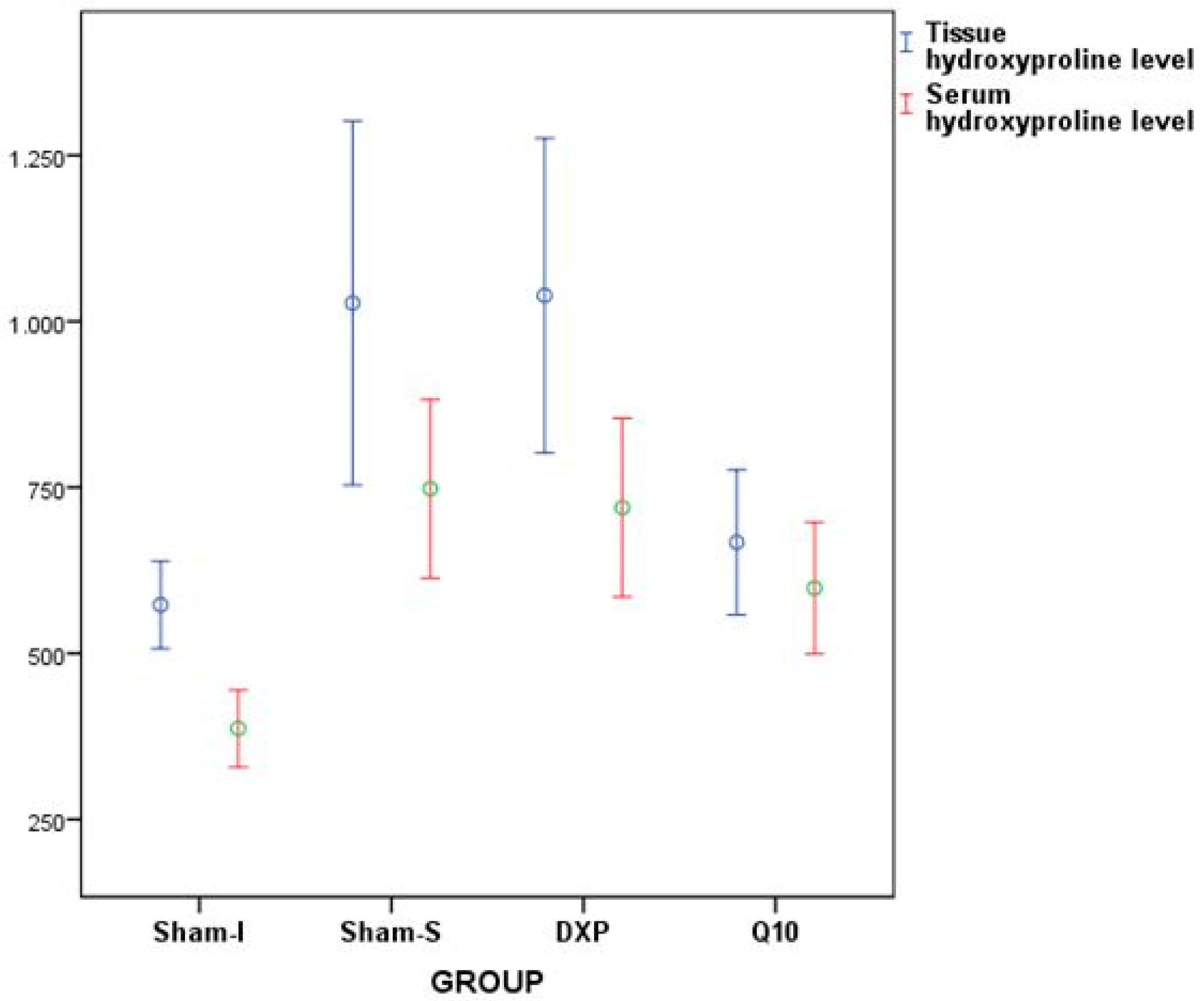
| Tissue hydroxyproline level | 446.37 | 623.99 | 573.06 | 78.92 | |
| Serum hydroxyproline level | 315.61 | 492.89 | 386.95 | 69.65 | |
| Sham-S | Burst pressure | 250.00 | 305.00 | 267.00 | 17.67 |
| Tissue hydroxyproline level | 485.12 | 1466.74 | 1027.66 | 328.06 | |
| Serum hydroxyproline level | 386.27 | 892.65 | 747.96 | 161.27 | |
| DXP | Burst pressure | 208.00 | 277.00 | 241.00 | 20.63 |
| Tissue hydroxyproline level | 653.27 | 1434.89 | 1039.09 | 283.23 | |
| Serum hydroxyproline level | 354.42 | 860.80 | 719.54 | 161.05 | |
| Q10 | Burst pressure | 201.00 | 270.00 | 234.25 | 20.10 |
| Tissue hydroxyproline level | 511.58 | 831.83 | 667.26 | 130.49 | |
| Serum hydroxyproline level | 469.58 | 830.66 | 598.36 | 118.75 |
| Variable | F | p |
|---|---|---|
| Burst pressure value | 6.797 | 0.008 |
| Tissue hydroxyproline level | 8.855 | <0.001 |
| Serum hydroxyproline level | 12.168 | <0.001 |
| Group (I/J) | Burst Pressure | Tissue HP Level | Serum HP Level |
|---|---|---|---|
| Sham-I/Sham-S | 0.006 | 0.003 | <0.001 |
| Sham-I/DXP | 0.016 | 0.002 | <0.001 |
| Sham-I/Q10 | 0.005 | 0.844 | 0.018 |
| Sham-S/DXP | 0.752 | 1.000 | 0.973 |
| Sham-S/Q10 | 0.371 | 0.020 | 0.135 |
| DXP/Q10 | 0.343 | 0.015 | 0.285 |
| Group | Variable | Min | Max | Median | SD |
|---|---|---|---|---|---|
| Sham-I | Reepithelization | 0 | 3 | 3 | 1.41 |
| Neovascularization | 3 | 4 | 4 | 0.35 | |
| Polymorphonuclear leukocyte density | 3 | 4 | 3 | 0.35 | |
| Lymphocyte density | 3 | 4 | 3 | 0.53 | |
| Muscular layer completeness | 1 | 2 | 1 | 0.51 | |
| Sham-S | Reepithelization | 3 | 3 | 3 | 0.00 |
| Neovascularization | 2 | 4 | 3 | 0.75 | |
| Polymorphonuclear leukocyte density | 2 | 3 | 2 | 0.46 | |
| Lymphocyte density | 3 | 4 | 3 | 0.46 | |
| Muscular layer completeness | 1 | 3 | 2.50 | 0.88 | |
| DXP | Reepithelization | 0 | 3 | 1.50 | 1.60 |
| Neovascularization | 2 | 4 | 4 | 0.74 | |
| Polymorphonuclear leukocyte density | 1 | 3 | 3 | 0.75 | |
| Lymphocyte density | 2 | 4 | 3 | 0.64 | |
| Muscular layer completeness | 1 | 3 | 2 | 0.83 | |
| Q10 | Reepithelization | 0 | 3 | 0 | 1.41 |
| Neovascularization | 3 | 4 | 4 | 0.51 | |
| Polymorphonuclear leukocyte density | 2 | 3 | 3 | 0.51 | |
| Lymphocyte density | 3 | 4 | 4 | 0.51 | |
| Muscular layer completeness | 1 | 3 | 1 | 0.91 |
| Variable | X2 | p |
|---|---|---|
| Reepithelization | 9.192 | 0.027 |
| Neovascularization | 7.577 | 0.056 |
| Polymorphonuclear leukocyte density | 9.616 | 0.022 |
| Lymphocyte density | 3.867 | 0.276 |
| Muscular layer completeness | 5.618 | 0.132 |
| Group | Reepithelization | PMNL |
|---|---|---|
| Sham-I/Sham-S | 0.064 | 0.003 |
| ShamI/DXP | 0.473 | 0.045 |
| Sham-I/Q10 | 0.135 | 0.044 |
| Sham-S/DXP | 0.025 | 0.289 |
| Sham-S/Q10 | 0.003 | 0.143 |
| DXP/Q10 | 0.473 | 0.854 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pehlivanlı, F.; Aydin, O.; Karaca, G.; Aydin, G.; Devrim, T.; Bulut, H.; Bakar, B.; Daphan, Ç.E. Healing of Ischemic Colon Anastomosis in Rats Could Be Provided by Administering Dexpanthenol or Coenzyme Q10. J. Clin. Med. 2018, 7, 161. https://doi.org/10.3390/jcm7070161
Pehlivanlı F, Aydin O, Karaca G, Aydin G, Devrim T, Bulut H, Bakar B, Daphan ÇE. Healing of Ischemic Colon Anastomosis in Rats Could Be Provided by Administering Dexpanthenol or Coenzyme Q10. Journal of Clinical Medicine. 2018; 7(7):161. https://doi.org/10.3390/jcm7070161
Chicago/Turabian StylePehlivanlı, Faruk, Oktay Aydin, Gökhan Karaca, Gülçin Aydin, Tuba Devrim, Huri Bulut, Bülent Bakar, and Çağatay Erden Daphan. 2018. "Healing of Ischemic Colon Anastomosis in Rats Could Be Provided by Administering Dexpanthenol or Coenzyme Q10" Journal of Clinical Medicine 7, no. 7: 161. https://doi.org/10.3390/jcm7070161
APA StylePehlivanlı, F., Aydin, O., Karaca, G., Aydin, G., Devrim, T., Bulut, H., Bakar, B., & Daphan, Ç. E. (2018). Healing of Ischemic Colon Anastomosis in Rats Could Be Provided by Administering Dexpanthenol or Coenzyme Q10. Journal of Clinical Medicine, 7(7), 161. https://doi.org/10.3390/jcm7070161





