Identifying Degenerative Brain Disease Using Rough Set Classifier Based on Wavelet Packet Method
Abstract
:1. Introduction
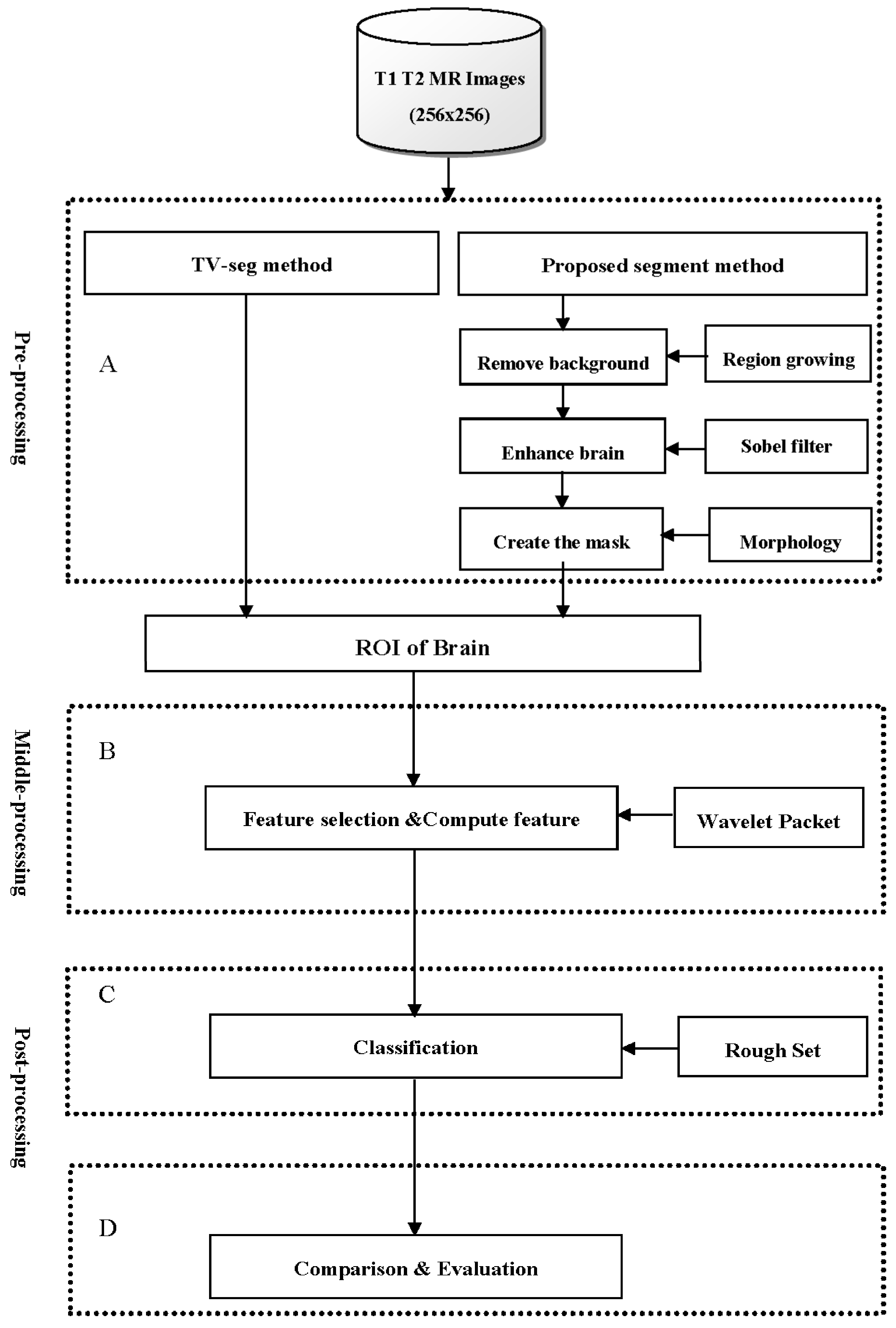
2. Materials and Methods
2.1. Segmentation Techniques
- Morphological operation
- Sobel Filter
2.2. TV-seg Algorithm
2.3. Discrete Wavelet Packet Transform
2.4. Rough Sets Theory
2.5. The Proposed Method
2.6. The Proposed Algorithm
| Algorithm 1. Feature calculation by discrete wavelet packet transform (DWPT) |
| Input: T1 and T2 Brain ROI images Step1: Apply bior2.2 filter in 2D DWPT to decompose Brain ROI Images Step2: Calculate the selected features according to the transformed coefficients Output: Generated nine feature values of each image after 2D DWPT |
3. Experiments and Results
4. Findings
5. Conclusions
Author Contributions
Conflicts of Interest
References
- McEvoy, L.K.; Holland, D.; Hagler, D.J., Jr.; Fennema-Notestine, C.; Brewer, J.B.; Dale, A.M. Mild Cognitive Impairment: Baseline and Longitudinal Structural MR Imaging Measures Improve Predictive Prognosis. Radiology 2011, 259, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, C.; Jiang, G.; Liu, W.; Tong, L. 3D texture analysis on MRI images of Alzheimer’s disease. Brain Imaging Behav. 2012, 6, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Filipovych, R.; Davatzikos, C. Semi-supervised pattern classification of medical images: Application to mild cognitive impairment (MCI). Neuroimage 2011, 55, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Guler, I.; Demirhan, A.; Karak, R. Interpretation of MR images using self-organizing maps and knowledge-based expert systems. Digit. Signal Process. 2009, 19, 668–677. [Google Scholar] [CrossRef]
- Chen, Y.B. A robust fully automatic scheme for general image Segmentation. Digit. Signal Process. 2010, 21, 87–99. [Google Scholar] [CrossRef]
- Chu, C.C.; Aggarwal, J.K. The integration of image segmentation maps using region and edge information. IEEE Trans. Pattern Anal. Mach. Intell. 1993, 15, 1241–1252. [Google Scholar] [CrossRef]
- Yu, X.; Yla-Jaaski, J.; Huttunen, O.; Vehkomaki, T.; Sipila, O.; Katila, T. Image segmentation combining region growing and edge detection. In Proceedings of the 11th IAPR International Conference on Pattern Recognition, The Hague, The Netherlands, 30 August–3 September 1992; Volume 3, pp. 481–484. [Google Scholar]
- Fan, J.; Yau, D.K.Y.; Elmagarmid, A.K.; Aref, W.G. Automatic Image Segmentation by Integrating Color-Edge Extraction and Seeded Region Growing. IEEE Trans. Image Process. 2001, 10, 1454–1466. [Google Scholar] [PubMed]
- Fan, S.L.X.; Man, Z.; Samur, R. Edge based region growing: A new image segmentation method. In Proceedings of the 2004 ACM SIGGRAPH International Conference on Virtual Reality Continuum and Its Applications in Industry, Singapore, 16–18 June 2004. [Google Scholar]
- Toulson, D.L.; Boyce, J.F. Segmentation of MR image using neural nets. Image Vis. Comput. 1992, 10, 324–348. [Google Scholar] [CrossRef]
- Brejl, M.; Sonka, M. Object Localization and Border Detection Criteria Design in Edge-Based Image Segmentation: Automated Learning from Examples. IEEE Trans. Med. Imaging 2000, 19, 973–985. [Google Scholar] [PubMed]
- Kazakova, N.; Margala, M.; Durdle, N.G. Sobel edge detection processor for a real-time volume rendering system. In Proceedings of the 2004 International Symposium on Circuits and Systems, Vancouver, BC, Canada, 23–26 May 2004; Volume 2, p. 913. [Google Scholar]
- Unger, M.; Pock, T.; Trobin, W.; Cremers, D.; Bischof, H. TVSeg-Interactive Total Variation Based Image Segmentation. In Proceedings of the British Machine Vision Conference (BMVC) 2008, England, UK, 1–4 September 2008. [Google Scholar]
- Caselles, V.; Kimmel, R.; Sapiro, G. Geodesic active contours. Int. J. Comput. Vis. 1997, 22, 61–79. [Google Scholar] [CrossRef]
- Haar, A. Theorie der orthogonalen funktionen-systeme. Math. Ann. 1910, 69, 331–371. [Google Scholar] [CrossRef]
- Meyer, Y. Ondettes et Functions Splines; Lectures; University of Torino: Turin, Italy, 1986. [Google Scholar]
- Mallat, S.G. A Theory for Multiresolution Signal Decomposition: The Wave Representation. Comm. Pure Appl. Math. 1988, 41, 674–693. [Google Scholar]
- Devasahayam, S.R. Signals and Systems in Biomedical Engineering; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Turkoglu, I.; Arslan, A.; Ilkay, E. An intelligent system for diagnosis of the heart valve diseases with wavelet packet neural Networks. Comput. Biol. Med. 2003, 33, 319–331. [Google Scholar] [CrossRef]
- Burrus, C.S.; Gopinath, R.A.; Guo, H. Introduction to Wavelet and Wavelet Transforms; Prentice-Hall: Upper Saddle River, NJ, USA, 1997. [Google Scholar]
- Pawlak, Z. Rough sets. Int. J. Comput. Inf. Sci. 1982, 11, 341–356. [Google Scholar] [CrossRef]
- Feng, L.; Li, T.; Ruan, D.; Gou, S. A vague-rough set approach for uncertain knowledge acquisition. Knowl. Based Syst. 2011, 24, 837–843. [Google Scholar] [CrossRef]
- Pun, C.M.; Zhu, H.M. Image Segmentation Using Discrete Cosine Texture Feature. Int. J. Comput. 2010, 4, 19–26. [Google Scholar]
- Charfi, M.; Nyeck, A.; Tosser, A. Focus Criterion. Electron. Lett. 1991, 27, 1233–1235. [Google Scholar] [CrossRef]
- Avci, E. Comparison of wavelet families for texture classification by using wavelet packet entropy adaptive network based fuzzy inference system. Appl. Soft Comput. 2008, 8, 225–231. [Google Scholar] [CrossRef]
- Harvard Medical School Website. Available online: http://med.harvard.edu/AANLIB/ (accessed on 26 May 2018).
- Quinlan, J.R. C4.5: Programs for Machine Learning; Morgan Kauffman: San Francisco, CA, USA, 1993. [Google Scholar]
- Baker, L.D.; McCallum, A.K. Distributional Clustering of Words for Text Classification. In Proceedings of the Sigir-98, 21st ACM International Conference on Research and Development in Information Retrieval, Melbourne, Australia, 24–28 August 1998; pp. 96–103. [Google Scholar]
- Vapnik, V. The Nature of Statistical Learning Theory; Springer: New York, NY, USA, 1995. [Google Scholar]
- Telagarapu, P.; Jagan, N.V.; Lakshmi, P.A.; Vijaya, S.G. Image Compression Using DCT and Wavelet Transformations. Int. J. Signal Process. Image Process. Pattern Recognit. 2011, 4, 61–74. [Google Scholar]
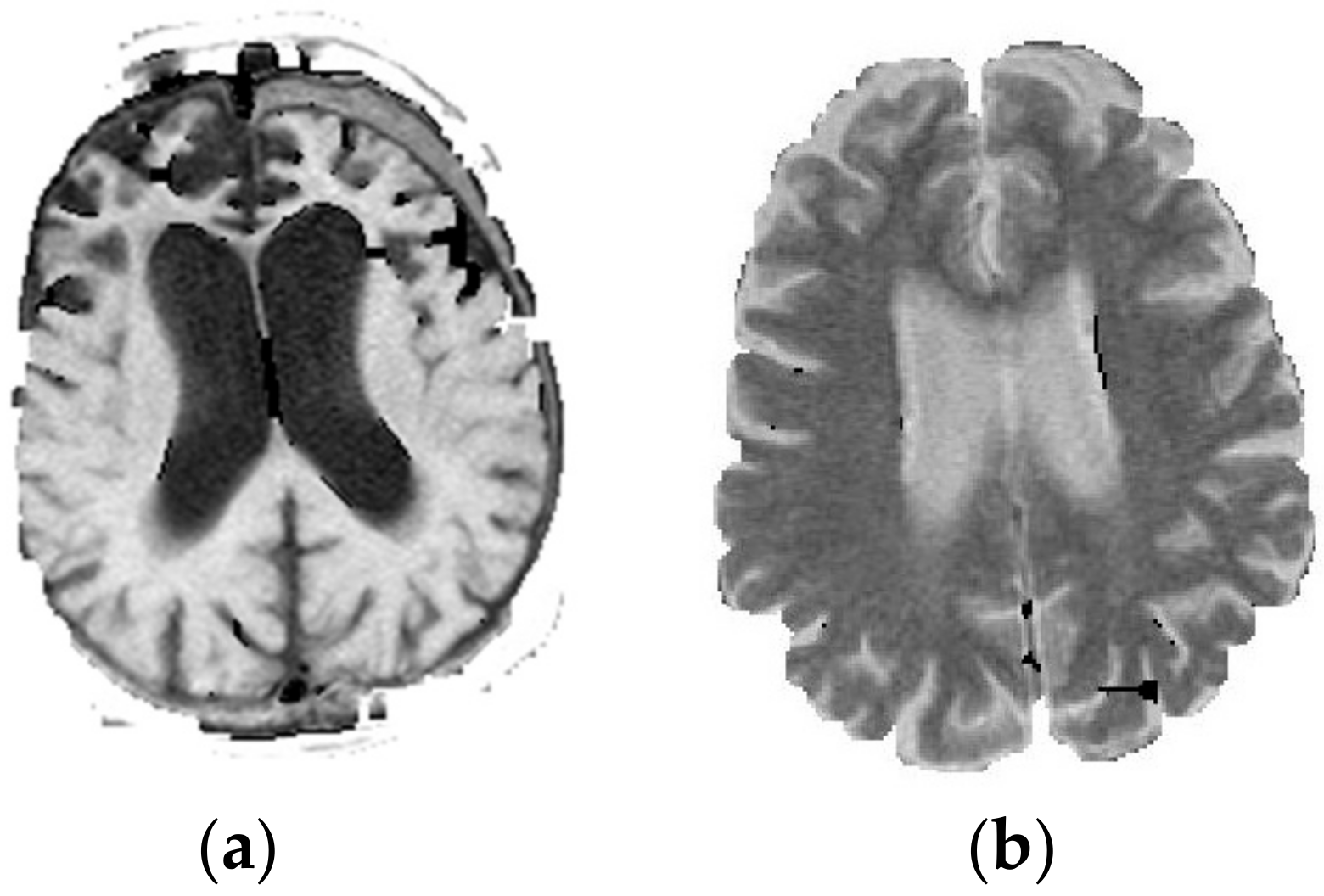
| T1 | T2 | |
|---|---|---|
| Normal | 14 | 40 |
| Abnormal | 28 | 60 |
| Total | 42 | 100 |
| Dataset | Segmentation Algorithm | RS | C4.5 | Naïve Bayes | SVM |
|---|---|---|---|---|---|
| T1 | Proposed | 99.89 (0.33) | 89.96 (16.26) | 94.99 (6.82) | 93.60 (10.20) |
| T1 | TV-seg | 94.25 (7.29) | 73.01 (9.01) | 77.83 (6.41) | 66.51 (3.19) |
| T2 | Proposed | 99.36 (1.22) | 93.53 (3.37) | 95.30 (2.85) | 95.90 (2.83) |
| T2 | TV-seg | 98.7 (2.57) | 91.81 (3.28) | 91.79 (3.55) | 90.94 (4.77) |
| Dataset | Method | RS | C4.5 | Naïve Bayes | SVM | |
|---|---|---|---|---|---|---|
| T1 | Proposed | DWPT | 99.89 (0.33.) | 89.96 (16.26) | 94.99 (6.82) | 93.60 (10.20) |
| T1 | Proposed | DCT | 97.03 (3.8) | 85.20 (8.73) | 86.54 (7.31) | 85.06 (11.04) |
| T1 | TV-seg | DWPT | 94.25 (7.29) | 73.01 (9.01) | 77.83 (6.41) | 66.51 (3.19) |
| T1 | TV-seg | DCT | 93.65 (6.23) | 71.26 (10.79) | 78.64 (7.47) | 65.75 (3.44) |
| T2 | Proposed | DWPT | 99.36 (1.22) | 93.53 (3.37) | 95.30 (2.85) | 95.90 (2.83) |
| T2 | Proposed | DCT | 97.79 (2.42) | 92.95 (3.01) | 95.89 (3.72) | 95.02 (3.92) |
| T2 | TV-seg | DWPT | 98.7 (2.57) | 91.81 (3.28) | 91.79 (3.55) | 90.94 (4.77) |
| T2 | TV-seg | DCT | 97.15 (4.05) | 87.69 (5.65) | 90.05 (5.65) | 86.22 (3.37) |
| Proposed vs. TV-seg | DWPT vs. DCT | |
|---|---|---|
| T1 | 5.635 *** | 7.836 *** |
| T2 | 4.488 *** | 6.149 *** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-H.; Liu, W.-X. Identifying Degenerative Brain Disease Using Rough Set Classifier Based on Wavelet Packet Method. J. Clin. Med. 2018, 7, 124. https://doi.org/10.3390/jcm7060124
Cheng C-H, Liu W-X. Identifying Degenerative Brain Disease Using Rough Set Classifier Based on Wavelet Packet Method. Journal of Clinical Medicine. 2018; 7(6):124. https://doi.org/10.3390/jcm7060124
Chicago/Turabian StyleCheng, Ching-Hsue, and Wei-Xiang Liu. 2018. "Identifying Degenerative Brain Disease Using Rough Set Classifier Based on Wavelet Packet Method" Journal of Clinical Medicine 7, no. 6: 124. https://doi.org/10.3390/jcm7060124
APA StyleCheng, C.-H., & Liu, W.-X. (2018). Identifying Degenerative Brain Disease Using Rough Set Classifier Based on Wavelet Packet Method. Journal of Clinical Medicine, 7(6), 124. https://doi.org/10.3390/jcm7060124





