Role of Stromal Paracrine Signals in Proliferative Diseases of the Aging Human Prostate
Abstract
1. Introduction
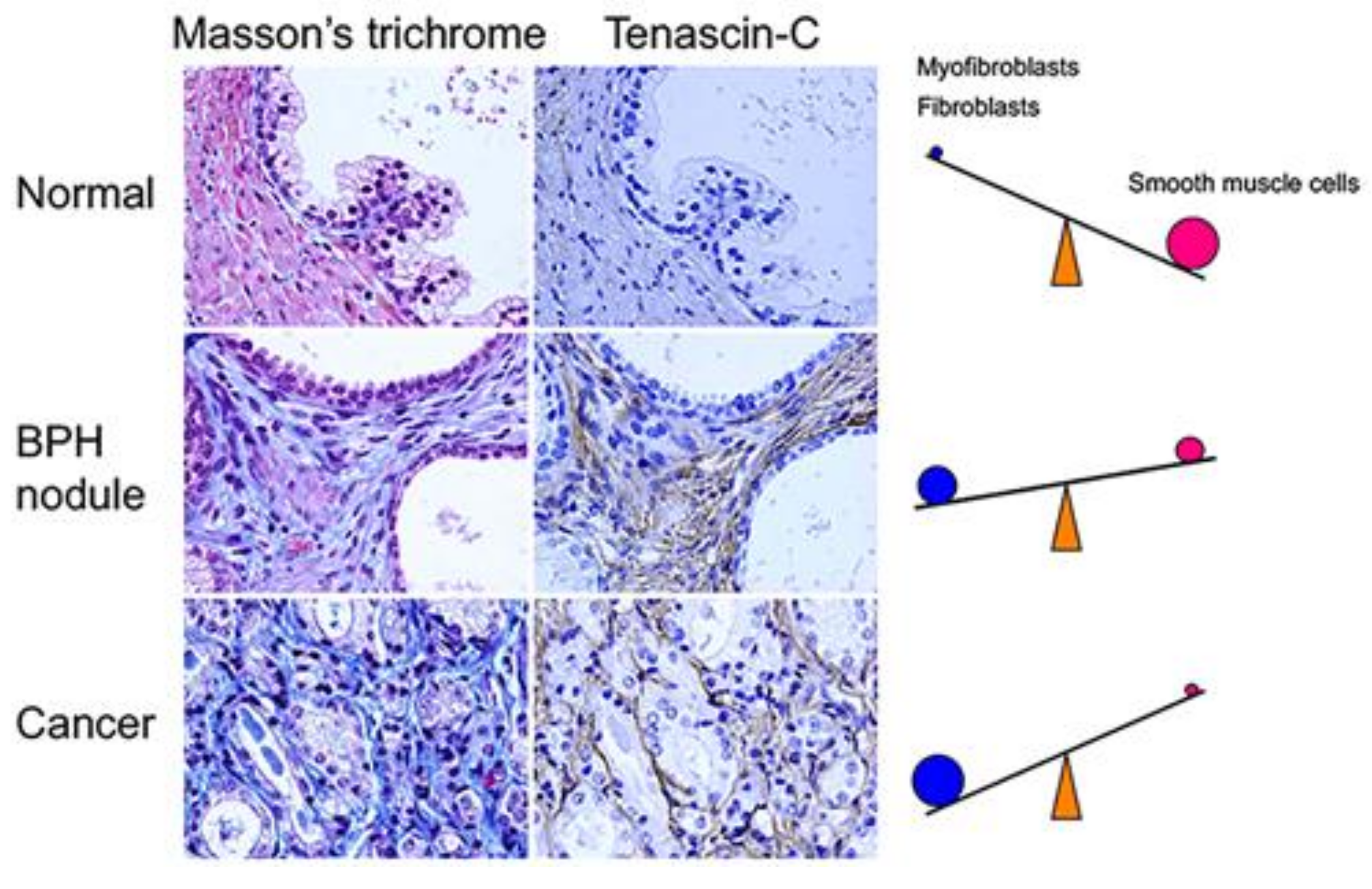
2. Benign Prostatic Hyperplasia (BPH)
3. Alteration of Stromal Structure by α1-Adrenoceptor Antagonists (α1-Blockers)
4. Aberrant Activation of Epithelial-Stromal Interactions in BPH
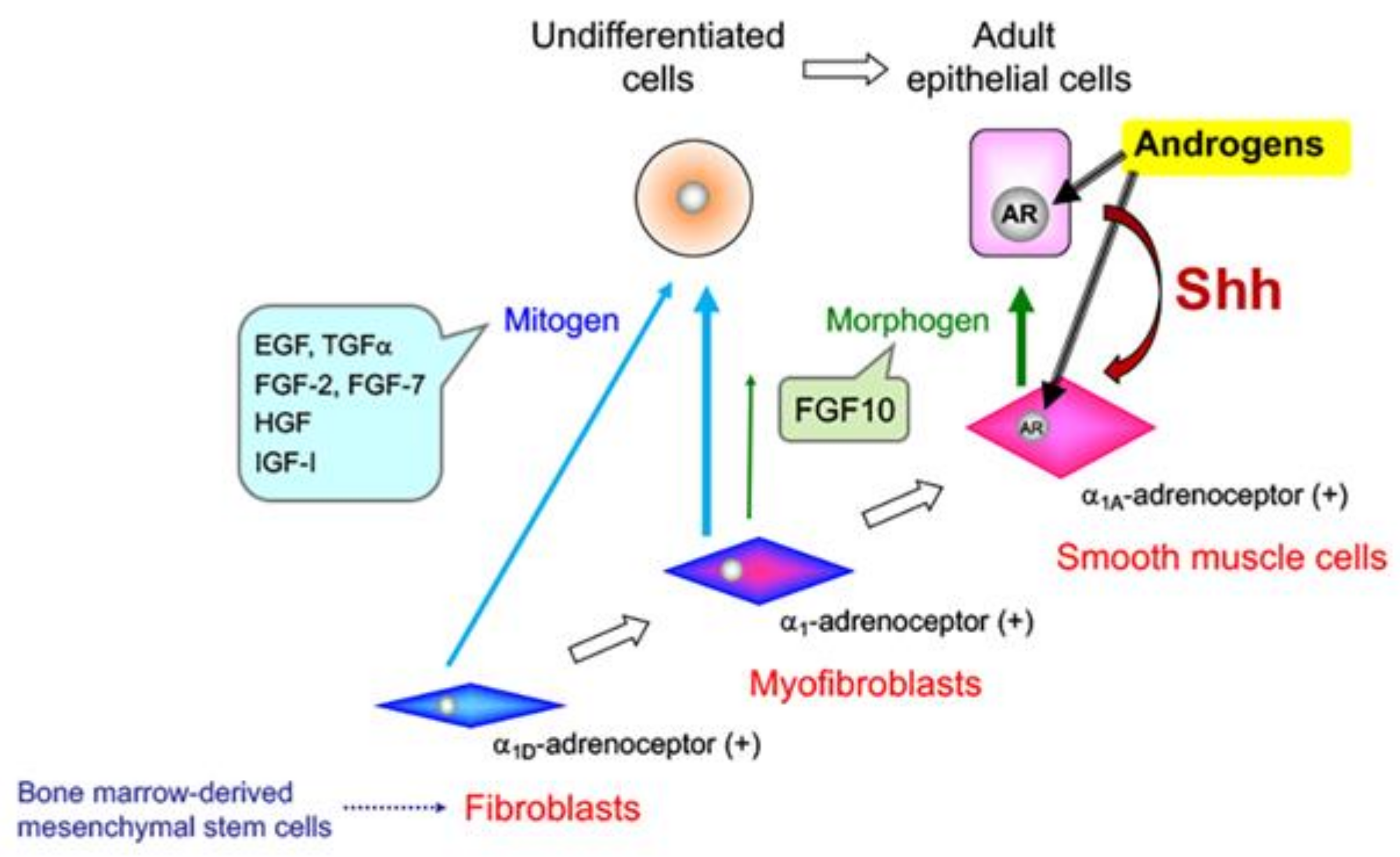
5. Effects of Sex Steroid Hormone Status on Basal Epithelial Cell Behavior in the Prostate
6. Prostate Cancer (PCa)
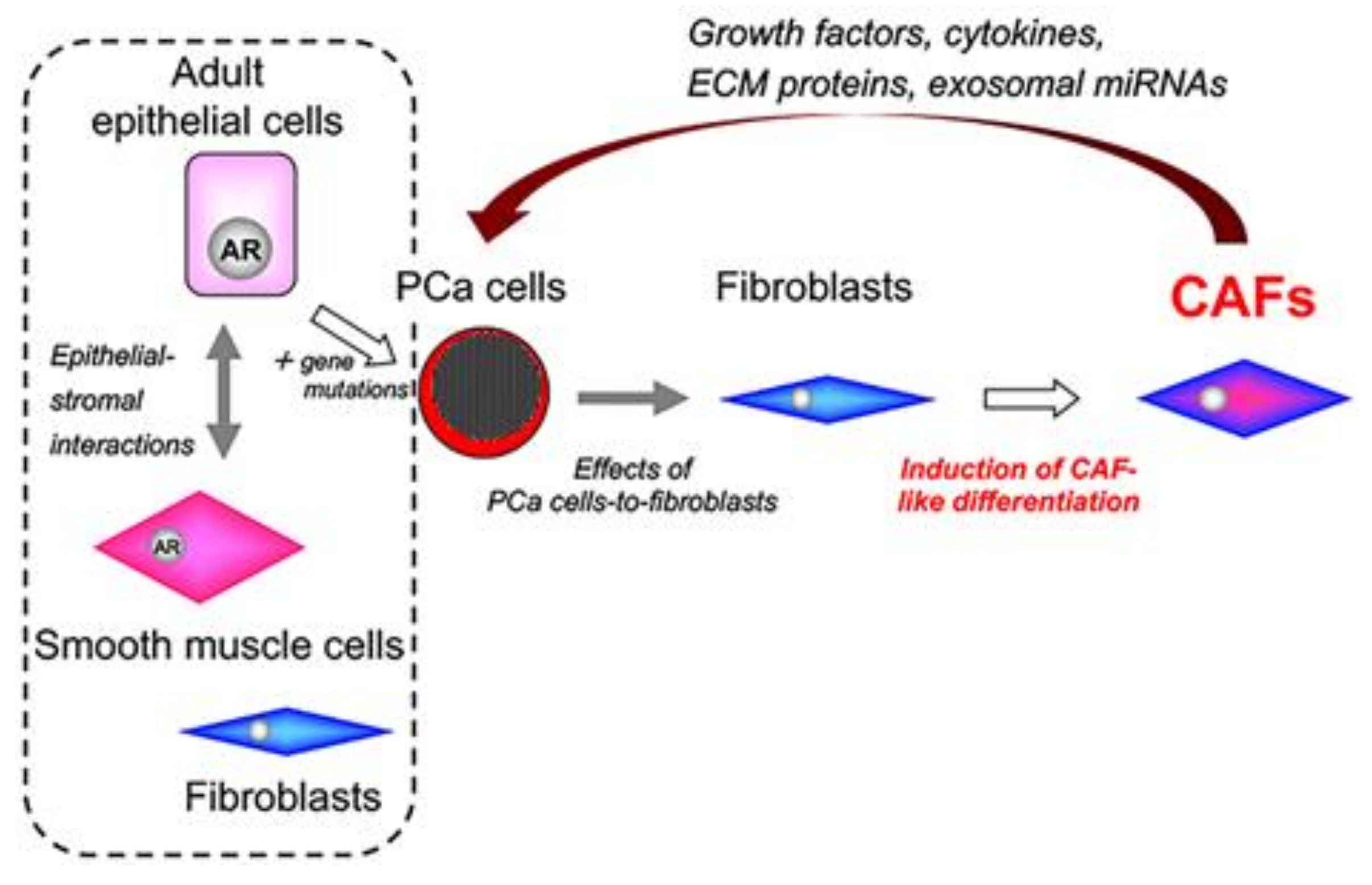
7. Tumor–Stromal Interactions in PCa
8. PCa Cell Lines with Different Levels of Androgen Sensitivity
9. Origins of Cell Populations Composed of Tumor Stroma
10. Characteristics of Cell Populations Composed of Tumor Stroma
11. Can We Discover New Biomarkers from Heterogeneous Stroma in PCa?
12. Role of CAF-Derived Exosomal miRNAs in Aberrant Activation of Tumor–Stromal Interactions
13. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cunha, G.R.; Donjacour, A.A.; Cooke, P.S.; Mee, S.; Bigsby, R.M.; Higgins, S.J.; Sugimura, Y. The endocrinology and developmental biology of the prostate. Endocr. Rev. 1987, 8, 338–362. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.R.; Chung, L.W.; Shannon, J.M.; Reese, B.A. Stromal-epithelial interactions in sex differentiation. Biol. Reprod. 1980, 22, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Simian, M.; Hirai, Y.; Navre, M.; Werb, Z.; Lochter, A.; Bissell, M.J. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development 2001, 128, 3117–3131. [Google Scholar] [PubMed]
- Cunha, G.R. Epithelial-stromal interactions in development of the urogenital tract. Int. Rev. Cytol. 1976, 47, 137–194. [Google Scholar] [PubMed]
- Ishii, K.; Imanaka-Yoshida, K.; Yoshida, T.; Sugimura, Y. Role of stromal tenascin-C in mouse prostatic development and epithelial cell differentiation. Dev. Biol. 2008, 324, 310–319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hayward, S.W.; Rosen, M.A.; Cunha, G.R. Stromal-epithelial interactions in the normal and neoplastic prostate. Br. J. Urol. 1997, 79 (Suppl. 2), 18–26. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.S.; Scarano, W.R.; Campos, S.G.; Santos, F.C.; Vilamaior, P.S.; Goes, R.M.; Taboga, S.R. Androgen receptor in the mongolian gerbil ventral prostate: Evaluation during different phases of postnatal development and following androgen blockage. Micron 2008, 39, 1312–1324. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.M.; Chung, L.W. Interaction between prostatic fibroblast and epithelial cells in culture: Role of androgen. Endocrinology 1989, 125, 2719–2727. [Google Scholar] [CrossRef] [PubMed]
- Ohlson, N.; Bergh, A.; Stattin, P.; Wikstrom, P. Castration-induced epithelial cell death in human prostate tissue is related to locally reduced IGF-1 levels. Prostate 2007, 67, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.I.; Misawa, A.; Inoue, S. Significance of microRNAs in androgen signaling and prostate cancer progression. Cancers 2017, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Hayward, S.W.; Cunha, G.R. The prostate: Development and physiology. Radiol. Clin. N. Am. 2000, 38, 1–14. [Google Scholar] [CrossRef]
- Cunha, G.R.; Ricke, W.; Thomson, A.; Marker, P.C.; Risbridger, G.; Hayward, S.W.; Wang, Y.Z.; Donjacour, A.A.; Kurita, T. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J. Steroid Biochem. Mol. Biol. 2004, 92, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, Y.; Foster, B.A.; Hom, Y.K.; Lipschutz, J.H.; Rubin, J.S.; Finch, P.W.; Aaronson, S.A.; Hayashi, N.; Kawamura, J.; Cunha, G.R. Keratinocyte growth factor (KGF) can replace testosterone in the ductal branching morphogenesis of the rat ventral prostate. Int. J. Dev. Biol. 1996, 40, 941–951. [Google Scholar] [PubMed]
- Cunha, G.R.; Hayward, S.W.; Dahiya, R.; Foster, B.A. Smooth muscle-epithelial interactions in normal and neoplastic prostatic development. Acta Anat. (Basel) 1996, 155, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Ito, K.; Suzuki, K.; Nakano, K.; Fukabori, Y.; Suzuki, R.; Kawabe, Y.; Honma, S.; Yamanaka, H. Changes in the endocrine environment of the human prostate transition zone with aging: Simultaneous quantitative analysis of prostatic sex steroids and comparison with human prostatic histological composition. Prostate 2000, 42, 45–55. [Google Scholar] [CrossRef]
- English, H.F.; Santen, R.J.; Isaacs, J.T. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate 1987, 11, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, E.; Cardoso, A.B.; Carvalho, H.F. Effects of long-term castration on the smooth muscle cell phenotype of the rat ventral prostate. J. Androl. 2007, 28, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Shidaifat, F.; Daradka, M.; Al-Omari, R. Effect of androgen ablation on prostatic cell differentiation in dogs. Endocr. Res. 2004, 30, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ishii, K.; Iwamoto, Y.; Sasaki, T.; Kanda, H.; Yamada, Y.; Arima, K.; Shiraishi, T.; Sugimura, Y. Activation of FGF2-FGFR signaling in the castrated mouse prostate stimulates the proliferation of basal epithelial cells. Biol. Reprod. 2013, 89, 81. [Google Scholar] [CrossRef] [PubMed]
- Vilamaior, P.S.; Taboga, S.R.; Carvalho, H.F. Modulation of smooth muscle cell function: Morphological evidence for a contractile to synthetic transition in the rat ventral prostate after castration. Cell Biol. Int. 2005, 29, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, Y.; Cunha, G.R.; Donjacour, A.A. Morphological and histological study of castration-induced degeneration and androgen-induced regeneration in the mouse prostate. Biol. Reprod. 1986, 34, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Tuohimaa, P.; Niemi, M. The effect of testosterone on cell renewal and mitotic cycles in sex accessory glands of castrated mice. Acta Endocrinol. (Copenh.) 1968, 58, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Morley, A.; Wright, N.; Appleton, D.; Alison, M. A cytokinetic analysis of the proliferative response to androgen in the prostatic complex of the castrated mouse. Biochem. Soc. Trans. 1973, 1, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.G.; Wang, B.E.; Johnson, L.; Gao, W.Q. Generation of a prostate from a single adult stem cell. Nature 2008, 456, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.T.; Coffey, D.S. Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl. 1989, 2, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.T. Prostate stem cells and benign prostatic hyperplasia. Prostate 2008, 68, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, A.; Hammerer, P.; Tubaro, A.; Schroder, F.H.; Castro, R. Is there evidence of a relationship between benign prostatic hyperplasia and prostate cancer? Findings of a literature review. Eur. Urol. 2009, 55, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Bostwick, D.G.; Cooner, W.H.; Denis, L.; Jones, G.W.; Scardino, P.T.; Murphy, G.P. The association of benign prostatic hyperplasia and cancer of the prostate. Cancer 1992, 70, 291–301. [Google Scholar] [CrossRef]
- Berry, S.J.; Coffey, D.S.; Walsh, P.C.; Ewing, L.L. The development of human benign prostatic hyperplasia with age. J. Urol. 1984, 132, 474–479. [Google Scholar] [CrossRef]
- Fujikawa, S.; Matsuura, H.; Kanai, M.; Fumino, M.; Ishii, K.; Arima, K.; Shiraishi, T.; Sugimura, Y. Natural history of human prostate gland: Morphometric and histopathological analysis of Japanese men. Prostate 2005, 65, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Ishigooka, M.; Hayami, S.; Hashimoto, T.; Suzuki, Y.; Katoh, T.; Nakada, T. Relative and total volume of histological components in benign prostatic hyperplasia: Relationships between histological components and clinical findings. Prostate 1996, 29, 77–82. [Google Scholar] [CrossRef]
- Ichiyanagi, O.; Sasagawa, I.; Ishigooka, M.; Suzuki, Y.; Nakada, T. Morphometric analysis of symptomatic benign prostatic hyperplasia with and without bladder outlet obstruction. Urol. Res. 2000, 28, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Djavan, B.; Marberger, M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur. Urol. 1999, 36, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Rhodes, N.P.; Ke, Y.; Foster, C.S. Influence of the alpha1-adrenergic antagonist, doxazosin, on noradrenaline-induced modulation of cytoskeletal proteins in cultured hyperplastic prostatic stromal cells. Prostate 1999, 38, 216–227. [Google Scholar] [CrossRef]
- Justulin, L.A., Jr.; Delella, F.K.; Felisbino, S.L. Doxazosin reduces cell proliferation and increases collagen fibers in rat prostatic lobes. Cell Tissue Res. 2008, 332, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Ishii, K.; Kanda, H.; Arase, S.; Yoshio, Y.; Hori, Y.; Soga, N.; Kise, H.; Arima, K.; Sugimura, Y. Structural changes in alpha1-adrenoceptor antagonist-treated human prostatic stroma. Clin. Exp. Med. 2010, 10, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-beta and fibrosis. World J. Gastroenterol. 2007, 13, 3056–3062. [Google Scholar] [CrossRef] [PubMed]
- Olumi, A.F.; Grossfeld, G.D.; Hayward, S.W.; Carroll, P.R.; Tlsty, T.D.; Cunha, G.R. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999, 59, 5002–5011. [Google Scholar] [PubMed]
- Bhowmick, N.A.; Chytil, A.; Plieth, D.; Gorska, A.E.; Dumont, N.; Shappell, S.; Washington, M.K.; Neilson, E.G.; Moses, H.L. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 2004, 303, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Bierhoff, E.; Vogel, J.; Benz, M.; Giefer, T.; Wernert, N.; Pfeifer, U. Stromal nodules in benign prostatic hyperplasia. Eur. Urol. 1996, 29, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.K.; Chandra, K. Morphogenesis of nodular hyperplasia–prostate. J. Urol. 1975, 113, 210–213. [Google Scholar] [CrossRef]
- McNeal, J.E. Origin and evolution of benign prostatic enlargement. Investig. Urol. 1978, 15, 340–345. [Google Scholar]
- Norman, J.T.; Cunha, G.R.; Sugimura, Y. The induction of new ductal growth in adult prostatic epithelium in response to an embryonic prostatic inductor. Prostate 1986, 8, 209–220. [Google Scholar] [CrossRef] [PubMed]
- De Marzo, A.M.; Nelson, W.G.; Meeker, A.K.; Coffey, D.S. Stem cell features of benign and malignant prostate epithelial cells. J. Urol. 1998, 160, 2381–2392. [Google Scholar] [CrossRef]
- Collins, A.T.; Habib, F.K.; Maitland, N.J.; Neal, D.E. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J. Cell Sci. 2001, 114, 3865–3872. [Google Scholar] [PubMed]
- Wang, Y.; Hayward, S.; Cao, M.; Thayer, K.; Cunha, G. Cell differentiation lineage in the prostate. Differentiation 2001, 68, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.L. Epithelial stem cells in human prostate growth and disease. Prostate Cancer Prostatic Dis. 2004, 7, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Kurita, T.; Medina, R.T.; Mills, A.A.; Cunha, G.R. Role of p63 and basal cells in the prostate. Development 2004, 131, 4955–4964. [Google Scholar] [CrossRef] [PubMed]
- Maitland, N.J.; Frame, F.M.; Polson, E.S.; Lewis, J.L.; Collins, A.T. Prostate cancer stem cells: Do they have a basal or luminal phenotype? Horm. Cancer 2011, 2, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Cleary, K.R.; Choi, H.Y.; Ayala, A.G. Basal cell hyperplasia of the prostate. Am. J. Clin. Pathol. 1983, 80, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Dermer, G.B. Basal cell proliferation in benign prostatic hyperplasia. Cancer 1978, 41, 1857–1862. [Google Scholar] [CrossRef]
- Rioux-Leclercq, N.C.; Epstein, J.I. Unusual morphologic patterns of basal cell hyperplasia of the prostate. Am. J. Surg. Pathol. 2002, 26, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Garcia, A.J.; Wu, M.; Lawson, D.A.; Witte, O.N.; Wu, H. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc. Natl. Acad. Sci. USA 2006, 103, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Mirosevich, J.; Bentel, J.M.; Zeps, N.; Redmond, S.L.; D’Antuono, M.F.; Dawkins, H.J. Androgen receptor expression of proliferating basal and luminal cells in adult murine ventral prostate. J. Endocrinol. 1999, 162, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, Y.; Sakurai, M.; Hayashi, N.; Yamashita, A.; Kawamura, J. Age-related changes of the prostate gland in the senescence-accelerated mouse. Prostate 1994, 24, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Leav, I.; Schelling, K.H.; Adams, J.Y.; Merk, F.B.; Alroy, J. Role of canine basal cells in prostatic post natal development, induction of hyperplasia, sex hormone-stimulated growth; and the ductal origin of carcinoma. Prostate 2001, 47, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Waltregny, D.; Leav, I.; Signoretti, S.; Soung, P.; Lin, D.; Merk, F.; Adams, J.Y.; Bhattacharya, N.; Cirenei, N.; Loda, M. Androgen-driven prostate epithelial cell proliferation and differentiation in vivo involve the regulation of p27. Mol. Endocrinol. 2001, 15, 765–782. [Google Scholar] [CrossRef] [PubMed]
- Nishi, N.; Oya, H.; Matsumoto, K.; Nakamura, T.; Miyanaka, H.; Wada, F. Changes in gene expression of growth factors and their receptors during castration-induced involution and androgen-induced regrowth of rat prostates. Prostate 1996, 28, 139–152. [Google Scholar] [CrossRef]
- Kyprianou, N.; Isaacs, J.T. Expression of transforming growth factor-beta in the rat ventral prostate during castration-induced programmed cell death. Mol. Endocrinol. 1989, 3, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.M.; Kovalenko, B.; Huang, Y.; Moscatelli, D. Vascular endothelial growth factor and angiopoietin are required for prostate regeneration. Prostate 2007, 67, 485–499. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donjacour, A.A.; Thomson, A.A.; Cunha, G.R. FGF-10 plays an essential role in the growth of the fetal prostate. Dev. Biol. 2003, 261, 39–54. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, F. Fgf signalling in prostate development, tissue homoeostasis and tumorigenesis. Biosci. Rep. 2010, 30, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Risbridger, G.; Wang, H.; Young, P.; Kurita, T.; Wang, Y.Z.; Lubahn, D.; Gustafsson, J.A.; Cunha, G. Evidence that epithelial and mesenchymal estrogen receptor-alpha mediates effects of estrogen on prostatic epithelium. Dev. Biol. 2001, 229, 432–442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tunn, U.; Senge, T.; Schenck, B.; Neumann, F. Biochemical and histological studies on prostates in castrated dogs after treatment with androstanediol, oestradiol and cyproterone acetate. Acta Endocrinol. (Copenh.) 1979, 91, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Timms, B.G.; Howdeshell, K.L.; Barton, L.; Bradley, S.; Richter, C.A.; vom Saal, F.S. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc. Natl. Acad. Sci. USA 2005, 102, 7014–7019. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Ishii, K.; Kanda, H.; Kanai, M.; Arima, K.; Wang, Y.; Sugimura, Y. Bisphenol a induces permanent squamous change in mouse prostatic epithelium. Differentiation 2007, 75, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Arase, S.; Ishii, K.; Igarashi, K.; Aisaki, K.; Yoshio, Y.; Matsushima, A.; Shimohigashi, Y.; Arima, K.; Kanno, J.; Sugimura, Y. Endocrine disrupter bisphenol a increases in situ estrogen production in the mouse urogenital sinus. Biol. Reprod. 2011, 84, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Ciana, P.; Ghisletti, S.; Mussi, P.; Eberini, I.; Vegeto, E.; Maggi, A. Estrogen receptor alpha, a molecular switch converting transforming growth factor-alpha-mediated proliferation into differentiation in neuroblastoma cells. J. Biol. Chem. 2003, 278, 31737–31744. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, P.J.; Leav, I.; Greenwood, J.; Kwan, P.W.; Ho, S.M. Involvement of transforming growth factor alpha (TGFalpha) and epidermal growth factor receptor (EGFR) in sex hormone-induced prostatic dysplasia and the growth of an androgen-independent transplantable carcinoma of the prostate. Carcinogenesis 1996, 17, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, Y.; Ishii, K.; Arase, S.; Hori, Y.; Nishikawa, K.; Soga, N.; Kise, H.; Arima, K.; Sugimura, Y. Effect of transforming growth factor alpha overexpression on urogenital organ development in mouse. Differentiation 2010, 80, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Gronberg, H. Prostate cancer epidemiology. Lancet 2003, 361, 859–864. [Google Scholar] [CrossRef]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. J. Urol. 2002, 168, 9–12. [Google Scholar] [CrossRef]
- Fizazi, K.; Higano, C.S.; Nelson, J.B.; Gleave, M.; Miller, K.; Morris, T.; Nathan, F.E.; McIntosh, S.; Pemberton, K.; Moul, J.W. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2013, 31, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Baldi, E.; Bonaccorsi, L.; Forti, G. Androgen receptor: Good guy or bad guy in prostate cancer invasion? Endocrinology 2003, 144, 1653–1655. [Google Scholar] [CrossRef] [PubMed]
- Jennbacken, K.; Tesan, T.; Wang, W.; Gustavsson, H.; Damber, J.E.; Welen, K. N-cadherin increases after androgen deprivation and is associated with metastasis in prostate cancer. Endocr. Relat. Cancer 2010, 17, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.S. Molecular states underlying androgen receptor activation: A framework for therapeutics targeting androgen signaling in prostate cancer. J. Clin. Oncol. 2012, 30, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Burchardt, T.; Burchardt, M.; Chen, M.W.; Cao, Y.; de la Taille, A.; Shabsigh, A.; Hayek, O.; Dorai, T.; Buttyan, R. Transdifferentiation of prostate cancer cells to a neuroendocrine cell phenotype in vitro and in vivo. J. Urol. 1999, 162, 1800–1805. [Google Scholar] [CrossRef]
- Yuan, T.C.; Veeramani, S.; Lin, F.F.; Kondrikou, D.; Zelivianski, S.; Igawa, T.; Karan, D.; Batra, S.K.; Lin, M.F. Androgen deprivation induces human prostate epithelial neuroendocrine differentiation of androgen-sensitive lncap cells. Endocr. Relat. Cancer 2006, 13, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.R. Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer 1994, 74, 1030–1044. [Google Scholar] [CrossRef]
- Noel, A.; Foidart, J.M. The role of stroma in breast carcinoma growth in vivo. J. Mammary Gland Biol. Neoplasia 1998, 3, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Ronnov-Jessen, L.; Petersen, O.W.; Bissell, M.J. Cellular changes involved in conversion of normal to malignant breast: Importance of the stromal reaction. Physiol. Rev. 1996, 76, 69–125. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Pujuguet, P.; Martin, F. Role of stromal myofibroblasts infiltrating colon cancer in tumor invasion. Pathol. Res. Pract. 1996, 192, 712–717. [Google Scholar] [CrossRef]
- Yang, F.; Tuxhorn, J.A.; Ressler, S.J.; McAlhany, S.J.; Dang, T.D.; Rowley, D.R. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005, 65, 8887–8895. [Google Scholar] [CrossRef] [PubMed]
- Tuxhorn, J.A.; McAlhany, S.J.; Dang, T.D.; Ayala, G.E.; Rowley, D.R. Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. Cancer Res. 2002, 62, 3298–3307. [Google Scholar] [PubMed]
- Tuxhorn, J.A.; Ayala, G.E.; Smith, M.J.; Smith, V.C.; Dang, T.D.; Rowley, D.R. Reactive stroma in human prostate cancer: Induction of myofibroblast phenotype and extracellular matrix remodeling. Clin. Cancer Res. 2002, 8, 2912–2923. [Google Scholar] [PubMed]
- Orimo, A.; Weinberg, R.A. Stromal fibroblasts in cancer: A novel tumor-promoting cell type. Cell Cycle 2006, 5, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wollan, P.; Bostwick, D.G. The extent and multicentricity of high-grade prostatic intraepithelial neoplasia in clinically localized prostatic adenocarcinoma. Hum. Pathol. 1997, 28, 143–148. [Google Scholar] [CrossRef]
- Franco, O.E.; Jiang, M.; Strand, D.W.; Peacock, J.; Fernandez, S.; Jackson, R.S., 2nd; Revelo, M.P.; Bhowmick, N.A.; Hayward, S.W. Altered TGF-beta signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011, 71, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Kiskowski, M.A.; Jackson, R.S., 2nd; Banerjee, J.; Li, X.; Kang, M.; Iturregui, J.M.; Franco, O.E.; Hayward, S.W.; Bhowmick, N.A. Role for stromal heterogeneity in prostate tumorigenesis. Cancer Res. 2011, 71, 3459–3470. [Google Scholar] [CrossRef] [PubMed]
- Ayala, G.; Tuxhorn, J.A.; Wheeler, T.M.; Frolov, A.; Scardino, P.T.; Ohori, M.; Wheeler, M.; Spitler, J.; Rowley, D.R. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin. Cancer Res. 2003, 9, 4792–4801. [Google Scholar] [PubMed]
- Yanagisawa, N.; Li, R.; Rowley, D.; Liu, H.; Kadmon, D.; Miles, B.J.; Wheeler, T.M.; Ayala, G.E. Stromogenic prostatic carcinoma pattern (carcinomas with reactive stromal grade 3) in needle biopsies predicts biochemical recurrence-free survival in patients after radical prostatectomy. Hum. Pathol. 2007, 38, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Schoepp, M.; Strose, A.J.; Haier, J. Dysregulation of miRNA expression in cancer associated fibroblasts (CAFs) and its consequences on the tumor microenvironment. Cancers 2017, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, T.; Furusato, B.; Takashima, Y.; Ravulapalli, S.; Dobi, A.; Srivastava, S.; McLeod, D.G.; Sesterhenn, I.A.; Ornstein, D.K.; Shirasawa, S. The increased expression of periostin during early stages of prostate cancer and advanced stages of cancer stroma. Prostate 2009, 69, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Peehl, D.M. Tumor-promoting phenotype of CD90hi prostate cancer-associated fibroblasts. Prostate 2009, 69, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- San Francisco, I.F.; DeWolf, W.C.; Peehl, D.M.; Olumi, A.F. Expression of transforming growth factor-beta 1 and growth in soft agar differentiate prostate carcinoma-associated fibroblasts from normal prostate fibroblasts. Int. J. Cancer 2004, 112, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Mizokami, A.; Tsunoda, T.; Iguchi, K.; Kato, M.; Hori, Y.; Arima, K.; Namiki, M.; Sugimura, Y. Heterogenous induction of carcinoma-associated fibroblast-like differentiation in normal human prostatic fibroblasts by co-culturing with prostate cancer cells. J. Cell. Biochem. 2011, 112, 3604–3611. [Google Scholar] [CrossRef] [PubMed]
- Mizokami, A.; Koh, E.; Izumi, K.; Narimoto, K.; Takeda, M.; Honma, S.; Dai, J.; Keller, E.T.; Namiki, M. Prostate cancer stromal cells and lncap cells coordinately activate the androgen receptor through synthesis of testosterone and dihydrotestosterone from dehydroepiandrosterone. Endocr. Relat. Cancer 2009, 16, 1139–1155. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, H.; Xie, Z.; Qian, W.; Zhau, H.E.; Chung, L.W.; Marshall, F.F.; Wang, R. Matched pairs of human prostate stromal cells display differential tropic effects on LNCaP prostate cancer cells. In Vitro Cell. Dev. Biol. Anim. 2010, 46, 538–546. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, C.C.; Bissell, M.J.; Barcellos-Hoff, M.H. The influence of the microenvironment on the malignant phenotype. Mol. Med. Today 2000, 6, 324–329. [Google Scholar] [CrossRef]
- Bhowmick, N.A.; Moses, H.L. Tumor-stroma interactions. Curr. Opin. Genet. Dev. 2005, 15, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Verona, E.V.; Elkahloun, A.G.; Yang, J.; Bandyopadhyay, A.; Yeh, I.T.; Sun, L.Z. Transforming growth factor-beta signaling in prostate stromal cells supports prostate carcinoma growth by up-regulating stromal genes related to tissue remodeling. Cancer Res. 2007, 67, 5737–5746. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chung, L.W.; Baseman, A.; Assikis, V.; Zhau, H.E. Molecular insights into prostate cancer progression: The missing link of tumor microenvironment. J. Urol. 2005, 173, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Placencio, V.R.; Sharif-Afshar, A.R.; Li, X.; Huang, H.; Uwamariya, C.; Neilson, E.G.; Shen, M.M.; Matusik, R.J.; Hayward, S.W.; Bhowmick, N.A. Stromal transforming growth factor-beta signaling mediates prostatic response to androgen ablation by paracrine WNT activity. Cancer Res. 2008, 68, 4709–4718. [Google Scholar] [CrossRef] [PubMed]
- Uemura, H.; Ishiguro, H.; Nagashima, Y.; Sasaki, T.; Nakaigawa, N.; Hasumi, H.; Kato, S.; Kubota, Y. Antiproliferative activity of angiotensin II receptor blocker through cross-talk between stromal and epithelial prostate cancer cells. Mol. Cancer Ther. 2005, 4, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Micke, P.; Ostman, A. Tumour-stroma interaction: Cancer-associated fibroblasts as novel targets in anti-cancer therapy? Lung Cancer 2004, 45 (Suppl. 2), S163–S175. [Google Scholar] [CrossRef] [PubMed]
- Ronnov-Jessen, L.; Petersen, O.W.; Koteliansky, V.E.; Bissell, M.J. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J. Clin. Investig. 1995, 95, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Eyden, B. The myofibroblast: Phenotypic characterization as a prerequisite to understanding its functions in translational medicine. J. Cell. Mol. Med. 2008, 12, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Stone, K.R.; Mickey, D.D.; Wunderli, H.; Mickey, G.H.; Paulson, D.F. Isolation of a human prostate carcinoma cell line (DU 145). Int. J. Cancer 1978, 21, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Horoszewicz, J.S.; Leong, S.S.; Chu, T.M.; Wajsman, Z.L.; Friedman, M.; Papsidero, L.; Kim, U.; Chai, L.S.; Kakati, S.; Arya, S.K.; et al. The lncap cell line—A new model for studies on human prostatic carcinoma. Prog. Clin. Biol. Res. 1980, 37, 115–132. [Google Scholar] [PubMed]
- Iguchi, K.; Ishii, K.; Nakano, T.; Otsuka, T.; Usui, S.; Sugimura, Y.; Hirano, K. Isolation and characterization of lncap sublines differing in hormone sensitivity. J. Androl. 2007, 28, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, K.; Hayakawa, Y.; Ishii, K.; Matsumoto, K.; Usui, S.; Sugimura, Y.; Hirano, K. Characterization of the low pH/low nutrient-resistant lncap cell subline LNCaP-F10. Oncol. Rep. 2012, 28, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Onishi, T.; Yamakawa, K.; Franco, O.E.; Kawamura, J.; Watanabe, M.; Shiraishi, T.; Kitazawa, S. Mitogen-activated protein kinase pathway is involved in alpha6 integrin gene expression in androgen-independent prostate cancer cells: Role of proximal SP1 consensus sequence. Biochim. Biophys. Acta 2001, 1538, 218–227. [Google Scholar] [CrossRef]
- Ishii, K.; Imamura, T.; Iguchi, K.; Arase, S.; Yoshio, Y.; Arima, K.; Hirano, K.; Sugimura, Y. Evidence that androgen-independent stromal growth factor signals promote androgen-insensitive prostate cancer cell growth in vivo. Endocr. Relat. Cancer 2009, 16, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.J.; Mishra, P.J.; Humeniuk, R.; Medina, D.J.; Alexe, G.; Mesirov, J.P.; Ganesan, S.; Glod, J.W.; Banerjee, D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008, 68, 4331–4339. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.; Dembinski, J.; Sasser, A.K.; Studeny, M.; Andreeff, M.; Marini, F. Mesenchymal stem cells in cancer: Tumor-associated fibroblasts and cell-based delivery vehicles. Int. J. Hematol. 2007, 86, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Park, J.S.; Chu, J.S.; Krakowski, A.; Luo, K.; Chen, D.J.; Li, S. Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor beta1 stimulation. J. Biol. Chem. 2004, 279, 43725–43734. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, K.; Otsuka, T.; Usui, S.; Ishii, K.; Onishi, T.; Sugimura, Y.; Hirano, K. Zinc and metallothionein levels and expression of zinc transporters in androgen-independent subline of LNCaP cells. J. Androl. 2004, 25, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Ishii, K.; Kanda, H.; Ogura, Y.; Kise, H.; Arima, K.; Sugimura, Y. Improvement in predicting tumorigenic phenotype of androgen-insensitive human lncap prostatic cancer cell subline in recombination with rat urogenital sinus mesenchyme. Cancer Sci. 2008, 99, 2435–2443. [Google Scholar] [CrossRef] [PubMed]
- Gleave, M.; Hsieh, J.T.; Gao, C.A.; von Eschenbach, A.C.; Chung, L.W. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res. 1991, 51, 3753–3761. [Google Scholar] [PubMed]
- Hayashi, N.; Cunha, G.R. Mesenchyme-induced changes in the neoplastic characteristics of the dunning prostatic adenocarcinoma. Cancer Res. 1991, 51, 4924–4930. [Google Scholar] [PubMed]
- Russell, P.J.; Bennett, S.; Stricker, P. Growth factor involvement in progression of prostate cancer. Clin. Chem. 1998, 44, 705–723. [Google Scholar] [PubMed]
- El Sheikh, S.S.; Domin, J.; Abel, P.; Stamp, G.; Lalani el, N. Phosphorylation of both EGFR and ErbB2 is a reliable predictor of prostate cancer cell proliferation in response to EGF. Neoplasia 2004, 6, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Gowardhan, B.; Douglas, D.A.; Mathers, M.E.; McKie, A.B.; McCracken, S.R.; Robson, C.N.; Leung, H.Y. Evaluation of the fibroblast growth factor system as a potential target for therapy in human prostate cancer. Br. J. Cancer 2005, 92, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Kawada, M.; Inoue, H.; Masuda, T.; Ikeda, D. Insulin-like growth factor i secreted from prostate stromal cells mediates tumor-stromal cell interactions of prostate cancer. Cancer Res. 2006, 66, 4419–4425. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cunha, G.R.; Alarid, E.T.; Turner, T.; Donjacour, A.A.; Boutin, E.L.; Foster, B.A. Normal and abnormal development of the male urogenital tract. Role of androgens, mesenchymal-epithelial interactions, and growth factors. J. Androl. 1992, 13, 465–475. [Google Scholar] [PubMed]
- Planz, B.; Oltean, H.; Deix, T.; Kirley, S.D.; Wang, Q.F.; McDougal, W.S.; Marberger, M. Effect of keratinocyte growth factor and activin on cell growth in the human prostatic cancer cell line LNCaP. World J. Urol. 2004, 22, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Halin, S.; Hammarsten, P.; Wikstrom, P.; Bergh, A. Androgen-insensitive prostate cancer cells transiently respond to castration treatment when growing in an androgen-dependent prostate environment. Prostate 2007, 67, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature 2004, 432, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Ao, M.; Franco, O.E.; Park, D.; Raman, D.; Williams, K.; Hayward, S.W. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007, 67, 4244–4253. [Google Scholar] [CrossRef] [PubMed]
- Ishii, G.; Hashimoto, H.; Asada, K.; Ito, T.; Hoshino, A.; Fujii, S.; Kojima, M.; Kuwata, T.; Harigaya, K.; Nagai, K.; et al. Fibroblasts associated with cancer cells keep enhanced migration activity after separation from cancer cells: A novel character of tumor educated fibroblasts. Int. J. Oncol. 2010, 37, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Joesting, M.S.; Perrin, S.; Elenbaas, B.; Fawell, S.E.; Rubin, J.S.; Franco, O.E.; Hayward, S.W.; Cunha, G.R.; Marker, P.C. Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer Res. 2005, 65, 10423–10430. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Klocker, H.; Bartsch, G.; Hobisch, A. Androgen receptors in prostate cancer. Endocr. Relat. Cancer 2002, 9, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.L.; Kyprianou, N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr. Relat. Cancer 2008, 15, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Coetzee, G.A. Prostate specific antigen gene regulation by androgen receptor. J. Cell. Biochem. 2004, 93, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Mawji, N.R.; Bruchovsky, N.; Sadar, M.D. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J. Biol. Chem. 2002, 277, 38087–38094. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Hobisch, A.; Cronauer, M.V.; Radmayr, C.; Trapman, J.; Hittmair, A.; Bartsch, G.; Klocker, H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994, 54, 5474–5478. [Google Scholar] [CrossRef] [PubMed]
- Gravina, G.L.; Mancini, A.; Ranieri, G.; Di Pasquale, B.; Marampon, F.; Di Clemente, L.; Ricevuto, E.; Festuccia, C. Phenotypic characterization of human prostatic stromal cells in primary cultures derived from human tissue samples. Int. J. Oncol. 2013, 42, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.J.; Welliver, R.C., Jr.; Chen, M.; Shtutman, M.; Godoy, A.; Smith, G.; Mian, B.M.; Buttyan, R. Effects of androgen receptor and androgen on gene expression in prostate stromal fibroblasts and paracrine signaling to prostate cancer cells. PLoS ONE 2011, 6, e16027. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, K.; Isotani, S.; Wang, R.; Fujisawa, M.; Gotoh, A.; Marshall, F.F.; Zhau, H.E.; Chung, L.W. Soluble factors derived from stroma activated androgen receptor phosphorylation in human prostate LNCaP cells: Roles of ERK/MAP kinase. Prostate 2009, 69, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Ishii, K.; Iwamoto, Y.; Kato, M.; Miki, M.; Kanda, H.; Arima, K.; Shiraishi, T.; Sugimura, Y. Fibroblasts prolong serum prostate-specific antigen decline after androgen deprivation therapy in prostate cancer. Lab. Investig. 2016, 96, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Cunha, G.R.; Wong, Y.C. Influence of male genital tract mesenchymes on differentiation of dunning prostatic adenocarcinoma. Cancer Res. 1990, 50, 4747–4754. [Google Scholar] [PubMed]
- Flaberg, E.; Markasz, L.; Petranyi, G.; Stuber, G.; Dicso, F.; Alchihabi, N.; Olah, E.; Csizy, I.; Jozsa, T.; Andren, O.; et al. High-throughput live-cell imaging reveals differential inhibition of tumor cell proliferation by human fibroblasts. Int. J. Cancer 2011, 128, 2793–2802. [Google Scholar] [CrossRef] [PubMed]
- Alkasalias, T.; Flaberg, E.; Kashuba, V.; Alexeyenko, A.; Pavlova, T.; Savchenko, A.; Szekely, L.; Klein, G.; Guven, H. Inhibition of tumor cell proliferation and motility by fibroblasts is both contact and soluble factor dependent. Proc. Natl. Acad. Sci. USA 2014, 111, 17188–17193. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Mishra, R.; Li, X.; Jackson, R.S., 2nd; Sharma, A.; Bhowmick, N.A. A reciprocal role of prostate cancer on stromal DNA damage. Oncogene 2014, 33, 4924–4931. [Google Scholar] [CrossRef] [PubMed]
- Shifrin, D.A., Jr.; Demory Beckler, M.; Coffey, R.J.; Tyska, M.J. Extracellular vesicles: Communication, coercion, and conditioning. Mol. Biol. Cell 2013, 24, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Pan, Y.; Li, W.; Sun, C.; Liu, J.; Xu, T.; Shu, Y. Extracellular vesicles-mediated noncoding RNAs transfer in cancer. J. Hematol. Oncol. 2017, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Volinia, S.; Calin, G.A.; Liu, C.G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Agarwal, P.; Bhowmick, N.A. MicroRNA applications for prostate, ovarian and breast cancer in the era of precision medicine. Endocr. Relat. Cancer 2017, 24, R157–R172. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Deng, J.J.; Gowda, P.S.; Rao, M.K.; Lin, C.L.; Chen, C.L.; Huang, T.; Sun, L.Z. Androgen receptor and microRNA-21 axis downregulates transforming growth factor beta receptor II (TGFBR2) expression in prostate cancer. Oncogene 2014, 33, 4097–4106. [Google Scholar] [CrossRef] [PubMed]
- Siu, M.K.; Chen, W.Y.; Tsai, H.Y.; Yeh, H.L.; Yin, J.J.; Liu, S.Y.; Liu, Y.N. Androgen receptor regulates src expression through microRNA-203. Oncotarget 2016, 7, 25726–25741. [Google Scholar] [CrossRef] [PubMed]
- Cannistraci, A.; Federici, G.; Addario, A.; Di Pace, A.L.; Grassi, L.; Muto, G.; Collura, D.; Signore, M.; De Salvo, L.; Sentinelli, S.; et al. C-met/miR-130b axis as novel mechanism and biomarker for castration resistance state acquisition. Oncogene 2017, 36, 3718–3728. [Google Scholar] [CrossRef] [PubMed]
- Ayub, S.G.; Kaul, D.; Ayub, T. An androgen-regulated miR-2909 modulates TGFbeta signalling through AR/miR-2909 axis in prostate cancer. Gene 2017, 631, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 2012, 1820, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Rabinowits, G.; Gercel-Taylor, C.; Day, J.M.; Taylor, D.D.; Kloecker, G.H. Exosomal microRNA: A diagnostic marker for lung cancer. Clin. Lung Cancer 2009, 10, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Schageman, J.; Zeringer, E.; Li, M.; Barta, T.; Lea, K.; Gu, J.; Magdaleno, S.; Setterquist, R.; Vlassov, A.V. The complete exosome workflow solution: From isolation to characterization of RNA cargo. BioMed Res. Int. 2013, 2013, 253957. [Google Scholar] [CrossRef] [PubMed]
- Cochetti, G.; Poli, G.; Guelfi, G.; Boni, A.; Egidi, M.G.; Mearini, E. Different levels of serum microRNAs in prostate cancer and benign prostatic hyperplasia: Evaluation of potential diagnostic and prognostic role. Onco Targets Ther. 2016, 9, 7545–7553. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Onishi, T.; Hoshina, A. Nadir PSA level and time to PSA nadir following primary androgen deprivation therapy are the early survival predictors for prostate cancer patients with bone metastasis. Prostate Cancer Prostatic Dis. 2011, 14, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Onishi, T.; Hoshina, A. Cutoff value of time to prostate-specific antigen nadir is inversely correlated with disease progression in advanced prostate cancer. Endocr. Relat. Cancer 2012, 19, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Aprelikova, O.; Green, J.E. MicroRNA regulation in cancer-associated fibroblasts. Cancer Immunol. Immunother. 2012, 61, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Josson, S.; Chung, L.W.; Gururajan, M. MicroRNAs and prostate cancer. Adv. Exp. Med. Biol. 2015, 889, 105–118. [Google Scholar] [PubMed]
- Ren, Q.; Liang, J.; Wei, J.; Basturk, O.; Wang, J.; Daniels, G.; Gellert, L.L.; Li, Y.; Shen, Y.; Osman, I.; et al. Epithelial and stromal expression of miRNAs during prostate cancer progression. Am. J. Transl. Res. 2014, 6, 329–339. [Google Scholar] [PubMed]
- Zhang, N.; Li, Z.; Bai, F.; Ji, N.; Zheng, Y.; Li, Y.; Chen, J.; Mao, X. MicroRNA expression profiles in benign prostatic hyperplasia. Mol. Med. Rep. 2018, 17, 3853–3858. [Google Scholar] [CrossRef] [PubMed]
- Paziewska, A.; Mikula, M.; Dabrowska, M.; Kulecka, M.; Goryca, K.; Antoniewicz, A.; Dobruch, J.; Borowka, A.; Rutkowski, P.; Ostrowski, J. Candidate diagnostic miRNAs that can detect cancer in prostate biopsy. Prostate 2018, 78, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Ahmad, M.K.; Srivatava, V.K.; Rastogi, N.; Serajuddin, M.; Kumar, S.; Mishra, D.P.; Sankhwar, S.N.; Mahdi, A.A. Evaluation of miR-711 as novel biomarker in prostate cancer progression. Asian Pac. J. Cancer Prev. 2017, 18, 2185–2191. [Google Scholar] [PubMed]
- Feng, S.; Qian, X.; Li, H.; Zhang, X. Combinations of elevated tissue miRNA-17-92 cluster expression and serum prostate-specific antigen as potential diagnostic biomarkers for prostate cancer. Oncol. Lett. 2017, 14, 6943–6949. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Endzelins, E.; Berger, A.; Melne, V.; Bajo-Santos, C.; Sobolevska, K.; Abols, A.; Rodriguez, M.; Santare, D.; Rudnickiha, A.; Lietuvietis, V.; et al. Detection of circulating miRNAs: Comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients. BMC Cancer 2017, 17, 730. [Google Scholar] [CrossRef] [PubMed]
- Bhagirath, D.; Yang, T.L.; Bucay, N.; Sekhon, K.; Majid, S.; Shahryari, V.; Dahiya, R.; Tanaka, Y.; Saini, S. MicroRNA-1246 is an exosomal biomarker for aggressive prostate cancer. Cancer Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Fredsoe, J.; Rasmussen, A.K.I.; Thomsen, A.R.; Mouritzen, P.; Hoyer, S.; Borre, M.; Orntoft, T.F.; Sorensen, K.D. Diagnostic and prognostic microRNA biomarkers for prostate cancer in cell-free urine. Eur. Urol. Focus 2017. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qin, S.; An, T.; Tang, Y.; Huang, Y.; Zheng, L. MiR-145 detection in urinary extracellular vesicles increase diagnostic efficiency of prostate cancer based on hydrostatic filtration dialysis method. Prostate 2017, 77, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
| Source | Prostatic Disease | Reference |
|---|---|---|
| Tissue | BPH | [170] |
| Tissue | BPH and PCa | [171] |
| Tissue | BPH and PCa | [172] |
| Tissue | BPH and PCa | [173] |
| Plasma EVs | BPH and PCa | [174] |
| Serum exosomes | BPH and PCa | [175] |
| Urine | BPH and PCa | [176] |
| Urinary EVs | BPH and PCa | [177] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, K.; Takahashi, S.; Sugimura, Y.; Watanabe, M. Role of Stromal Paracrine Signals in Proliferative Diseases of the Aging Human Prostate. J. Clin. Med. 2018, 7, 68. https://doi.org/10.3390/jcm7040068
Ishii K, Takahashi S, Sugimura Y, Watanabe M. Role of Stromal Paracrine Signals in Proliferative Diseases of the Aging Human Prostate. Journal of Clinical Medicine. 2018; 7(4):68. https://doi.org/10.3390/jcm7040068
Chicago/Turabian StyleIshii, Kenichiro, Sanai Takahashi, Yoshiki Sugimura, and Masatoshi Watanabe. 2018. "Role of Stromal Paracrine Signals in Proliferative Diseases of the Aging Human Prostate" Journal of Clinical Medicine 7, no. 4: 68. https://doi.org/10.3390/jcm7040068
APA StyleIshii, K., Takahashi, S., Sugimura, Y., & Watanabe, M. (2018). Role of Stromal Paracrine Signals in Proliferative Diseases of the Aging Human Prostate. Journal of Clinical Medicine, 7(4), 68. https://doi.org/10.3390/jcm7040068







