Long-Term Oncologic Outcomes, Opioid Use, and Complications after Esophageal Cancer Surgery
Abstract
:1. Introduction
2. Materials and Methods
Statistical Methods
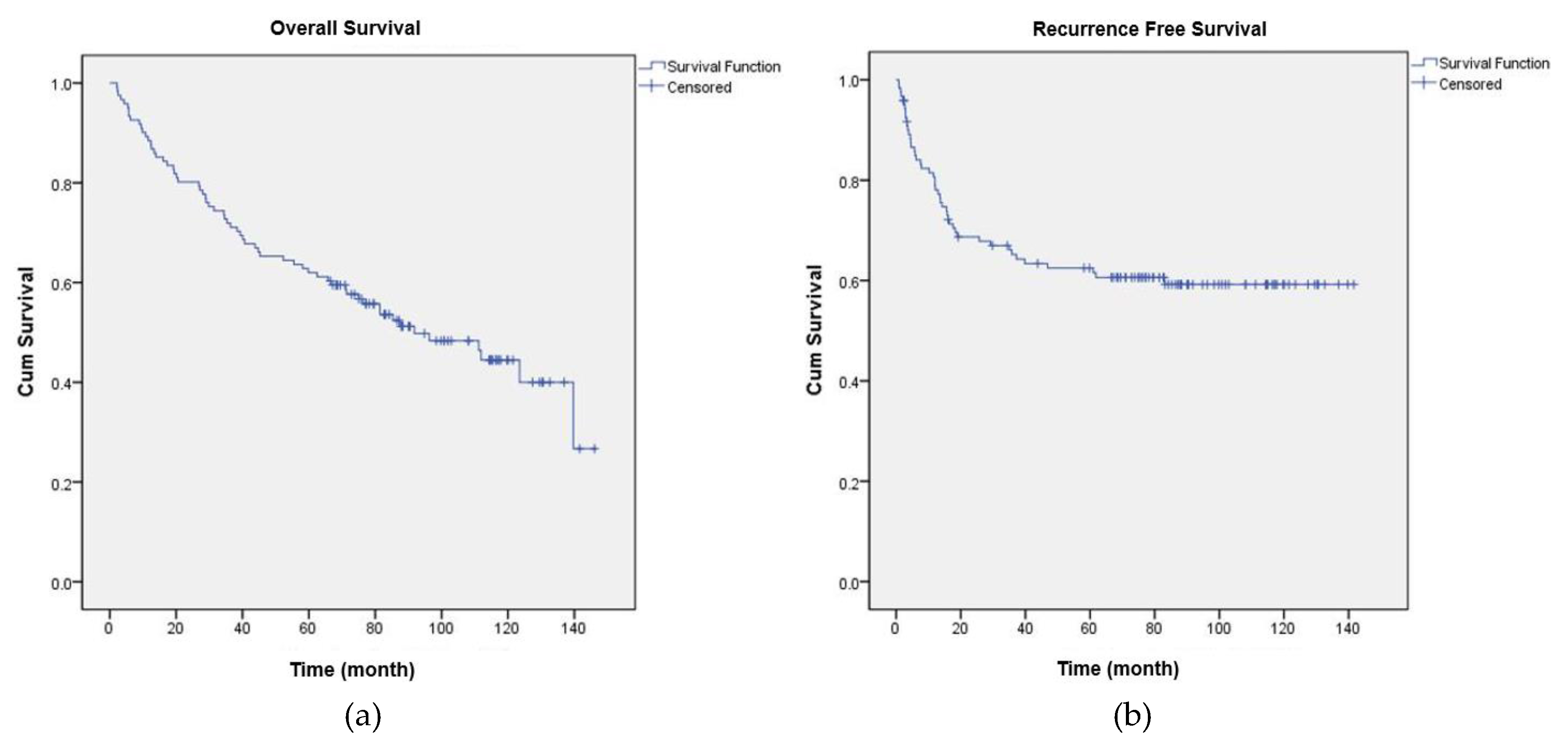
3. Results
3.1. Overall Survival
3.2. Recurrence Free Survival
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Opioid | Administration Route | Dose Equivalent to 10 mg of Oral Morphine (mg) |
|---|---|---|
| Morphine | Oral | 10 |
| Morphine | Intravenous | 3.3 |
| Morphine | Epidural | 0.33 |
| Hydromorphone | Oral | 2 |
| Fentanyl | Intravenous | 0.03 |
| Oxycodone | Oral | 7 |
| Codeine | Oral | 80 |
| Tramadol | Oral | 40 |
Appendix B
| Postoperative Complication | n | % |
|---|---|---|
| Pulmonary complication | 28 | 38% |
| Anastomtic leakage | 13 | 18% |
| Wound infection of dehiscence | 18 | 25% |
| Urinary tract infection | 1 | 1% |
| Chylothorax | 2 | 3% |
| New-onset arrythmia | 2 | 3% |
| Ileus | 2 | 3% |
| Acute kidney injury | 4 | 5% |
| Graft failure | 3 | 4% |
References
- Enzinger, P.C.; Mayer, R.J. Esophageal cancer. N. Engl. J. Med. 2003, 349, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Epidemiology of esophageal cancer. World J. Gastroenterol. 2013, 19, 5598–5606. [Google Scholar] [CrossRef] [PubMed]
- Worni, M.; Castleberry, A.W.; Gloor, B.; Pietrobon, R.; Haney, J.C.; D’Amico, T.A.; Akushevich, I.; Berry, M.F. Trends and outcomes in the use of surgery and radiation for the treatment of locally advanced esophageal cancer: A propensity score adjusted analysis of the surveillance, epidemiology, and end results registry from 1998 to 2008. Dis. Esophagus 2014, 27, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Budach, W.; Meyer, H.J.; Cervantes, A.; ESMO Guidelines Working Group. Esophageal cancer: Clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21 (Suppl. 5), v46–v49. [Google Scholar] [CrossRef] [PubMed]
- Blom, R.L.; van Heijl, M.; Bemelman, W.A.; Hollmann, M.W.; Klinkenbijl, J.H.; Busch, O.R.; van Berge Henegouwen, M.I. Initial experiences of an enhanced recovery protocol in esophageal surgery. World J. Surg. 2013, 37, 2372–2378. [Google Scholar] [CrossRef] [PubMed]
- Cata, J.P.; Gottumukkala, V.; Sessler, D.I. How regional analgesia might reduce postoperative cancer recurrence. Eur. J. Pain Suppl. 2011, 5, 345–355. [Google Scholar] [CrossRef]
- Lerut, T.; Moons, J.; Coosemans, W.; Van Raemdonck, D.; de Leyn, P.; Decaluwe, H.; Decker, G.; Nafteux, P. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: Role of systematic grading of complications using the modified clavien classification. Ann. Surg. 2009, 250, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Markar, S.; Gronnier, C.; Duhamel, A.; Mabrut, J.Y.; Bail, J.P.; Carrere, N.; Lefevre, J.H.; Brigand, C.; Vaillant, J.C.; Adham, M.; et al. The impact of severe anastomotic leak on long-term survival and cancer recurrence after surgical resection for esophageal malignancy. Ann. Surg. 2015, 262, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Bugada, D.; Bellini, V.; Fanelli, A.; Marchesini, M.; Compagnone, C.; Baciarello, M.; Allegri, M.; Fanelli, G. Future perspectives of eras: A narrative review on the new applications of an established approach. Surg. Res. Pract. 2016. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibanes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Benzon, H.; Rathmell, J.P.; Wu, C.L.; Turk, D.C.; Argoff, C.E. Raj’s Practical Management of Pain. Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Jacox, A.; Carr, D.; Payne, R.; Berde, C.; Breitbart, W.; Cain, J.; Chapman, C.; Cleeland, C.; Ferrell, B.; Finley, R. Management of cancer pain-adults. Am. Fam. Physician 1994, 49, 1853–1868. [Google Scholar]
- Cata, J.P.; Keerty, V.; Keerty, D.; Feng, L.; Norman, P.H.; Gottumukkala, V.; Mehran, J.R.; Engle, M. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med. 2014, 3, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Maher, D.P.; Wong, W.; White, P.F.; McKenna, R., Jr.; Rosner, H.; Shamloo, B.; Louy, C.; Wender, R.; Yumul, R.; Zhang, V. Association of increased postoperative opioid administration with non-small-cell lung cancer recurrence: A retrospective analysis. Br. J. Anaesth. 2014, 113 (Suppl. 1), i88–i94. [Google Scholar] [CrossRef] [PubMed]
- Sessler, D.I. Does regional analgesia reduce the risk of cancer recurrence? A hypothesis. Eur. J. Cancer. Prev. 2008, 17, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Xu, Q.X.; Liao, L.D.; Xu, X.E.; Wu, J.Y.; Wu, Z.Y.; Shen, J.H.; Li, E.M.; Xu, L.Y. Association of mu-opioid receptor expression with lymph node metastasis in esophageal squamous cell carcinoma. Dis. Esophagus 2015, 28, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, H.; Nakao, J.; Matsutani, T.; Kuwahara, H.; Taga, M.; Ogawa, R. Usefulness of the Clavien-Dindo classification in understanding the limitations and indications of larynx-preserving esophageal reconstruction. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1113. [Google Scholar] [CrossRef] [PubMed]
- Beilin, B.; Shavit, Y.; Trabekin, E.; Mordashev, B.; Mayburd, E.; Zeidel, A.; Bessler, H. The effects of postoperative pain management on immune response to surgery. Anesth. Analg. 2003, 97, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Bourke, C.D.; Berkley, J.A.; Prendergast, A.J. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, C.; Makino, H.; Kimura, J.; Oshima, T.; Fujii, S.; Takagawa, R.; Kosaka, T.; Ono, H.A.; Akiyama, H. Impact of lymph-node metastasis site in patients with thoracic esophageal cancer. J. Surg. Oncol. 2010, 101, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Z.; Yang, Z.; Shang, B.; Liu, X.; Chen, G. Prospective study of adjuvant radiotherapy on preventing lymph node metastasis after Ivor-Lewis esophagectomy in esophageal cancer. Ann. Surg. Oncol. 2013, 20, 2721–2726. [Google Scholar] [CrossRef] [PubMed]
- O’Bryant, C.L.; Linnebur, S.A.; Yamashita, T.E.; Kutner, J.S. Inconsistencies in opioid equianalgesic ratios: Clinical and research implications. J. Pain. Palliat. Care. Pharmacother. 2008, 22, 282–290. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Number (%) | Mean (SD) | |
|---|---|---|---|
| Total | 121 | ||
| Age (years) | 63.5 (8.9) | ||
| Body mass index (kg m−2) | 22.5 (3.2) | ||
| Sex: Male | 116 (95.9%) | ||
| ASA classification | |||
| 1 | 28 (23.1%) | ||
| 2 | 77 (63.6%) | ||
| 3 | 16 (13.2%) | ||
| Operation time (min) | 416.7 (127.1) | ||
| Estimated blood loss (mL) | 512.4 (573.8) | ||
| Location of tumor | |||
| Upper | 24 (19.8%) | ||
| Middle | 18 (14.9%) | ||
| Lower | 79 (65.3%) | ||
| Pathology of tumor | |||
| Squamous cell carcinoma | 111 (91.7%) | ||
| Adenocarcinoma | 10 (8.3%) | ||
| Pathologic cancer stage | |||
| IA, IB | 40 (33.0%) | ||
| IIA, IIB | 58 (47.0%) | ||
| IIIA, IIIB, IIIC | 23 (19.0%) | ||
| Pathologic tumor stage | |||
| T1 | 50 (41.3%) | ||
| T2 | 25 (20.7%) | ||
| T3 | 46 (38.0%) | ||
| Pathologic lymph node stage | |||
| N0 | 72 (59.5%) | ||
| N1 | 49 (40.5%) | ||
| Adjuvant chemotherapy | 43 (35.5%) | ||
| Adjuvant radiotherapy | 19 (15.7%) | ||
| Postoperative complication | 46 (38.0%) | ||
| Clavien-dindo classification | |||
| None | 75 (61.2%) | ||
| 1 + 2 | 18 (14.9%) | ||
| 3a + 3b | 18 (14.9%) | ||
| 4a + 4b + 5 | 10 (8.3%) | ||
| ICU readmission | 10 (8.3%) | ||
| Epidural analgesia | 7 (5.8%) | ||
| Intraoperative remifentanil dose (mcg) | 1284.6 (1363.8) | ||
| Opioid consumption in POD 0–7, MEDD (mg) | 884.0 (458.5) | ||
| Univariate Cox Regression Model | Multivariate Cox Regression Model | ||||
|---|---|---|---|---|---|
| Variables | Hazard ratio (95% CI) | p value | Hazard Ratio (95% CI) | p value | |
| Age | 1.028 (0.998–1.060) | 0.072 | 1.040 (1.005–1.075) | 0.023 | |
| Sex: male | 1.247 (0.304–5.108) | 0.759 | |||
| Body mass index | 0.879 (0.811–0.954) | 0.002 | 0.864 (0.793–0.941) | 0.001 | |
| ASA classification | |||||
| 2 (vs 1) | 2.197 (1.030–4.684) | 0.042 | 2.259 (0.984–5.186) | 0.055 | |
| 3 (vs 1) | 2.919 (1.191–7.151) | 0.019 | 1.938 (0.752–4.991) | 0.171 | |
| Epidural analgesia | 1.756 (0.702–4.392) | 0.229 | |||
| Length of hospital stay | 1.008 (0.999–1.017) | 0.074 | 0.996 (0.982–1.010) | 0.562 | |
| ICU readmission | 1.362 (0.585–3.168) | 0.473 | |||
| Operation time | 1.001 (1.000–1.003) | 0.136 | |||
| Estimated blood loss | 1.000 (1.000–1.000) | 0.783 | |||
| Pathology of tumor: squamous cell carcimoma | 1.247 (0.452–3.439) | 0.670 | |||
| Location of tumor | |||||
| Middle (vs upper) | 1.371 (0.645–2.912) | 0.412 | |||
| Lower (vs upper) | 0.622 (0.344–1.125) | 0.116 | |||
| Pathologic cancer stage | |||||
| IIA, IIB (vs IA, IB) | 2.514 (1.359–4.651) | 0.003 | 1.841 (0.889–3.815) | 0.101 | |
| IIIA, IIIB, IIIC (vs IA, IB) | 2.271 (1.076–4.793) | 0.031 | 1.920 (0.877–4.204) | 0.103 | |
| Pathologic tumor stage | |||||
| T2 (vs T1) | 1.288 (0.621–2.761) | 0.496 | 1.570 (0.716–3.446) | 0.260 | |
| T3 (vs T1) | 2.474 (1.418–4.316) | 0.001 | 2.737 (1.473–5.085) | 0.001 | |
| Pathologic lymph node stage | |||||
| N1 (vs N0) | 1.315 (0.799–2.165) | 0.281 | |||
| Adjuvant chemotherapy | 1.452 (0.878–2.402) | 0.146 | |||
| Adjuvant radiotherapy | 3.035 (1.723–5.345) | <0.001 | 2.441 (1.310–4.551) | 0.005 | |
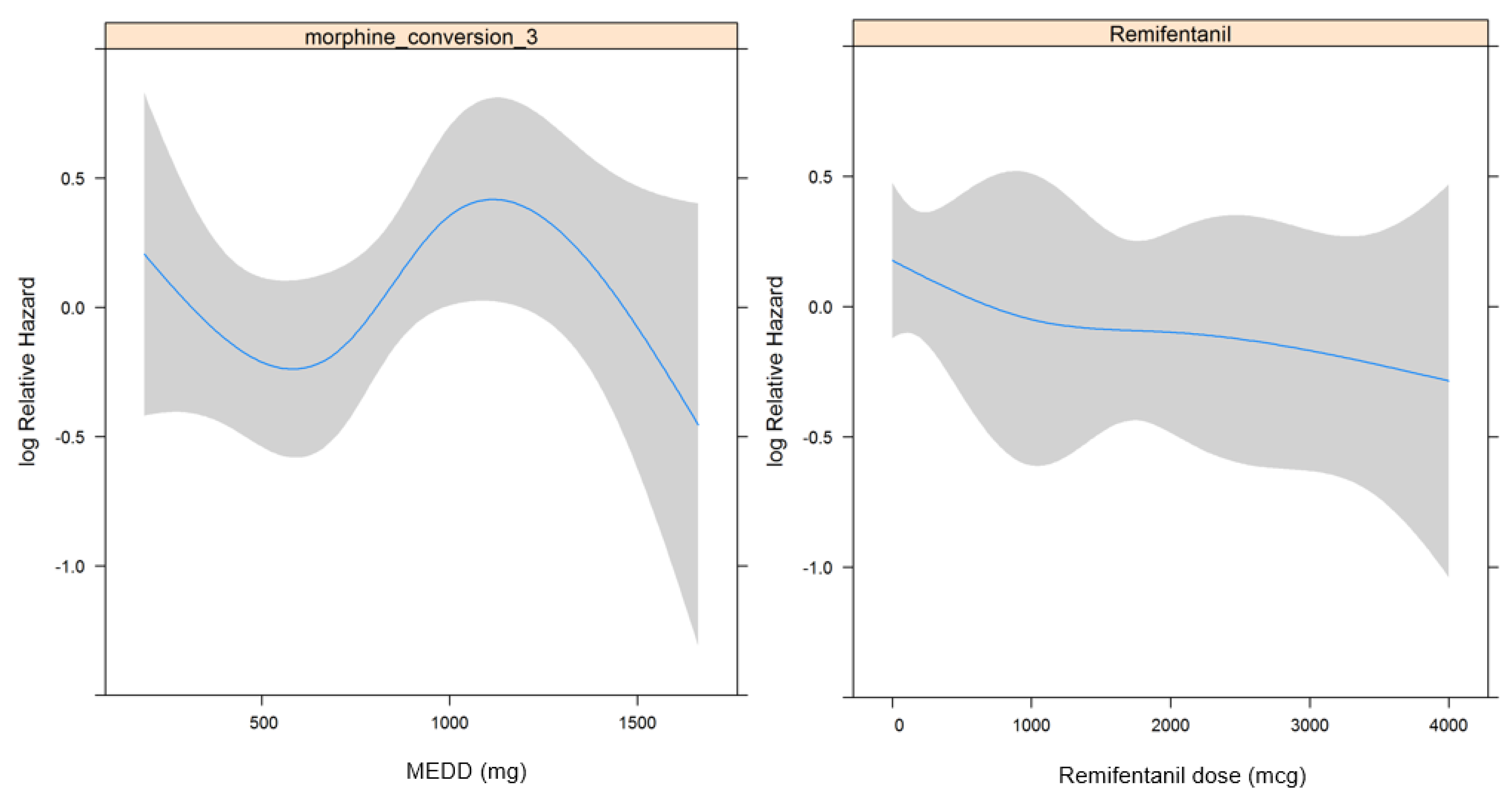
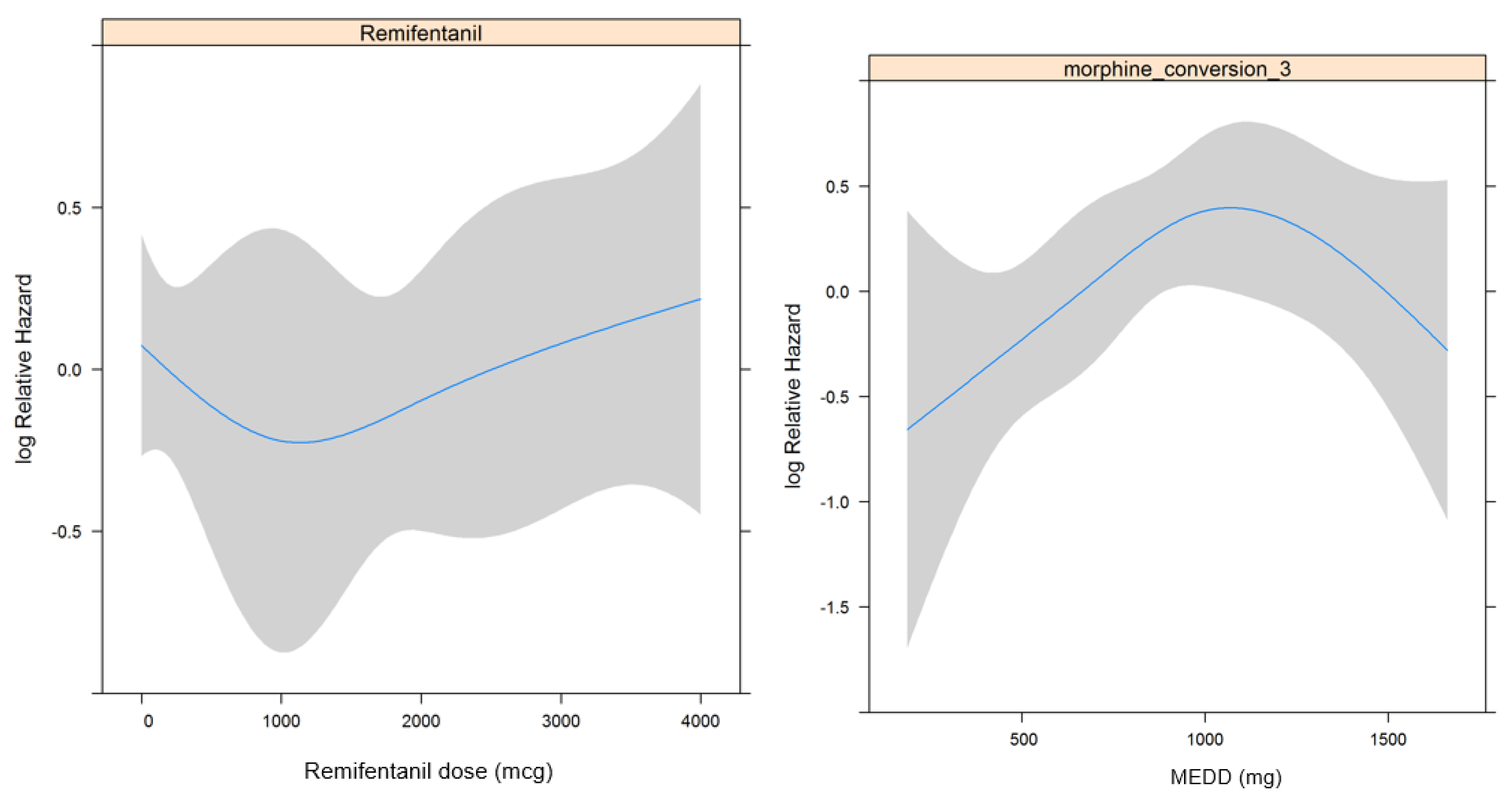
| Intraoperative remifentanil (mcg) | 1.000 (1.000–1.000) | 0.235 | |||
| Opioid consumption in POD 0–7, MEDD (mg) | 1.000 (0.999–1.001) | 0.520 | |||
| ≤630 (25%) | 1 | 0.697 | |||
| ≤843 (50%) | 0.923 (0.452–1.884) | ||||
| ≤1125 (75%) | 1.333 (0.681–2.612) | ||||
| > 1125 | 0.950 (0.456–1.982) | ||||
| Clavien-dindo classification | |||||
| 1 + 2 (vs no complication) | 2.182 (1.123–4.239) | 0.021 | 2.009 (1.014–3.979) | 0.046 | |
| 3a + 3b (vs no complication) | 4.351 (2.324–8.145) | <0.001 | 5.759 (2.642–12.554) | <0.001 | |
| 4a + 4b + 5 (vs no complication) | 3.137 (1.358–7.247) | 0.007 | 3.982 (1.244–12.749) | 0.020 | |
| Univariate Cox Regression Model | Multivaiate Cox Regression Model | ||||
|---|---|---|---|---|---|
| Variables | Hazard Ratio (95% CI) | p value | Hazard Ratio (95% CI) | p value | |
| Age | 1.010 (0.976–1.045) | 0.576 | |||
| Sex: male | 0.914 (0.222–3.770) | 0.901 | |||
| Body Mass Index | 0.843 (0.771–0.923) | <0.001 | 0.862 (0.789–0.941) | 0.001 | |
| ASA classification | |||||
| 2 (vs 1) | 2.473 (1.039–5.885) | 0.041 | 1.783 (0.726–4.381) | 0.207 | |
| 3 (vs 1) | 1.954 (0.629–6.067) | 0.246 | 1.100 (0.342–3.534) | 0.873 | |
| Epidural analgesia | 1.695 (0.608–4.725) | 0.313 | |||
| Length of hospital stay | 1.003 (0.990–1.015) | 0.684 | |||
| ICU readmission | 1.265 (0.393–4.075) | 0.693 | |||
| Operation time | 0.999 (0.997–1.002) | 0.552 | |||
| Estimated blood loss | 1.000 (0.999–1.000) | 0.770 | |||
| Pathology of tumor : Squamous cell carcimoma | 1.362 (0.423–4.387) | 0.605 | |||
| Location of tumor | |||||
| Middle (vs upper) | 1.305 (0.540–3.151) | 0.555 | |||
| Lower (vs upper) | 0.691 (0.343–1.394) | 0.302 | |||
| Pathologic cancer stage | |||||
| IIA, IIB (vs IA, IB) | 2.574 (1.199–5.528) | 0.015 | 2.094 (0.807–5.435) | 0.129 | |
| IIIA, IIIB, IIIC (vs IA, IB) | 3.748 (1.595–8.803) | 0.002 | 1.885 (0.673–5.283) | 0.228 | |
| Pathologic tumor stage | |||||
| T2 (vs T1) | 1.563 (0.638–3.827) | 0.328 | 1.489 (0.557–3.984) | 0.428 | |
| T3 (vs T1) | 4.067 (2.048–8.075) | <0.001 | 3.234 (1.505–6.947) | 0.003 | |
| Pathologic lymph node stage | |||||
| N1 (vs N0) | 1.486 (0.838–2.636) | 0.175 | |||
| Adjuvant chemotherapy | 2.657 (1.491–4.734) | <0.001 | 1.447 (0.730–2.870) | 0.290 | |
| Adjuvant radiotherapy | 3.859 (2.062–7.220) | <0.001 | 2.768 (1.369–5.596) | 0.005 | |
| Intraoperative remifentanil (mcg) | 1.000 (1.000–1.000) | 0.838 | |||
| Opioid consumption in POD 0–7, MEDD (mg) | 0.818 | ||||
| ≤684 (33%) | 1 | ||||
| ≤1020.6 (67%) | 2.385 (1.122–5.072) | 0.024 | |||
| >1020.6 | 1.689 (0.766–3.722) | 0.194 | |||
| Clavien-dindo classification | |||||
| 1 + 2 (vs no complication) | 2.622 (1.269–5.417) | 0.009 | 2.336 (1.096–3.984) | 0.028 | |
| 3a + 3b + 4a + 4b + 5 (vs no complication) | 2.457 (1.255–4.812) | 0.009 | 2.040 (0.994–4.183) | 0.052 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, T.K.; Kim, K.; Jheon, S.H.; Do, S.-H.; Hwang, J.-W.; Jeon, Y.-T.; Kim, K.; Song, I.-A. Long-Term Oncologic Outcomes, Opioid Use, and Complications after Esophageal Cancer Surgery. J. Clin. Med. 2018, 7, 33. https://doi.org/10.3390/jcm7020033
Oh TK, Kim K, Jheon SH, Do S-H, Hwang J-W, Jeon Y-T, Kim K, Song I-A. Long-Term Oncologic Outcomes, Opioid Use, and Complications after Esophageal Cancer Surgery. Journal of Clinical Medicine. 2018; 7(2):33. https://doi.org/10.3390/jcm7020033
Chicago/Turabian StyleOh, Tak Kyu, Kwhanmien Kim, Sang Hoon Jheon, Sang-Hwan Do, Jung-Won Hwang, Young-Tae Jeon, Kooknam Kim, and In-Ae Song. 2018. "Long-Term Oncologic Outcomes, Opioid Use, and Complications after Esophageal Cancer Surgery" Journal of Clinical Medicine 7, no. 2: 33. https://doi.org/10.3390/jcm7020033
APA StyleOh, T. K., Kim, K., Jheon, S. H., Do, S.-H., Hwang, J.-W., Jeon, Y.-T., Kim, K., & Song, I.-A. (2018). Long-Term Oncologic Outcomes, Opioid Use, and Complications after Esophageal Cancer Surgery. Journal of Clinical Medicine, 7(2), 33. https://doi.org/10.3390/jcm7020033




