N-Terminal Pro-Brain Natriuretic Peptide Predicts Long-Term Technique Failure in Patients Undergoing Peritoneal Dialysis
Abstract
1. Introduction
2. Methods
2.1. Ethical Approval
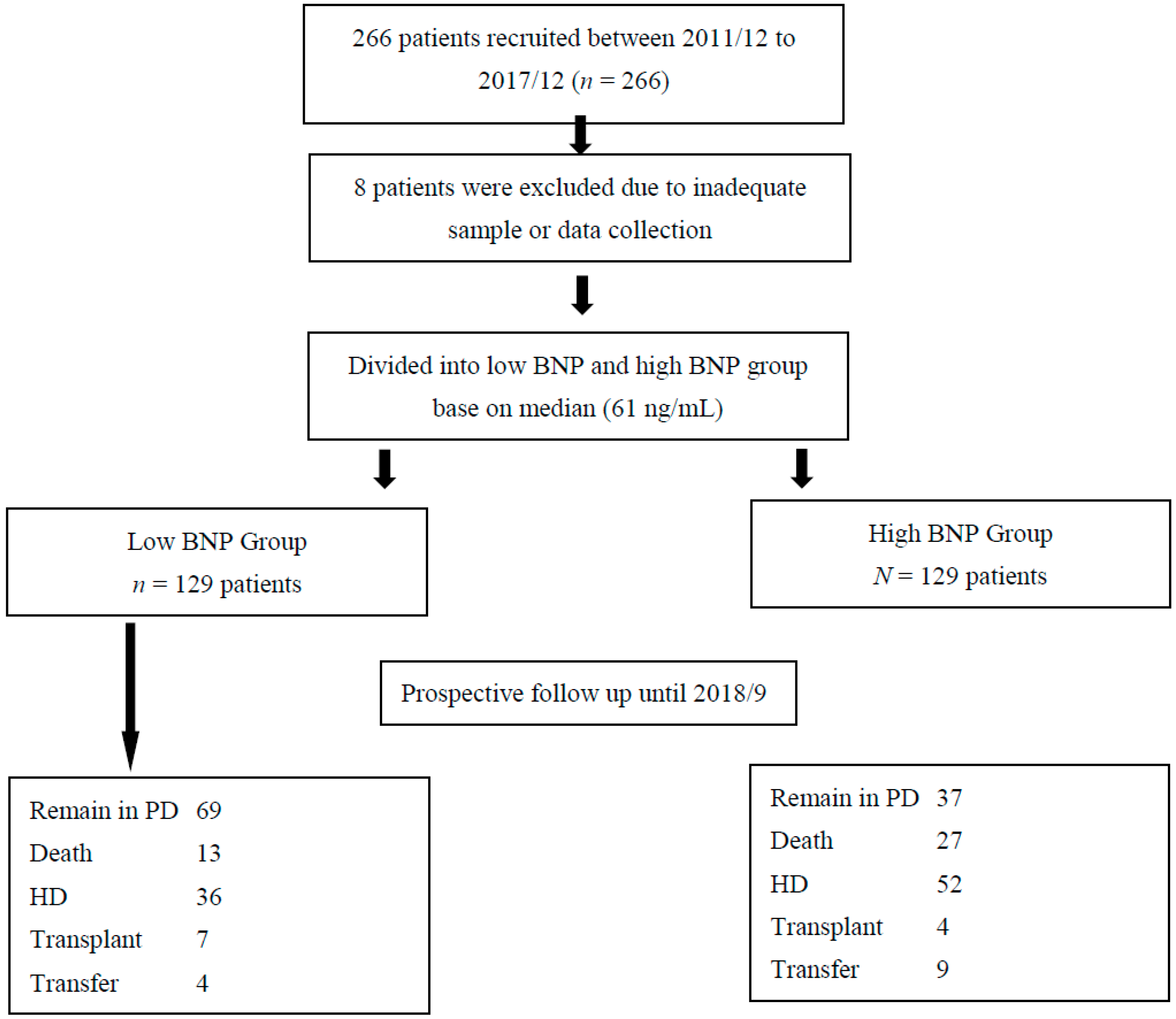
2.2. Establishment of the Index PD Cohort
2.3. Endpoints
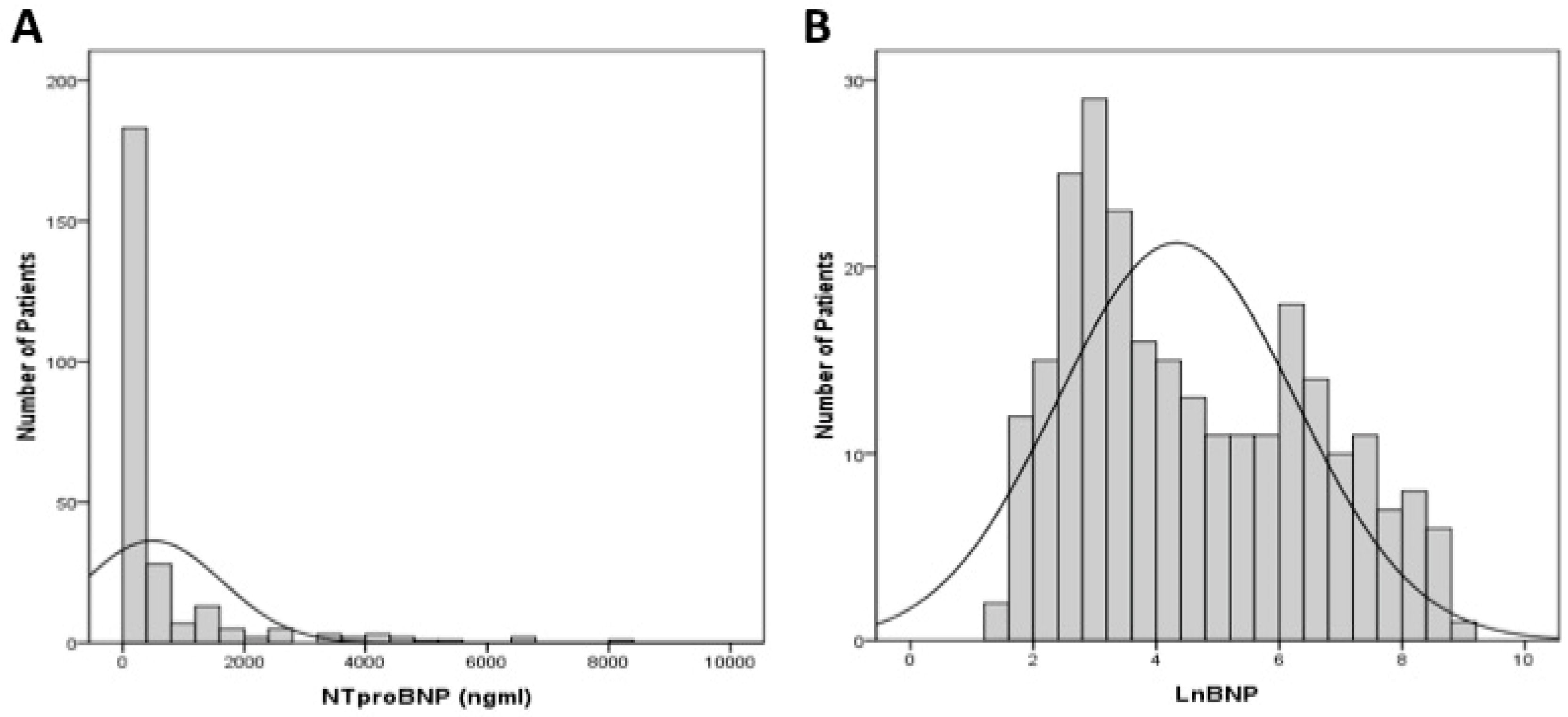
2.4. Statistical Analysis
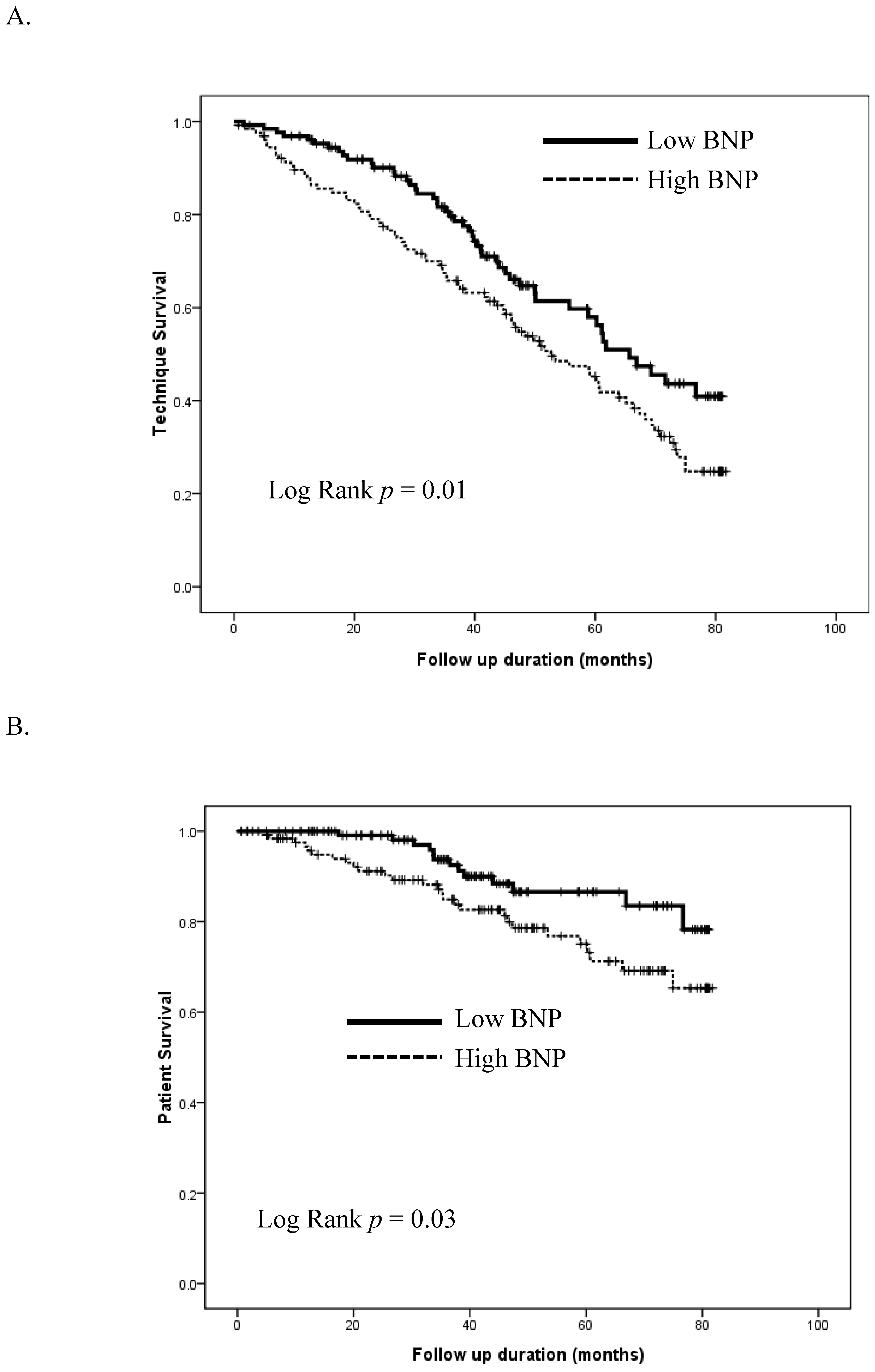
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beddhu, S.; Greene, T.; Boucher, R.; Cushman, W.C.; Wei, G.; Stoddard, G.; Ix, J.H.; Chonchol, M.; Kramer, H.; Cheung, A.K.; et al. Intensive systolic blood pressure control and incident chronic kidney disease in people with and without diabetes mellitus: Secondary analyses of two randomised controlled trials. Lancet Diabetes Endocrinol. 2018, 6, 555–563. [Google Scholar] [CrossRef]
- Chao, C.-T.; Wang, J.; Wu, H.-Y.; Huang, J.-W.; Chien, K.-L. Age modifies the risk factor profiles for acute kidney injury among recently diagnosed type 2 diabetic patients: A population-based study. GeroScience 2018, 40, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-T.; Wang, J.; Chien, K.-L. Both pre-frailty and frailty increase healthcare utilization and adverse health outcomes in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2018, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K.; Sangha, H.; Khanna, R. Peritoneal Dialysis First: Rationale. Clin. J. Am. Soc. Nephrol. 2011, 6, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Vonesh, E.F.; Snyder, J.J.; Foley, R.N.; Collins, A.J. Mortality studies comparing peritoneal dialysis and hemodialysis: What do they tell us? Kidney. Int. 2006, 70, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Li, P.K.-T.; Chow, K.M.; Van de Luijtgaarden, M.W.; Johnson, D.W.; Jager, K.J.; Mehrotra, R.; Naicker, S.; Pecoits-Filho, R.; Yu, X.Q.; Lameire, N. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 2016, 13, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-C.; Guo, A.; Just, P.M.; Mujais, S. Impact of initial dialysis modality and modality switches on Medicare expenditures of end-stage renal disease patients. Kidney Int. 2005, 68, 319–329. [Google Scholar] [CrossRef]
- See, E.J.; Johnson, D.W.; Hawley, C.M.; Pascoe, E.M.; Badve, S.V.; Boudville, N.; Clayton, P.A.; Sud, K.; Polkinghorne, K.R.; Borlace, M.; et al. Risk Predictors and Causes of Technique Failure within the First Year of Peritoneal Dialysis: An Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) Study. Am. J. Kidney Dis. 2018, 72, 188–197. [Google Scholar] [CrossRef]
- Nadeau-Fredette, A.-C.; Johnson, D.W.; Hawley, C.M.; Pascoe, E.M.; Cho, Y.; Clayton, P.A.; Borlace, M.; Badve, S.V.; Sud, K.; Boudville, N.; et al. Center-Specific Factors Associated with Peritonitis Risk—A Multi-Center Registry Analysis. Perit. Dial. Int. 2016, 36, 509–518. [Google Scholar] [CrossRef]
- Htay, H.; Cho, Y.; Pascoe, E.M.; Darssan, D.; Nadeau-Fredette, A.C.; Hawley, C.; Clayton, P.A.; Borlace, M.; Badve, S.V.; Sud, K.; et al. Multicenter Registry Analysis of Center Characteristics Associated with Technique Failure in Patients on Incident Peritoneal Dialysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 1090–1099. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Hung, K.-Y.; Huang, T.-M.; Hu, F.-C.; Peng, Y.-S.; Huang, J.-W.; Lin, S.-L.; Chen, Y.-M.; Chu, T.-S.; Tsai, T.-J.; et al. Safety Issues of Long-Term Glucose Load in Patients on Peritoneal Dialysis—A 7-Year Cohort Study. PLoS ONE 2012, 7, e30337. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.; Park, M.; Kim, Y.; Lee, H.; Kim, D.K.; Joo, K.W.; Kim, Y.S.; Cho, E.J.; Ahn, C.; et al. Lower Education Level Is a Risk Factor for Peritonitis and Technique Failure but Not a Risk for Overall Mortality in Peritoneal Dialysis under Comprehensive Training System. PLoS ONE 2017, 12, e0169063. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.-P.; Wang, H.; Du, F.-H.; Wang, T. The standard deviation of extracellular water/intracellular water is associated with all-cause mortality and technique failure in peritoneal dialysis patients. Int. Urol. Nephrol. 2016, 48, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, R.; Ventura, M.D.; Ávila-Díaz, M.; Hinojosa-Heredia, H.; Mendez-Duran, A.; Cueto-Manzano, A.; Cisneros, A.; Ramos, A.; Madonia-Juseino, C.; Belio-Caro, F.; et al. NT-proBNP, fluid volume overload and dialysis modality are independent predictors of mortality in ESRD patients. Nephrol. Dial. Transplant. 2010, 25, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, I.; Sipahioglu, M.H.; Orscelik, O.; Unal, A.; Celik, A.; Abbas, S.R.; Zhu, F.; Tokgoz, B.; Dogan, A.; Oymak, O.; et al. The Association between Arterial Stiffness and Fluid Status in Peritoneal Dialysis Patients. Perit. Dial. Int. 2014, 34, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Lee, M.J.; Kwon, Y.E.; Park, K.S.; Park, J.T.; Han, S.H.; Yoo, T.H.; Kim, Y.L.; Yang, C.W.; Kim, N.H.; et al. Which Biomarker is the Best for Predicting Mortality in Incident Peritoneal Dialysis Patients: NT-ProBNP, Cardiac TnT, or hsCRP? A Prospective Observational Study. Medicine 2015, 94, e1636. [Google Scholar] [CrossRef] [PubMed]
- Caetano, C.; Valente, A.; Oliveira, T.; Garagarza, C. Body Composition and Mortality Predictors in Hemodialysis Patients. J. Ren. Nutr. 2016, 26, 81–86. [Google Scholar] [CrossRef]
- Chao, C.-T.; Chan, D.-C.; Huang, J.-W. Frail Phenotype Might Be Associated With Higher Appendicular but Not Truncal Fat among End-Stage Renal Disease Patients. J. Pain Symptom Manag. 2017, 53, e1–e4. [Google Scholar] [CrossRef]
- Shen, J.I.; Mitani, A.A.; Saxena, A.B.; Goldstein, B.A.; Winkelmayer, W.C. Determinants of Peritoneal Dialysis Technique Failure in Incident US Patients. Perit. Dial. Int. 2013, 33, 155–166. [Google Scholar] [CrossRef]
- Matsui, M.; Akai, Y.; Samejima, K.I.; Tsushima, H.; Tanabe, K.; Morimoto, K.; Tagawa, M.; Saito, Y. Prognostic Value of Predialysis Indices for Technique Failure and Mortality in Peritoneal Dialysis Patients. Ther. Apher. Dial. 2017, 21, 493–499. [Google Scholar] [CrossRef]
- Hsieh, Y.-P.; Chang, C.-C.; Kor, C.-T.; Yang, Y.; Wen, Y.K.; Chiu, P.F.; Lin, C.C. Relationship between uric acid and technique failure in patients on continuous ambulatory peritoneal dialysis: A long-term observational cohort study. BMJ Open 2017, 7, e010816. [Google Scholar] [CrossRef]
- Lee, J.-A.; Kim, D.-H.; Yoo, S.-J.; Oh, D.J.; Yu, S.H.; Kang, E.T. Association between serum N-terminal pro-brain natriuretic peptide concentration and left ventricular dysfunction and extracellular water in continuous ambulatory peritoneal dialysis patients. Perit. Dial. Int. 2006, 26, 360–365. [Google Scholar] [PubMed]
- Wang, A.Y.; Lam, C.W.; Yu, C.M.; Wang, M.; Chan, I.H.; Zhang, Y.; Lui, S.F.; Sanderson, J.E. N-Terminal Pro-Brain Natriuretic Peptide: An Independent Risk Predictor of Cardiovascular Congestion, Mortality, and Adverse Cardiovascular Outcomes in Chronic Peritoneal Dialysis Patients. J. Am. Soc. Nephrol. 2007, 18, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, R.; Amato, D.; Mujais, S.; Vonesh, E.; Ramos, A.; Correa-Rotter, R.; Horl, W.H. Predictive Value of Brain Natriuretic Peptides in Patients on Peritoneal Dialysis: Results from the ADEMEX Trial. Clin. J. Am. Soc. Nephrol. 2008, 3, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Yun, N.R.; Ahn, C.Y.; Lee, W.S.; Kim, H.L. Relationship between Serum N-Terminal Pro-Brain Natriuretic Peptide Level and Left Ventricular Dysfunction and Extracellular Water in Continuous Ambulatory Peritoneal Dialysis Patients. Electrolyte Blood Press. 2008, 6, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Papakrivopoulou, E.; Lillywhite, S.; Davenport, A. Is N-terminal probrain-type natriuretic peptide a clinically useful biomarker of volume overload in peritoneal dialysis patients? Nephrol. Dial. Transplant. 2012, 27, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Kerschbaum, J.; Konig, P.; Rudnicki, M. Risk Factors Associated with Peritoneal-Dialysis-Related Peritonitis. Int. J. Nephrol. 2012, 2012, 483250. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-T.; Shiao, C.-C.; Huang, J.-W.; Hung, K.Y.; Chuang, H.F.; Chen, Y.M.; Wu, K.D.; Tsai, T.J. Predictors of faster decline of residual renal function in Taiwanese peritoneal dialysis patients. Perit. Dial. Int. 2008, 28 Suppl. 3, S191–S195. [Google Scholar]
- Liao, C.-T.; Chen, Y.-M.; Shiao, C.-C.; Hu, F.-C.; Huang, J.-W.; Kao, T.-W.; Chuang, H.-F.; Hung, K.-Y.; Wu, K.-D.; Tsai, T.-J. Rate of decline of residual renal function is associated with all-cause mortality and technique failure in patients on long-term peritoneal dialysis. Nephrol. Dial. Transplant. 2009, 24, 2909–2914. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, X.; Xiang, S.; Yang, X.; Lin, J.; Zhang, X.; Shou, Z.; Chen, J. Risk Factors and Outcomes of Early-Onset Peritonitis in Chinese Peritoneal Dialysis Patients. Kidney Blood Press. Res. 2017, 42, 1266–1276. [Google Scholar] [CrossRef]
- Moon, S.J.; Han, S.H.; Kim, D.K.; Lee, J.E.; Kim, B.S.; Kang, S.W.; Choi, K.H.; Lee, H.Y.; Han, D.S. Risk factors for adverse outcomes after peritonitis-related technique failure. Perit. Dial. Int. 2008, 28, 352–360. [Google Scholar] [PubMed]
- Li, P.K.; Ng, J.K.; McIntyre, C.W. Inflammation and Peritoneal Dialysis. Semin. Nephrol. 2017, 37, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.A.; Seifert, M.; Sugatani, T. Pathophysiology of the chronic kidney disease-mineral bone disorder. Curr. Opin. Nephrol. Hypertens. 2015, 24, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-T.; Liu, Y.-P.; Su, S.-F.; Yeh, H.-Y.; Chen, H.-Y.; Lee, P.-J.; Chen, W.-J.; Lee, Y.-M.; Huang, J.-W.; Chiang, C.-K.; et al. Circulating microRNA-125b predicts the presence and progression of uremic vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-T.; Hsu, Y.-H.; Chang, P.-Y.; He, Y.-T.; Ueng, R.-S.; Lai, C.-F.; Chiang, C.-K.; Huang, J.-W.; Huang, S.-J. Simple self-report FRAIL scale might be more closely associated with dialysis complications than other frailty screening instruments in rural chronic dialysis patients. Nephrology 2015, 20, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Fahim, M.A.; Hayen, A.; Horvath, A.R.; Dimeski, G.; Coburn, A.; Johnson, D.W.; Hawley, C.M.; Campbell, S.B.; Craig, J.C. N-Terminal Pro–B-Type Natriuretic Peptide Variability in Stable Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2015, 10, 620–629. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 258) | Low BNP (n = 129) | High BNP (n = 129) | p-Value | |
|---|---|---|---|---|
| Demographic profile | ||||
| Age | 54 ± 12 | 54 ± 13 | 53 ± 12 | 0.33 |
| Gender (F/M) | 138/120 | 63/66 | 75/54 | 0.14 |
| PD vintage (months) | 42 ± 40 | 33 ± 36 | 50 ± 42 | <0.01 |
| BMI (kg/m2) | 23.6 ± 3.5 | 23.8 ± 3.5 | 23.4 ± 3.6 | 0.39 |
| Cause of ESRD | 0.75 | |||
| DMN | 47 (18) | 22 (17) | 25 (19) | |
| CGN | 150 (58) | 79 (61) | 71 (55) | |
| HTN | 20 (8) | 10 (8) | 10 (8) | |
| Others | 41 (16) | 18 (14) | 23 (18) | |
| Comorbidity | ||||
| Diabetes mellitus | 63 (24) | 33 (26) | 30 (23) | 0.98 |
| Hypertension | 217 (84) | 106 (82) | 111 (86) | 0.87 |
| Heart failure | 26 (10) | 4 (3) | 22 (17) | <0.01 |
| Coronary artery disease | 32 (12) | 14 (11) | 18 (14) | 0.9 |
| Cirrhosis | 2 (1) | 0 (0) | 2 (2) | 0.57 |
| Cancer (any) | 15 (6) | 6 (5) | 9 (7) | 0.89 |
| Medications | ||||
| Diuretics | 28 (11) | 12 (9) | 16 (12) | 0.89 |
| Statin | 56 (22) | 30 (23) | 26 (20) | 0.95 |
| Beta-blocker | 126 (49) | 53 (41) | 73 (57) | 0.1 |
| ACEI/ARB | 103 (40) | 45 (35) | 58 (45) | 0.43 |
| Physical examination | ||||
| BP systolic (mmHg) | 141 ± 22 | 139 ± 19 | 144 ± 25 | 0.11 |
| BP diastolic (mmHg) | 83 ± 13 | 83 ± 12 | 82 ± 15 | 0.86 |
| PD variables | ||||
| PET results | ||||
| Low | 10 (4) | 4 (3) | 6 (5) | 0.94 |
| Low Average | 95 (37) | 52 (40) | 43 (33) | 0.72 |
| High Average | 127 (49) | 61 (47) | 66 (51) | 0.94 |
| High | 26 (10) | 12 (9) | 14 (11) | 0.98 |
| Fourth hour Glucose D/D0 | 0.37 ± 0.08 | 0.37 ± 0.08 | 0.36 ± 0.08 | 0.43 |
| Fourth hour Creatinine D/P | 0.68 ± 0.11 | 0.68 ± 0.11 | 0.69 ± 0.11 | 0.44 |
| Peritoneal Kt/V | 1.77 ± 0.37 | 1.68 ± 0.4 | 1.86 ± 0.3 | <0.01 |
| Renal Kt/V | 0.23 ± 0.33 | 0.33 ± 0.37 | 0.13 ± 0.26 | <0.01 |
| Total Kt/V | 2 ± 0.22 | 2.01 ± 0.24 | 2 ± 0.21 | 0.68 |
| nPNA | 1 ± 0.2 | 1.01 ± 0.18 | 0.99 ± 0.22 | 0.45 |
| Laboratory profiles | ||||
| Hb (g/dL) | 10 ± 1.5 | 10.3 ± 1.2 | 9.7 ± 1.7 | <0.01 |
| Albumin (gm/dL) | 3.9 ± 0.4 | 3.9 ± 0.3 | 3.8 ± 0.4 | 0.13 |
| BUN (mg/dL) | 67 ± 16 | 69 ± 16 | 65 ± 17 | 0.06 |
| Creatinine (mg/dL) | 12.2 ± 2.8 | 12.3 ± 2.9 | 12.1 ± 2.7 | 0.74 |
| PTH (pg/mL) | 457 ± 423 | 472 ± 478 | 443 ± 362 | 0.57 |
| CRP (mg/dL) | 0.69 ± 1.72 | 0.66 ± 2 | 0.72 ± 1.39 | 0.77 |
| NT pro-BNP (ng/mL) | 582 ± 1216 | 23 ± 14 | 1141 ± 1530 | <0.01 |
| Body composition parameters | ||||
| Cardiac/thoracic ratio (%) | 49 ± 7 | 48 ± 6 | 50 ± 7 | <0.01 |
| Relative OH (%) | 10.3 ± 9.4 | 7.3 ± 8.7 | 13.3 ± 9.2 | <0.01 |
| ECW (L) | 14.6 ± 3.2 | 14.6 ± 3.3 | 14.6 ± 3.1 | 0.87 |
| ICW (L) | 15.5 ± 3.5 | 16.1 ± 3.8 | 15 ± 3.2 | <0.01 |
| ECW/ICW | 1 ± 0.1 | 0.9 ± 0.1 | 1 ± 0.1 | <0.01 |
| Follow-up duration (months) | 43 ± 24 | 43 ± 22 | 44 ± 25 | 0.91 |
| PD peritonitis incidence (per 100 months) | 1.73 | 1.66 | 1.8 | 0.25 |
| Switch to HD (%) | ||||
| PD peritonitis | 46 (18) | 23 (18) | 23 (18) | 1 |
| Abdominal surgery | 18 (7) | 8 (6) | 10 (8) | 0.97 |
| Catheter dysfunction | 15 (6) | 2 (2) | 13 (10) | 0.04 |
| UF failure | 3 (1) | 1 (1) | 2 (2) | 0.95 |
| Others | 5 (2) | 2 (2) | 3 (2) | 0.98 |
| Ln(NT-proBNP) | p-Value | |
|---|---|---|
| Age | −0.1 | 0.11 |
| Gender | 0.23 | <0.01 |
| PD vintage | −0.05 | 0.47 |
| Renal Kt/V | −0.33 | <0.01 |
| Hb (g/dL) | −0.19 | <0.01 |
| Albumin (gm/dL) | −0.1 | 0.11 |
| Cardiac/thoracic ratio | 0.21 | <0.01 |
| CRP (mg/dL) | 0.04 | 0.54 |
| Rel. OH (%) | 0.43 | <0.01 |
| BP systolic (mmHg) | 0.13 | 0.046 |
| BP diastolic (mmHg) | 0.03 | 0.63 |
| ECW/ICW | 0.35 | <0.01 |
| Variable | Remaining on PD (n = 130) | Technique Failure (n = 128) | p-Value | Survivors (n = 218) | Non-Survivors (n = 40) | p-Value |
|---|---|---|---|---|---|---|
| Age | 52 ± 11 | 55 ± 13 | 0.03 | 52 ± 12 | 61 ± 11 | <0.01 |
| Gender (F/M) | 76/54 | 62/66 | 0.46 | 123/95 | 15/25 | 0.18 |
| PD vintage (months) | 41 ± 43 | 43 ± 37 | 0.62 | 42 ± 39 | 43 ± 45 | 0.81 |
| BMI (kg/m2) | 23.3 ± 3.6 | 23.9 ± 3.4 | 0.15 | 23.5 ± 3.5 | 23.9 ± 3.5 | 0.56 |
| Etiology of ESRD (%) | ||||||
| DMN | 19 (15) | 28 (22) | 0.52 | 34 (16) | 13 (33) | 0.09 |
| CGN | 86 (66) | 64 (50) | 0.08 | 132 (61) | 18 (45) | 0.34 |
| HTN | 7 (5) | 13 (10) | 0.56 | 16 (7) | 4 (10) | 0.95 |
| Others | 18 (14) | 23 (18) | 0.85 | 36 (17) | 5 (13) | 0.94 |
| Diabetes mellitus | 28 (22) | 35 (27) | 0.76 | 47 (22) | 16 (40) | 0.1 |
| Hypertension | 108 (83) | 109 (85) | 0.98 | 184 (84) | 33 (83) | 0.99 |
| Heart failure | 9 (7) | 17 (13) | 0.41 | 22 (11) | 4 (10) | 1.00 |
| Coronary artery disease | 13 (10) | 19 (15) | 0.71 | 24 (11) | 8 (20) | 0.47 |
| Cirrhosis | 1 (1) | 1 (1) | 1.00 | 2 (1) | 0 (0) | 0.95 |
| Cancer (any) | 4 (3) | 11 (9) | 0.31 | 9 (4) | 6 (15) | 0.06 |
| Diuretics use | 11 (8) | 17 (13) | 0.67 | 22 (10) | 6 (15) | 0.84 |
| Statin use | 29 (22) | 27 (21) | 0.99 | 48 (22) | 8 (20) | 0.99 |
| Beta-blocker use | 59 (45) | 67 (52) | 0.74 | 109 (50) | 17 (43) | 0.86 |
| ACEI/ARB use | 56 (43) | 47 (37) | 0.78 | 91 (42) | 12 (30) | 0.58 |
| BP systolic (mmHg) | 138 ± 22 | 144 ± 22 | 0.03 | 142 ± 23 | 139 ± 20 | 0.43 |
| BP diastolic (mmHg) | 82 ± 13 | 84 ± 14 | 0.37 | 83 ± 14 | 78 ± 12 | 0.03 |
| Fourth hour Glucose D/D0 | 0.37 ± 0.07 | 0.37 ± 0.08 | 0.74 | 0.37 ± 0.08 | 0.38 ± 0.07 | 0.41 |
| Fourth hour Creatinine D/P | 0.69 ± 0.1 | 0.68 ± 0.11 | 0.89 | 0.69 ± 0.11 | 0.67 ± 0.09 | 0.39 |
| Peritoneal Kt/V | 1.76 ± 0.39 | 1.78 ± 0.35 | 0.76 | 1.78 ± 0.37 | 1.71 ± 0.35 | 0.3 |
| Renal Kt/V | 0.25 ± 0.34 | 0.22 ± 0.32 | 0.39 | 0.24 ± 0.33 | 0.22 ± 0.36 | 0.83 |
| Total Kt/V | 2.01 ± 0.22 | 1.99 ± 0.23 | 0.4 | 2.02 ± 0.23 | 1.93 ± 0.21 | 0.03 |
| nPNA | 0.99 ± 0.19 | 1.01 ± 0.22 | 0.42 | 1 ± 0.19 | 1.03 ± 0.26 | 0.44 |
| Hb (g/dL) | 10 ± 1.4 | 9.9 ± 1.6 | 0.56 | 10 ± 1.5 | 9.8 ± 1.2 | 0.37 |
| Albumin (g/dL) | 3.9 ± 0.3 | 3.9 ± 0.4 | 0.64 | 3.9 ± 0.4 | 3.8 ± 0.5 | 0.34 |
| BUN (mg/dL) | 67 ± 15 | 67 ± 18 | 0.97 | 67 ± 16 | 67 ± 18 | 0.8 |
| Creatinine (mg/dL) | 12.3 ± 2.9 | 12 ± 2.7 | 0.37 | 12.2 ± 2.7 | 12 ± 3.2 | 0.69 |
| PTH (pg/mL) | 488 ± 465 | 426 ± 375 | 0.24 | 479 ± 430 | 339 ± 369 | 0.05 |
| CRP (mg/dL) | 0.4 ± 1.1 | 1 ± 2.2 | 0.01 | 0.5 ± 1.12 | 1.7 ± 3.4 | 0.03 |
| NT pro-BNP (ng/mL) | 415 ± 1017 | 751 ± 1373 | 0.03 | 467 ± 1064 | 1206 ± 1724 | 0.01 |
| Cardiac/thoracic ratio (%) | 48 ± 6 | 50 ± 7 | <0.01 | 49 ± 7 | 51 ± 7 | 0.05 |
| Relative OH (%) | 9.7 ± 9.1 | 11 ± 9.7 | 0.27 | 10.2 ± 9.2 | 11 ± 10.5 | 0.62 |
| ECW (L) | 14.4 ± 3.3 | 14.9 ± 3.1 | 0.22 | 14.6 ± 3.2 | 14.8 ± 3.4 | 0.62 |
| ICW (L) | 15.6 ± 3.7 | 15.5 ± 3.4 | 0.96 | 15.6 ± 3.5 | 15.1 ± 3.9 | 0.35 |
| ECW/ICW | 0.9 ± 0.1 | 1 ± 0.1 | 0.02 | 0.9 ± 0.1 | 1 ± 0.1 | <0.01 |
| Follow-up duration | 50 ± 24 | 37 ± 21 | <0.01 | 45 ± 24 | 36 ± 19 | 0.01 |
| Technique Failure | |||
| Variable | B ± SE | HR | p-Value |
| Age | 0.01 ± 0.01 | 1.01 | 0.12 |
| Male (vs. female) | 0.65 ± 0.2 | 1.91 | <0.01 |
| Diabetes mellitus | −0.07 ± 0.24 | 0.94 | 0.54 |
| Ln (NT-proBNP) | 0.13 ± 0.05 | 1.13 | <0.01 |
| CT ratio (%) | 0.04 ± 0.01 | 1.04 | <0.01 |
| CRP (mg/dL) | 0.01 ± 0.004 | 1.01 | <0.01 |
| Mortality | |||
| Variable | B ± SE | HR | p-Value |
| Age | 0.09 ± 0.02 | 1.1 | <0.01 |
| Male (vs. female) | 1.12 ± 0.38 | 3.05 | <0.01 |
| Diabetes mellitus | 0.02 ± 0.39 | 1.02 | 0.95 |
| CRP (mg/dL) | 0.02 ± 0.004 | 1.02 | <0.01 |
| Ln(NT-proBNP) | 0.44 ± 0.1 | 1.56 | <0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, C.-T.; Chiang, C.-K.; Huang, J.-W.; Hung, K.-Y. N-Terminal Pro-Brain Natriuretic Peptide Predicts Long-Term Technique Failure in Patients Undergoing Peritoneal Dialysis. J. Clin. Med. 2018, 7, 557. https://doi.org/10.3390/jcm7120557
Chao C-T, Chiang C-K, Huang J-W, Hung K-Y. N-Terminal Pro-Brain Natriuretic Peptide Predicts Long-Term Technique Failure in Patients Undergoing Peritoneal Dialysis. Journal of Clinical Medicine. 2018; 7(12):557. https://doi.org/10.3390/jcm7120557
Chicago/Turabian StyleChao, Chia-Ter, Chih-Kang Chiang, Jenq-Wen Huang, and Kuan-Yu Hung. 2018. "N-Terminal Pro-Brain Natriuretic Peptide Predicts Long-Term Technique Failure in Patients Undergoing Peritoneal Dialysis" Journal of Clinical Medicine 7, no. 12: 557. https://doi.org/10.3390/jcm7120557
APA StyleChao, C.-T., Chiang, C.-K., Huang, J.-W., & Hung, K.-Y. (2018). N-Terminal Pro-Brain Natriuretic Peptide Predicts Long-Term Technique Failure in Patients Undergoing Peritoneal Dialysis. Journal of Clinical Medicine, 7(12), 557. https://doi.org/10.3390/jcm7120557





