Association between Hemoglobin and Hemoglobin A1c: A Data-Driven Analysis of Health Checkup Data in Japan
Abstract
1. Introduction
2. Experimental Section
2.1. Participants
2.2. Statistical Analysis
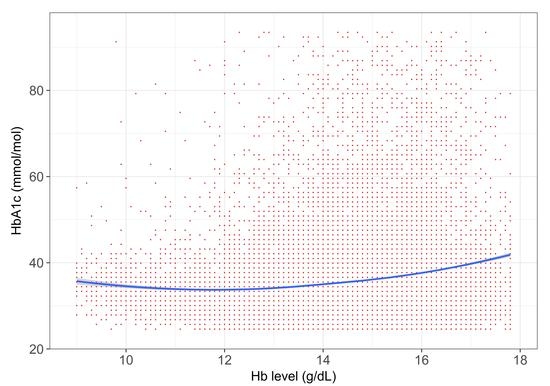
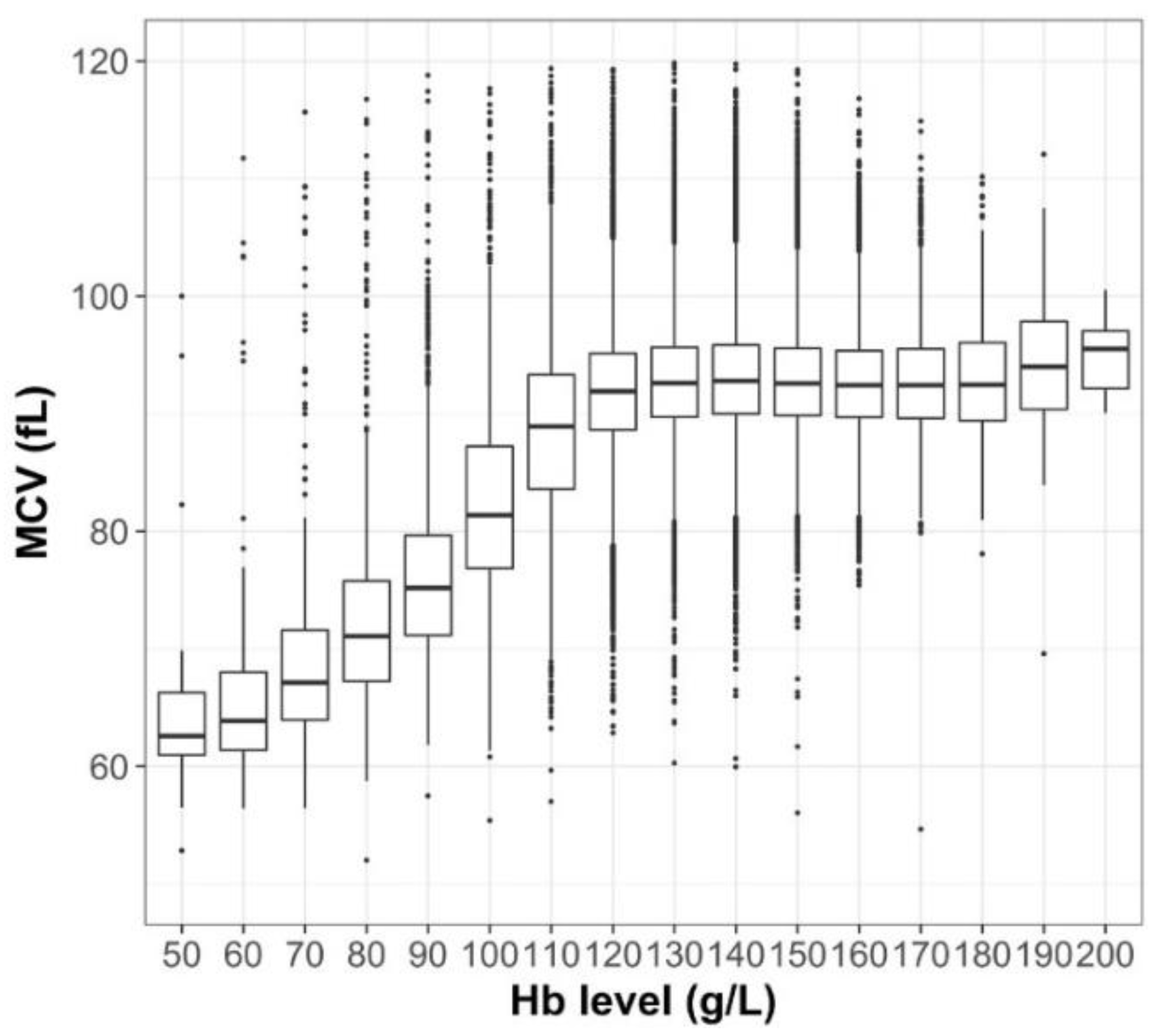
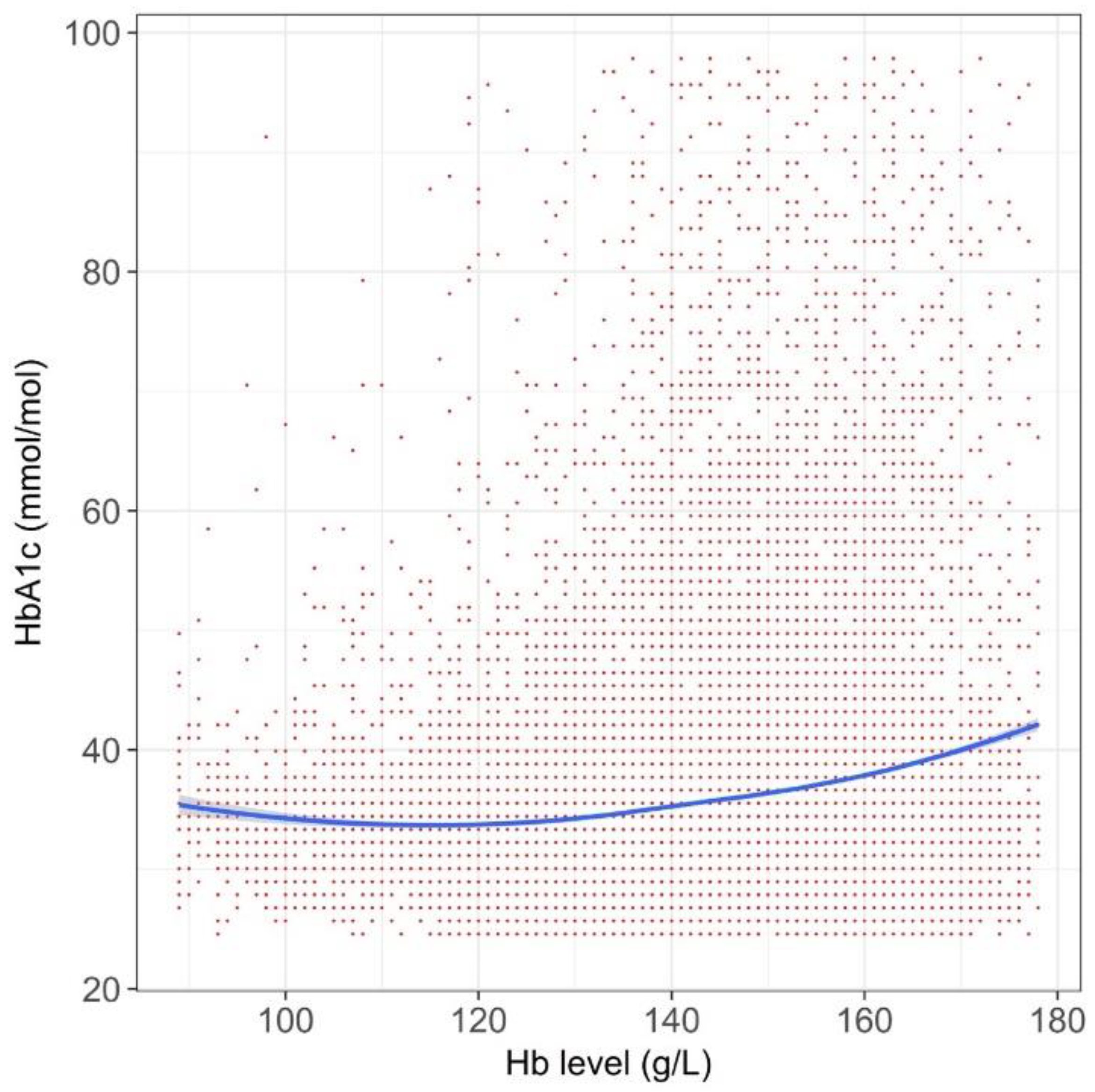
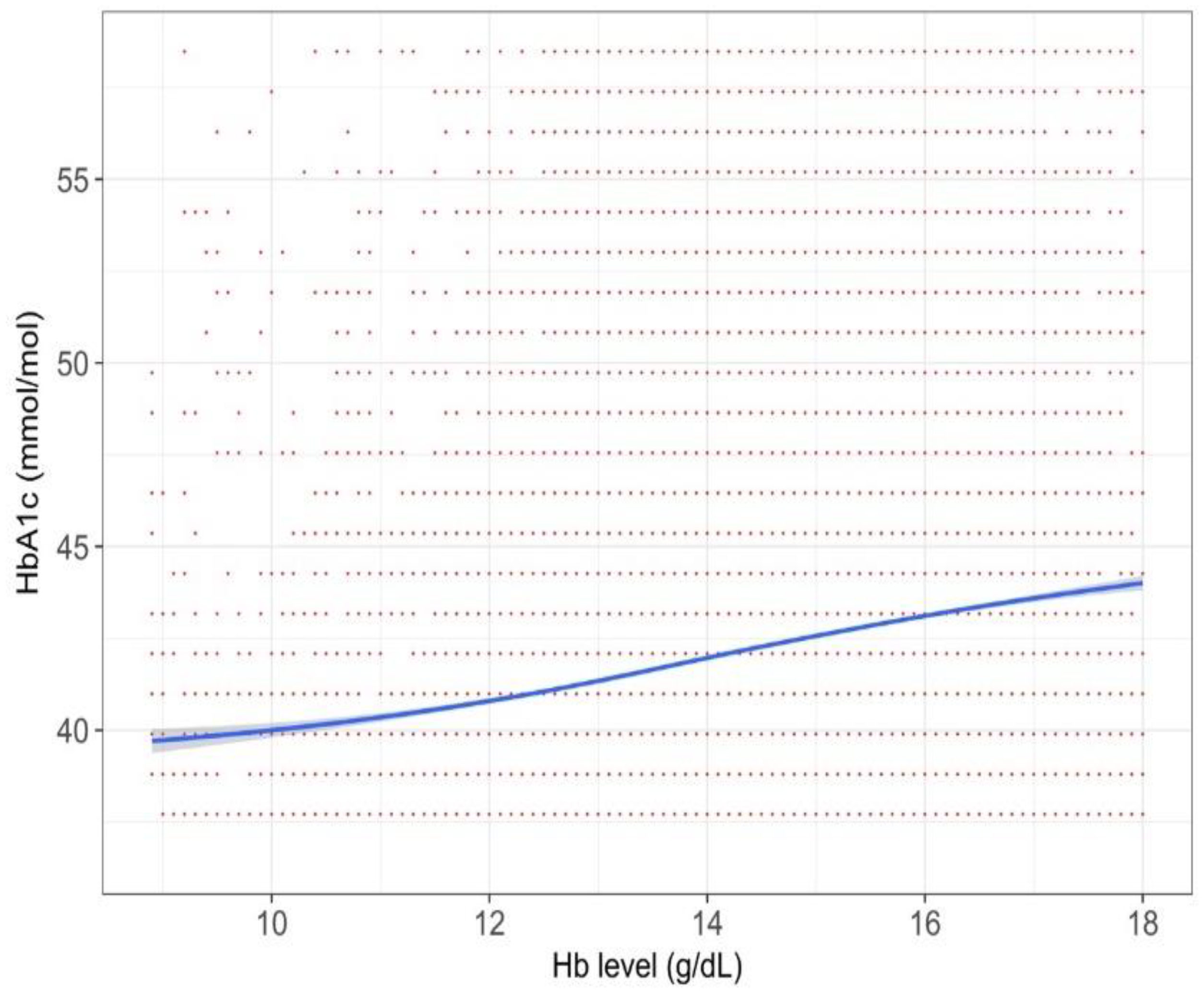
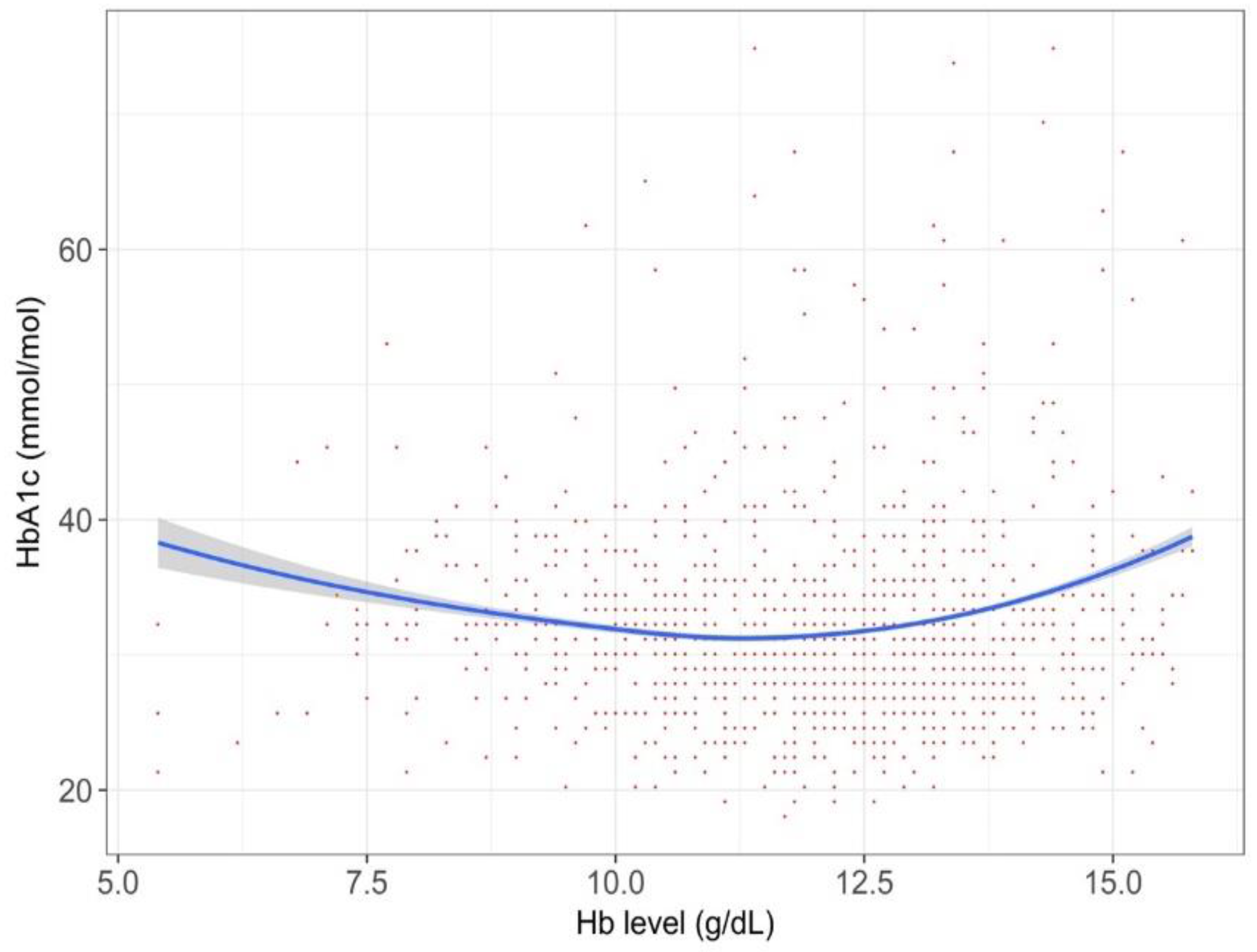
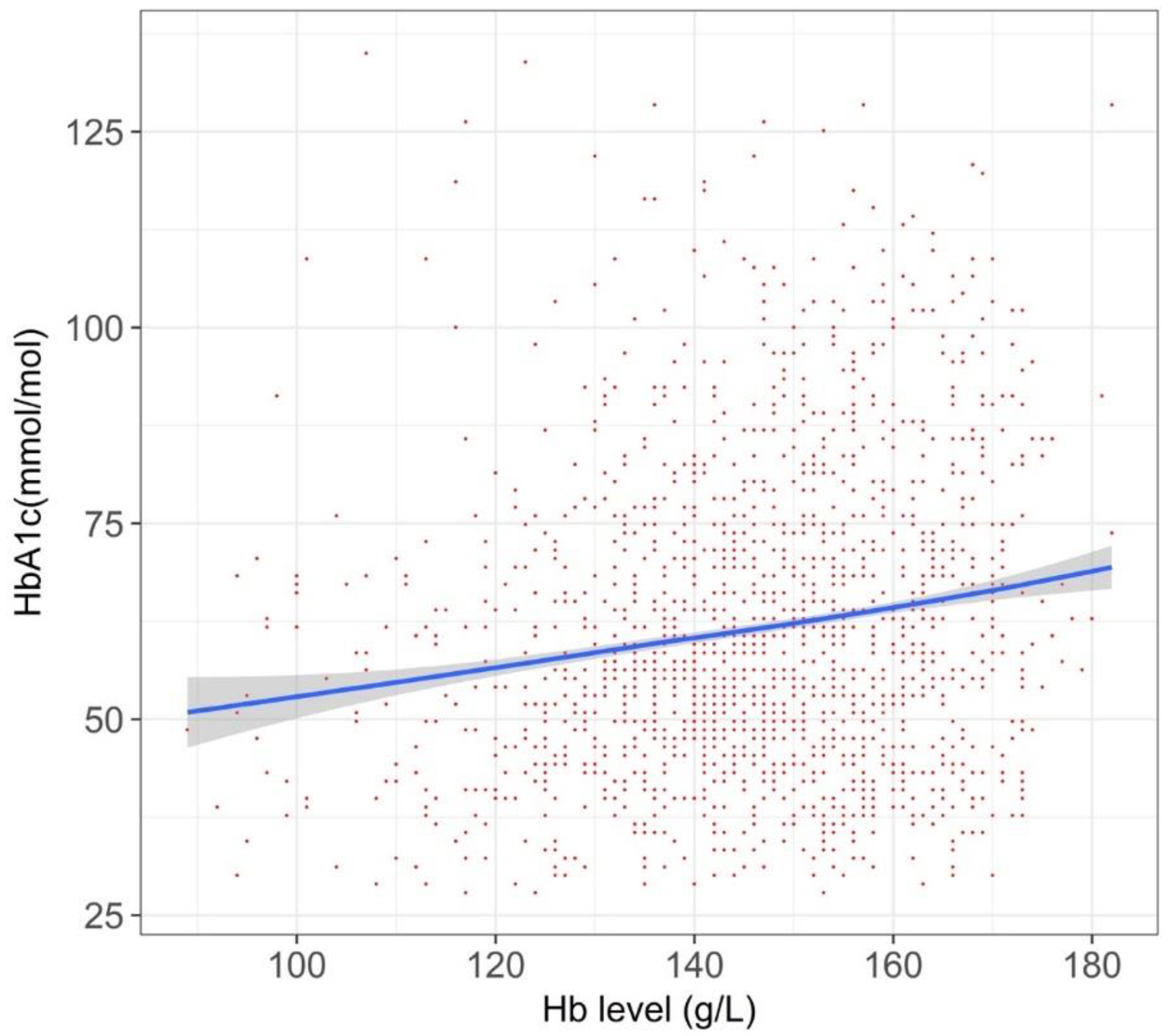
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Unnikrishnan, R.; Pradeepa, R.; Joshi, S.R.; Mohan, V. Type 2 diabetes: Demystifying the global epidemic. Diabetes 2017, 66, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas–8th Edition. Available online: http://www.diabetesatlas.org/ (accessed on 29 November 2018).
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Welsh, K.J.; Kirkman, M.S.; Sacks, D.B. Role of glycated proteins in the diagnosis and management of diabetes: Research gaps and future directions. Diabetes care 2016, 39, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Executive summary: Standards of medical care in diabetes—2009. Diabetes Care 2009, 32, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Radin, M.S. Pitfalls in hemoglobin A1c measurement: When results may be misleading. J. Gen. Intern. Med. 2014, 29, 388–394. [Google Scholar] [CrossRef] [PubMed]
- English, E.; Idris, I.; Smith, G.; Dhatariya, K.; Kilpatrick, E.S.; John, W.G. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: A systematic review. Diabetologia 2015, 58, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Son, J.I.; Rhee, S.Y.; Woo, J.T.; Hwang, J.K.; Chin, S.O.; Chon, S.; Oh, S.; Kim, S.W.; Kim, Y.S. Hemoglobin a1c may be an inadequate diagnostic tool for diabetes mellitus in anemic subjects. Diabetes Metab J. 2013, 37, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Shanthi, B.; Revathy, C.; Manjula Devi, A.J.; Subhashree. Effect of iron deficiency on glycation of haemoglobin in nondiabetics. J. Clin. Diagn. Res. 2013, 7, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Coban, E.; Ozdogan, M.; Timuragaoglu, A. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. Acta Haematol. 2004, 112, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Mishra, T.K.; Singh, T.; Gupta, N. Effect of iron deficiency anemia on hemoglobin A1c levels. Ann. Lab. Med. 2012, 32, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Seto, K.; Kawakami, K. Pharmacoepidemiology in Japan: Medical databases and research achievements. J. Pharm. Health Care Sci. 2015, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.N. General Additive Models: An Introduction with R, Second Edition; CRC Press: Boca Raton, Fl, USA, 2017. [Google Scholar]
- Nafiu, O.O.; Gillespie, B.W.; Tsodikov, A. Continuous variable transformation in anesthesia: Useful clinical shorthand, but threat to research. Anesthesiology 2015, 123, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Conlin, P.R.; Colburn, J.; Aron, D.; Pries, R.M.; Tschanz, M.P.; Pogach, L. Synopsis of the 2017 U.S. Department of veterans affairs/U.S. Department of defense clinical practice guideline: Management of type 2 diabetes mellitus. Ann. Intern. Med. 2017, 167, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Royston, P. The cost of dichotomising continuous variables. BMJ 2006, 332, 1080. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.F.; Pimentel, A.L.; Camargo, J.L. Effect of iron deficiency anaemia on HbA1c levels is dependent on the degree of anaemia. Clin Biochem. 2016, 49, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Mayo Clinic Laboratories. Test ID: HbA1c. Available online: https://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/82080 (accessed on 28 November 2018).
- Schnedl, W.J.; Reisinger, E.C.; Pieber, T.R.; Lipp, R.W.; Schreiber, F.; Hopmeier, P.; Rejs, G.J. Hemoglobin sherwood forest detected by high performance liquid chromatography for hemoglobin A(1c). Am. J. Clin. Pathol. 1995, 104, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Lahousen, T.; Roller, R.E.; Lipp, R.W.; Schnedl, W.J. Silent haemoglobin variants and determination of HbA1c with the HPLC Bio-Rad Variant II. J. Clin. Pathol. 2002, 55, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Bergenstal, R.M.; Gal, R.L.; Connor, C.G.; Gubitosi-Klug, R.; Kruger, D.; Olson, B.A.; Willi, S.M.; Aleppo, G.; Weinstock, R.S.; Wood, J.; et al. Racial Differences in the Relationship of Glucose Concentrations and Hemoglobin A1c Levels. Ann. Intern. Med. 2017, 167, 95–102. [Google Scholar] [CrossRef] [PubMed]

| Parameters | All T2DM Participants (n = 55,420) | HbA1c around Diagnostic Threashold 1,2 (n = 18,478) | Current Drug Users 1,3 (n = 6253) | Iron-Supplement Cohort 1 (n = 956) | T1DM Cohort (n = 598) | Iron-Supplement Cohort (n = 956) |
|---|---|---|---|---|---|---|
| Age 3 (years) | 46.5 (10.7) | 51.2 (9.3) | 51.4 (9.3) | 44.2 (8.3) | 48.4 (10.1) | 44.2 (8.3) |
| Sex (male) | 59.4% | 66.4% | 77.4% | 17.8% | 72.2% | 17.8% |
| BMI 3 (kg/m2) | 23.8 (4.3) | 25.3 (4.5) | 25.9 (4.7) | 22.3 (4.2) | 24.7 (4.7) | 22.3 (4.2) |
| Hb 4 (g/L) | 143 (16) | 144 (16) | 148 (15) | 118 (19) | 141 (17) | 11.8 (19) |
| HbA1c 3 (mmol/mol) | 35 (11) | 41 (5) | 52 (16) | 33 (9) | 63 (21) | 33 (9) |
| fBG 3 (mg/dL) | 104 (33) | 114 (27) | 149 (55) | 97 (29) | 168 (74) | 97 (29) |
| Smoking | 32.1% | 32.2% | 37.9% | 21.8% | 40.3% | 21.8% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeuchi, M.; Kawakami, K. Association between Hemoglobin and Hemoglobin A1c: A Data-Driven Analysis of Health Checkup Data in Japan. J. Clin. Med. 2018, 7, 539. https://doi.org/10.3390/jcm7120539
Takeuchi M, Kawakami K. Association between Hemoglobin and Hemoglobin A1c: A Data-Driven Analysis of Health Checkup Data in Japan. Journal of Clinical Medicine. 2018; 7(12):539. https://doi.org/10.3390/jcm7120539
Chicago/Turabian StyleTakeuchi, Masato, and Koji Kawakami. 2018. "Association between Hemoglobin and Hemoglobin A1c: A Data-Driven Analysis of Health Checkup Data in Japan" Journal of Clinical Medicine 7, no. 12: 539. https://doi.org/10.3390/jcm7120539
APA StyleTakeuchi, M., & Kawakami, K. (2018). Association between Hemoglobin and Hemoglobin A1c: A Data-Driven Analysis of Health Checkup Data in Japan. Journal of Clinical Medicine, 7(12), 539. https://doi.org/10.3390/jcm7120539