Global Expression Profiling Identifies a Novel Hyaluronan Synthases 2 Gene in the Pathogenesis of Lower Extremity Varicose Veins
Abstract
1. Introduction
2. Methods
2.1. Patient Selection
2.2. Surgical Removal of Venous Samples
2.3. Sample Preparation and RNA Sequencing
2.4. Reverse Transcription Polymerase Chain Reaction
2.5. Bioinformatics Analysis of RNA Sequence Data
2.6. Screening of Differentially Expressed Genes (DEGs)
2.7. Pathway and Network Analysis
2.8. Zebrafish Model
2.9. Antisense Morpholino Design and Microinjection
2.10. Statistical Methods
3. Results
3.1. Global Expressional Profiling of VV
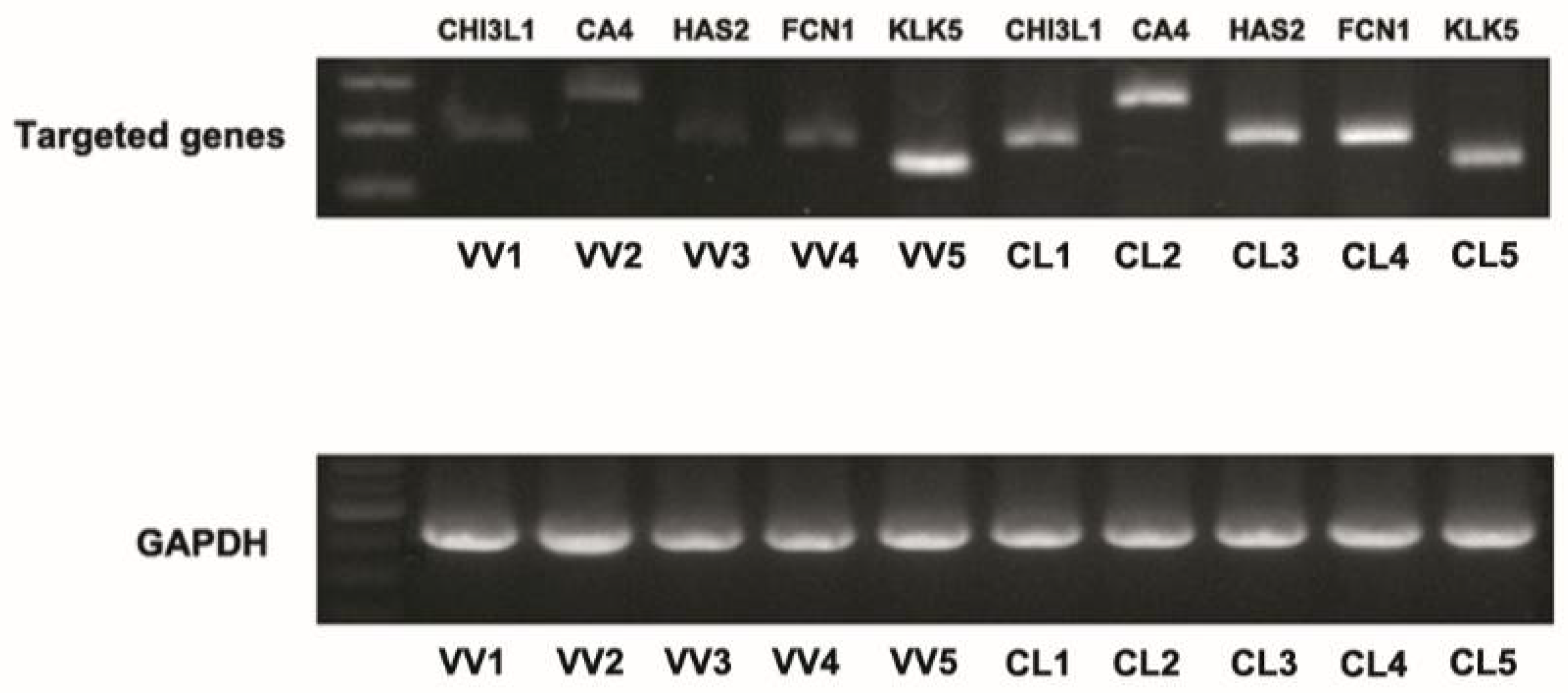
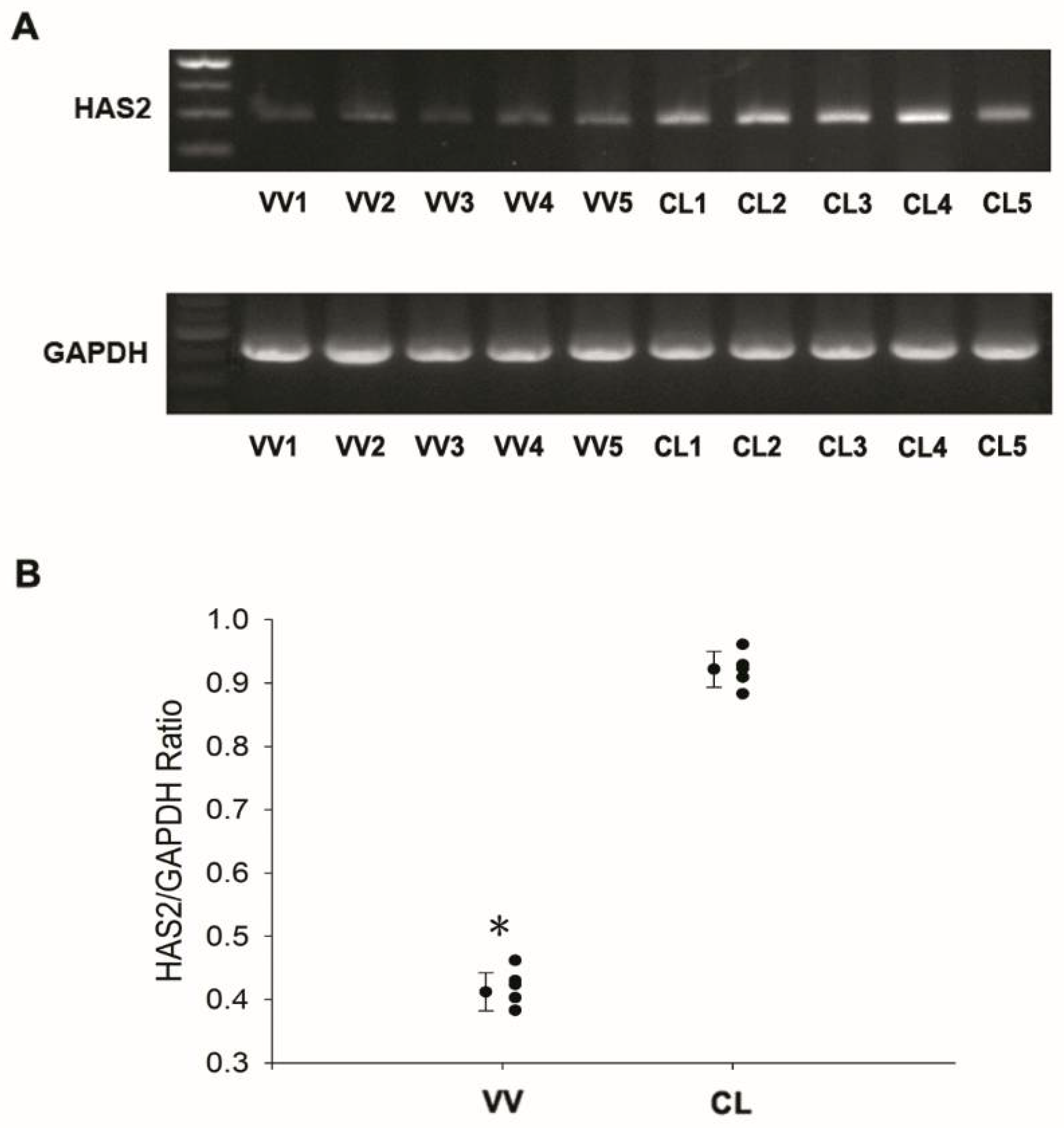
3.2. Downregulation of Hyaluronan Synthases 2 in Venous Tissues from Patients with VV
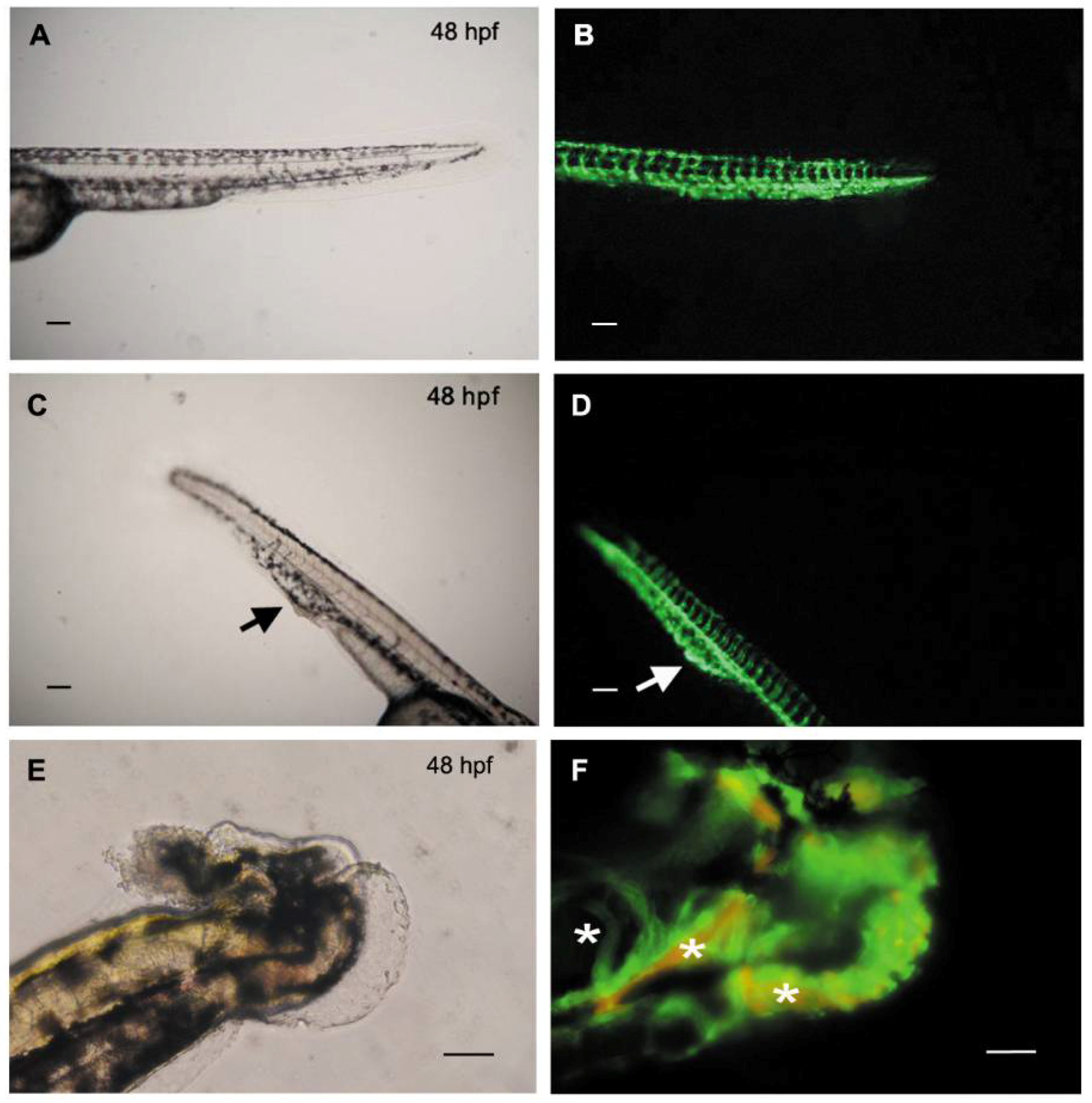
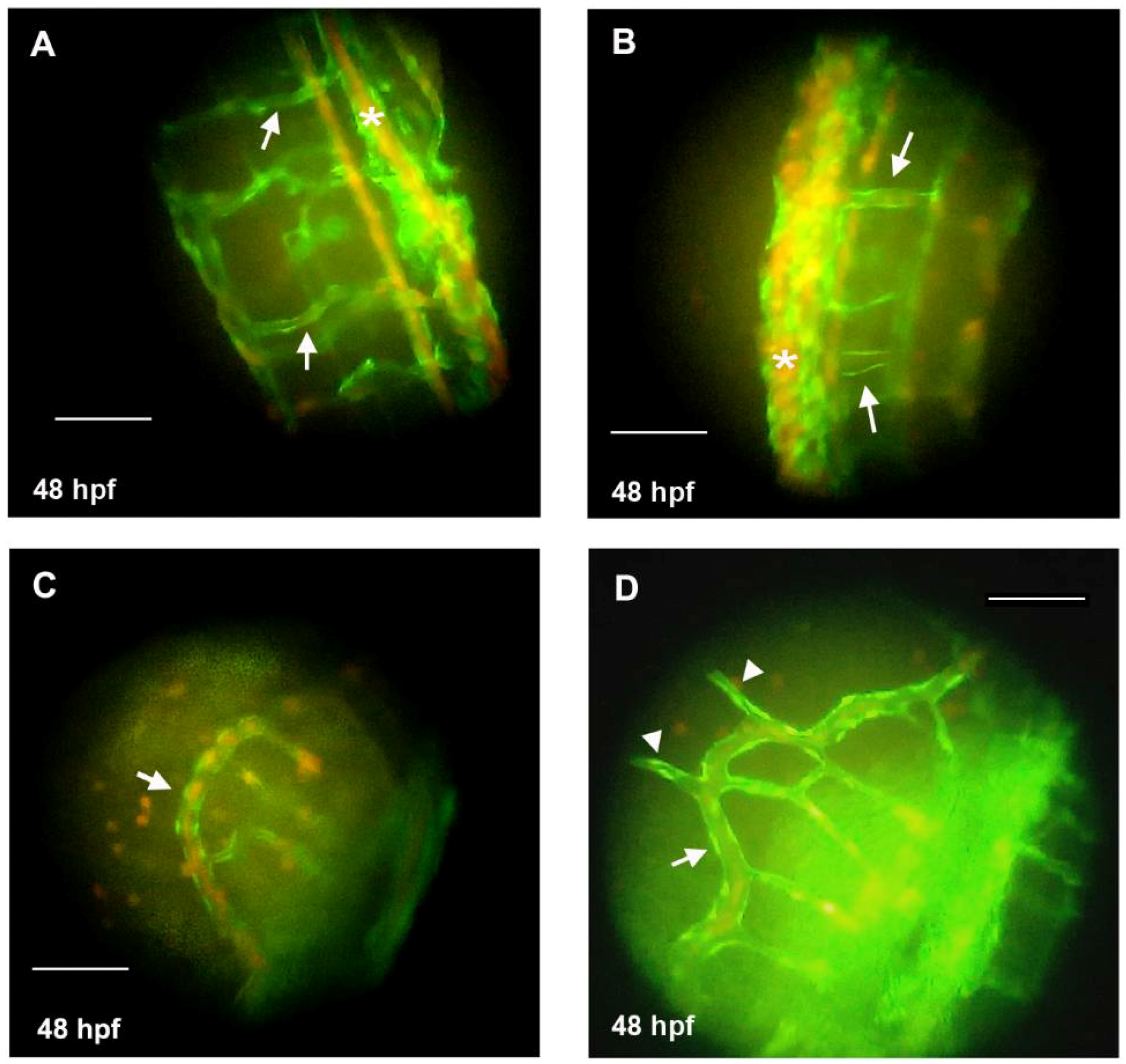
3.3. Knockdown of HAS2 Results in Venous Dilation and Blood Flow Stasis in Zebrafish
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oklu, R.; Habito, R.; Mayr, M.; Deipolyi, A.R.; Albadawi, H.; Hesketh, R.; Walker, T.G.; Linskey, K.R.; Long, C.A.; Wicky, S.; et al. Pathogenesis of varicose veins. J. Vasc. Interv. Radiol. 2012, 23, 33–39. [Google Scholar] [CrossRef]
- Li, X.; Jiang, X.Y.; Ge, J.; Wang, J.; Chen, G.J.; Xu, L.; Xie, D.Y.; Yuan, T.Y.; Zhang, D.S.; Zhang, H.; et al. Aberrantly expressed lncRNAs in primary varicose great saphenous veins. PLoS ONE 2014, 9, e86156. [Google Scholar] [CrossRef]
- Meissner, M.H.; Gloviczki, P.; Bergan, J.; Kistner, R.L.; Morrison, N.; Pannier, F.; Pappas, P.J.; Rabe, E.; Raju, S.; Villavicencio, J.L. Primary chronic venous disorders. J. Vasc. Surg. 2007, 46 (Suppl. S), 54s–67s. [Google Scholar] [CrossRef]
- Heit, J.A.; Rooke, T.W.; Silverstein, M.D.; Mohr, D.N.; Lohse, C.M.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., 3rd. Trends in the incidence of venous stasis syndrome and venous ulcer: A 25-year population-based study. J. Vasc. Surg. 2001, 33, 1022–1027. [Google Scholar] [CrossRef]
- Brand, F.N.; Dannenberg, A.L.; Abbott, R.D.; Kannel, W.B. The epidemiology of varicose veins: The Framingham Study. Am. J. Prev. Med. 1988, 4, 96–101. [Google Scholar] [CrossRef]
- Bergan, J.J.; Schmid-Schonbein, G.W.; Smith, P.D.; Nicolaides, A.N.; Boisseau, M.R.; Eklof, B. Chronic venous disease. N. Engl. J. Med. 2006, 355, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Beebe-Dimmer, J.L.; Pfeifer, J.R.; Engle, J.S.; Schottenfeld, D. The epidemiology of chronic venous insufficiency and varicose veins. Ann. Epidemiol. 2005, 15, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Krysa, J.; Jones, G.T.; van Rij, A.M. Evidence for a genetic role in varicose veins and chronic venous insufficiency. Phlebology 2012, 27, 329–335. [Google Scholar] [CrossRef]
- Naik, B.; Kumar, M.; Khanna, A.K.; Suman, P.K. Clinico-histopathological study of varicose vein and role of matrix metalloproteinases-1, matrix metalloproteinases-9 and tissue inhibitor of matrix metalloproteinase-1 in varicose vein formation. Indian J. Pathol. Microbiol. 2016, 59, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, W.; Raffetto, J.D.; Khalil, R.A. Matrix Metalloproteinases in Remodeling of Lower Extremity Veins and Chronic Venous Disease. Prog. Mol. Biol. Transl. Sci. 2017, 147, 267–299. [Google Scholar] [CrossRef]
- Xu, Y.; Bei, Y.; Li, Y.; Chu, H. Phenotypic and functional transformation in smooth muscle cells derived from varicose veins. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.M.; Zhao, Y.; Zhang, X.M.; Zhu, T.; Fu, W.G. Polymorphisms in MMP-9 and TIMP-2 in Chinese patients with varicose veins. J. Surg. Res. 2011, 168, e143–e148. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.N.; Andraska, E.A.; Obi, A.T.; Wakefield, T.W. Pathophysiology of varicose veins. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Mannello, F. Pathophysiology of chronic venous disease. Int. Angiol. J. Int. Union Angiol. 2014, 33, 212–221. [Google Scholar]
- Bharath, V.; Kahn, S.R.; Lazo-Langner, A. Genetic polymorphisms of vein wall remodeling in chronic venous disease: A narrative and systematic review. Blood 2014, 124, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.Y.; Andrew, T.; Spector, T.D.; Jeffery, S. Linkage to the FOXC2 region of chromosome 16 for varicose veins in otherwise healthy, unselected sibling pairs. J. Med. Genet. 2005, 42, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Mellor, R.H.; Brice, G.; Stanton, A.W.; French, J.; Smith, A.; Jeffery, S.; Levick, J.R.; Burnand, K.G.; Mortimer, P.S. Mutations in FOXC2 are strongly associated with primary valve failure in veins of the lower limb. Circulation 2007, 115, 1912–1920. [Google Scholar] [CrossRef]
- Matousek, V.; Prerovsky, I. A contribution to the problem of the inheritance of primary varicose veins. Hum. Hered. 1974, 24, 225–235. [Google Scholar]
- Hauge, M.; Gundersen, J. Genetics of varicose veins of the lower extremities. Hum. Hered. 1969, 19, 573–580. [Google Scholar] [CrossRef]
- Brice, G.; Mansour, S.; Bell, R.; Collin, J.R.; Child, A.H.; Brady, A.F.; Sarfarazi, M.; Burnand, K.G.; Jeffery, S.; Mortimer, P.; et al. Analysis of the phenotypic abnormalities in lymphoedema-distichiasis syndrome in 74 patients with FOXC2 mutations or linkage to 16q24. J. Med. Genet. 2002, 39, 478–483. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, G.; Huang, J.; Zhou, D.; Huang, X.; Shen, L. Analysis of the association between an insertion/deletion polymorphism within the 3’ untranslated region of COL1A2 and chronic venous insufficiency. Ann. Vasc. Surg. 2013, 27, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, R.; Malkowski, A.; Gacko, M.; Sobolewski, K. Influence of thrombophlebitis on TGF-beta1 and its signaling pathway in the vein wall. Folia Histochem. Cytobiol. 2010, 48, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Yang, X.; Kaplan, L.M.; Molony, C.; Schadt, E.E. Integrating pathway analysis and genetics of gene expression for genome-wide association studies. Am. J. Hum. Genet. 2010, 86, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Schunkert, H.; Konig, I.R.; Kathiresan, S.; Reilly, M.P.; Assimes, T.L.; Holm, H.; Preuss, M.; Stewart, A.F.; Barbalic, M.; Gieger, C.; et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 2011, 43, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Lotta, L.A. Genome-wide association studies in atherothrombosis. Eur. J. Intern. Med. 2010, 21, 74–78. [Google Scholar] [CrossRef]
- Hardy, J.; Singleton, A. Genomewide association studies and human disease. N. Engl. J. Med. 2009, 360, 1759–1768. [Google Scholar] [CrossRef]
- Shadrina, A.S.; Smetanina, M.A.; Sokolova, E.A.; Shamovskaya, D.V.; Sevost’ianova, K.S.; Shevela, A.I.; Soldatsky, E.Y.; Seliverstov, E.I.; Demekhova, M.Y.; Shonov, O.A.; et al. Allele rs2010963 C of the VEGFA gene is associated with the decreased risk of primary varicose veins in ethnic Russians. Phlebology 2018, 33, 27–35. [Google Scholar] [CrossRef]
- Kundu, S.; Lurie, F.; Millward, S.F.; Padberg, F., Jr.; Vedantham, S.; Elias, S.; Khilnani, N.M.; Marston, W.; Cardella, J.F.; Meissner, M.H.; et al. Recommended reporting standards for endovenous ablation for the treatment of venous insufficiency: Joint statement of The American Venous Forum and The Society of Interventional Radiology. J. Vasc. Surg. 2007, 46, 582–589. [Google Scholar] [CrossRef]
- Brittenden, J.; Cotton, S.C.; Elders, A.; Ramsay, C.R.; Norrie, J.; Burr, J.; Campbell, B.; Bachoo, P.; Chetter, I.; Gough, M.; et al. A Randomized Trial Comparing Treatments for Varicose Veins. N. Engl. J. Med. 2014, 371, 1218–1227. [Google Scholar] [CrossRef]
- Sufian, S.; Arnez, A.; Labropoulos, N.; Nguyen, K.; Satwah, V.; Marquez, J.; Chowla, A.; Lakhanpal, S. Radiofrequency ablation of the great saphenous vein, comparing one versus two treatment cycles for the proximal vein segment. Phlebology 2015, 30, 724–728. [Google Scholar] [CrossRef]
- Sufian, S.; Arnez, A.; Labropoulos, N.; Lakhanpal, S. Endovenous heat-induced thrombosis after ablation with 1470 nm laser: Incidence, progression, and risk factors. Phlebology 2015, 30, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.T.; Hsieh, C.S.; Chang, S.N.; Chuang, E.Y.; Ueng, K.C.; Tsai, C.F.; Lin, T.H.; Wu, C.K.; Lee, J.K.; Lin, L.Y.; et al. Genome-wide screening identifies a KCNIP1 copy number variant as a genetic predictor for atrial fibrillation. Nat. Commun. 2016, 7, 10190. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, C.; Li, Y.; Lam, T.W.; Yiu, S.M.; Kristiansen, K.; Wang, J. SOAP2: An improved ultrafast tool for short read alignment. Bioinformatics 2009, 25, 1966–1967. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lin, Y.T.; Lee, G.H. Novel and unexpected functions of zebrafish CCAAT box binding transcription factor (NF-Y) B subunit during cartilages development. Bone 2009, 44, 777–784. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Hen, G.; Nicenboim, J.; Mayseless, O.; Asaf, L.; Shin, M.; Busolin, G.; Hofi, R.; Almog, G.; Tiso, N.; Lawson, N.D.; et al. Venous-derived angioblasts generate organ-specific vessels during zebrafish embryonic development. Development 2015, 142, 4266–4278. [Google Scholar] [CrossRef]
- Camenisch, T.D.; Spicer, A.P.; Brehm-Gibson, T.; Biesterfeldt, J.; Augustine, M.L.; Calabro, A., Jr.; Kubalak, S.; Klewer, S.E.; McDonald, J.A. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J. Clin. Investig. 2000, 106, 349–360. [Google Scholar] [CrossRef] [PubMed]
| Variables | VV (n = 5) | Controls (n = 5) |
|---|---|---|
| Age (yr) | 69.6 ± 14.7 | 75 ± 11.4 |
| BMI | 26.1 ± 2.8 | 22.6 ± 1.8 |
| Male | 2 | 5 |
| DM | 0 | 3 |
| HTN | 2 | 3 |
| HLP | 1 | 4 |
| Smoke | 0 | 2 |
| Gene | Log2 Ratio | Regulation | Location | Type(s) |
|---|---|---|---|---|
| (VV/CTL) | (VV/CTL) | |||
| CRABP1 | 11.98 | Up | Cytoplasm | transporter |
| SNORA77 | 10.16 | Up | Other | other |
| DPEP1 | 9.69 | Up | Cytoplasm | peptidase |
| PSORS1C1 | 9.56 | Up | Other | other |
| CST2 | 9.32 | Up | Extracellular Space | other |
| HLA-DQA2 | 8.68 | Up | Plasma Membrane | transmembrane receptor |
| CLIC3 | 8.46 | Up | Nucleus | ion channel |
| WFDC10B | 8.20 | Up | Extracellular Space | other |
| KLK5 | 7.77 | Up | Extracellular Space | peptidase |
| CPNE7 | 7.51 | Up | Cytoplasm | transporter |
| CHAD | 7.39 | Up | Extracellular Space | other |
| WFDC3 | 7.16 | Up | Extracellular Space | other |
| S100A8 | −12.33 | Down | Cytoplasm | other |
| GSTT1 | −11.46 | Down | Cytoplasm | enzyme |
| CXCL1 | −10.01 | Down | Extracellular Space | cytokine |
| DAB1 | −9.99 | Down | Cytoplasm | other |
| CHI3L1 | −9.98 | Down | Extracellular Space | enzyme |
| CSF3 | −9.96 | Down | Extracellular Space | cytokine |
| CXCL8 | −9.56 | Down | Extracellular Space | cytokine |
| FCGR3A/FCGR3B | −9.28 | Down | Plasma Membrane | transmembrane receptor |
| S100A12 | −8.95 | Down | Cytoplasm | other |
| CD33 | −8.70 | Down | Plasma Membrane | other |
| FCN1 | −8.53 | Down | Extracellular Space | other |
| PQLC2L | −8.10 | Down | Other | other |
| AQP9 | −7.93 | Down | Plasma Membrane | transporter |
| SELE | −7.85 | Down | Plasma Membrane | transmembrane receptor |
| HAS2 | −7.69 | Down | extracellular matrix | enzyme |
| CA4 | −7.63 | Down | Plasma Membrane | enzyme |
| IL6 | −7.62 | Down | Extracellular Space | cytokine |
| THEM5 | −7.58 | Down | Cytoplasm | enzyme |
| ZNF385B | −7.52 | Down | Nucleus | other |
| SRGAP2B | −7.39 | Down | Other | other |
| FCGR2C | −7.18 | Down | Plasma Membrane | transmembrane receptor |
| IL1R2 | −7.13 | Down | Plasma Membrane | transmembrane receptor |
| ALAS2 | −7.08 | Down | Cytoplasm | enzyme |
| HHATL | −7.08 | Down | Cytoplasm | enzyme |
| Diseases or Functions Annotation | p-Value * | Predicted Activation State | Molecules |
|---|---|---|---|
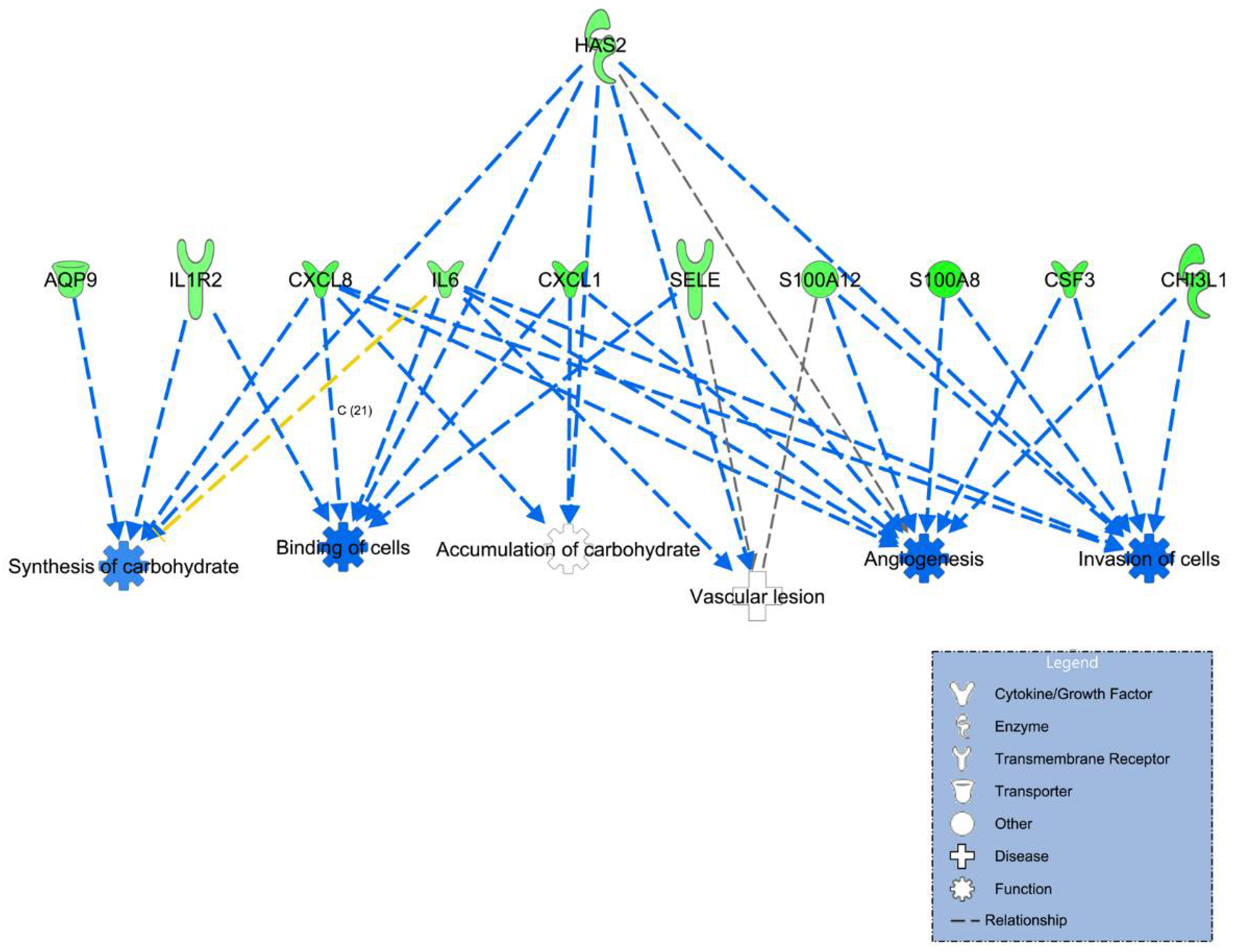
| Angiogenesis | 8.48 × 10−5 | Decreased | CHI3L1, CSF3, CXCL1, CXCL8, HAS2, IL6, S100A12, S100A8, SELE |
| Binding of cells | 3.07 × 10−4 | Decreased | CXCL1, CXCL8, HAS2, IL1R2, IL6, SELE |
| Invasion of cells | 3.43 × 10−4 | Decreased | CHI3L1, CSF3, CXCL1, CXCL8, HAS2, IL6, S100A12, S100A8 |
| Vascular lesion | 7.74 × 10−4 | HAS2, IL6, S100A12, SELE | |
| Synthesis of carbohydrate | 1.32 × 10−3 | AQP9, CXCL8, HAS2, IL1R2, IL6 | |
| Accumulation of carbohydrate | 1.93 × 10−3 | CXCL1, CXCL8, HAS2 |
| Injection Dose (μM) | Injected Embryos | Survival Embryos 1 | GFP-Positive Embryos | Embryos without Defective Vascular Phenotypes (%) * | Embryos with Defective Vascular Phenotypes (%) * |
|---|---|---|---|---|---|
| (Survival Rates %) 2 | |||||
| 250 | 231 | 145 (62%) | 132 | 84 (63%) | 48 (37%) |
| 375 | 563 | 254 (45%) | 240 | 68 (28%) | 172 (72%) |
| 500 | 502 | 49 (9%) | 28 | 16 (57%) | 12 (43%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-S.; Tsai, C.-T.; Chen, Y.-H.; Chang, S.-N.; Hwang, J.-J.; Chuang, E.Y.; Wu, I.-H. Global Expression Profiling Identifies a Novel Hyaluronan Synthases 2 Gene in the Pathogenesis of Lower Extremity Varicose Veins. J. Clin. Med. 2018, 7, 537. https://doi.org/10.3390/jcm7120537
Hsieh C-S, Tsai C-T, Chen Y-H, Chang S-N, Hwang J-J, Chuang EY, Wu I-H. Global Expression Profiling Identifies a Novel Hyaluronan Synthases 2 Gene in the Pathogenesis of Lower Extremity Varicose Veins. Journal of Clinical Medicine. 2018; 7(12):537. https://doi.org/10.3390/jcm7120537
Chicago/Turabian StyleHsieh, Chia-Shan, Chia-Ti Tsai, Yau-Hung Chen, Sheng-Nan Chang, Juey-Jen Hwang, Eric Y. Chuang, and I-Hui Wu. 2018. "Global Expression Profiling Identifies a Novel Hyaluronan Synthases 2 Gene in the Pathogenesis of Lower Extremity Varicose Veins" Journal of Clinical Medicine 7, no. 12: 537. https://doi.org/10.3390/jcm7120537
APA StyleHsieh, C.-S., Tsai, C.-T., Chen, Y.-H., Chang, S.-N., Hwang, J.-J., Chuang, E. Y., & Wu, I.-H. (2018). Global Expression Profiling Identifies a Novel Hyaluronan Synthases 2 Gene in the Pathogenesis of Lower Extremity Varicose Veins. Journal of Clinical Medicine, 7(12), 537. https://doi.org/10.3390/jcm7120537






