Mucolytic Agents and Statins Use is Associated with a Lower Risk of Acute Exacerbations in Patients with Bronchiectasis-Chronic Obstructive Pulmonary Disease Overlap
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Design and Population
2.3. Potential Confounders and Classification of Severity
2.4. Effect of Exposure to Co-Medications
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Population
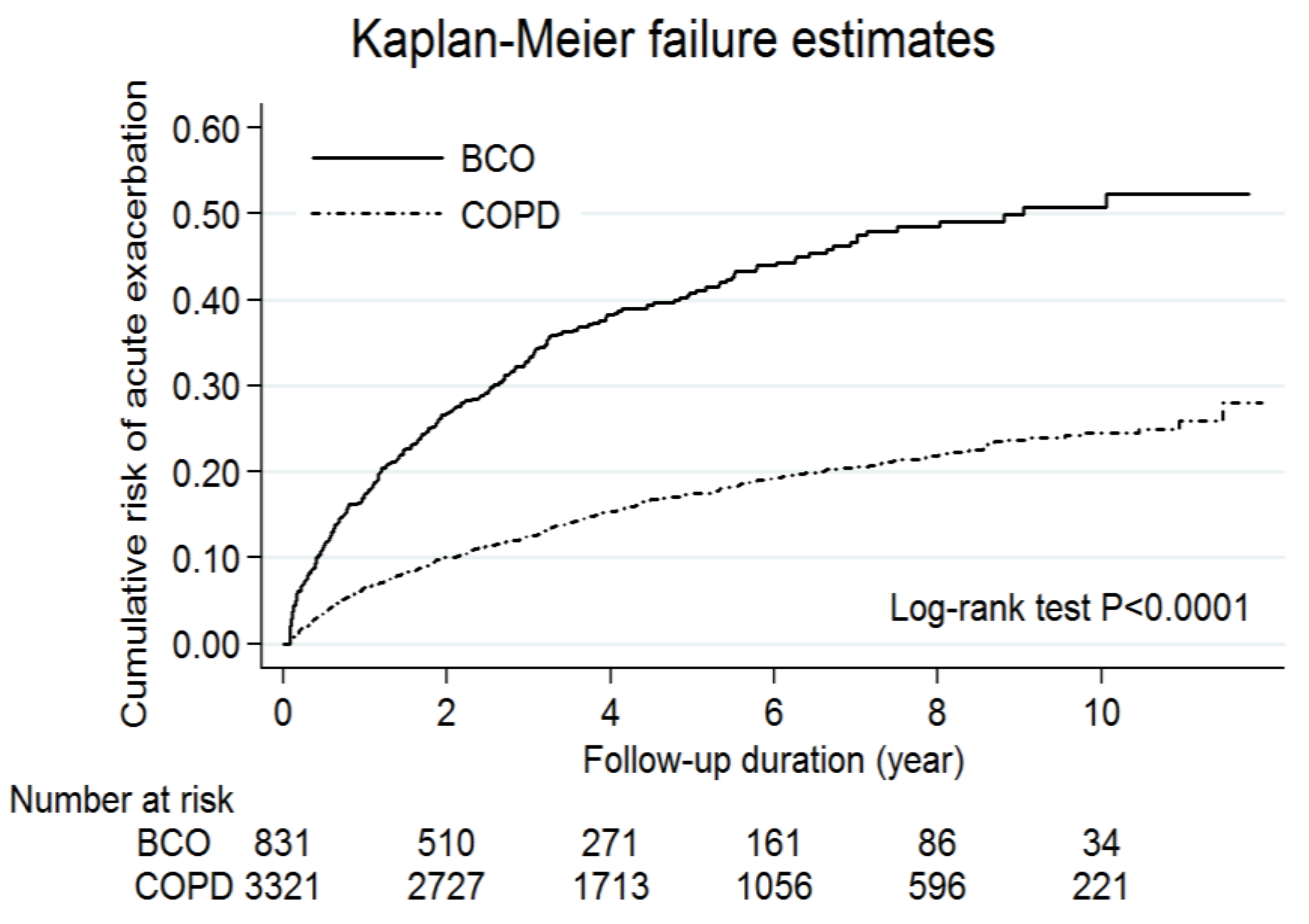
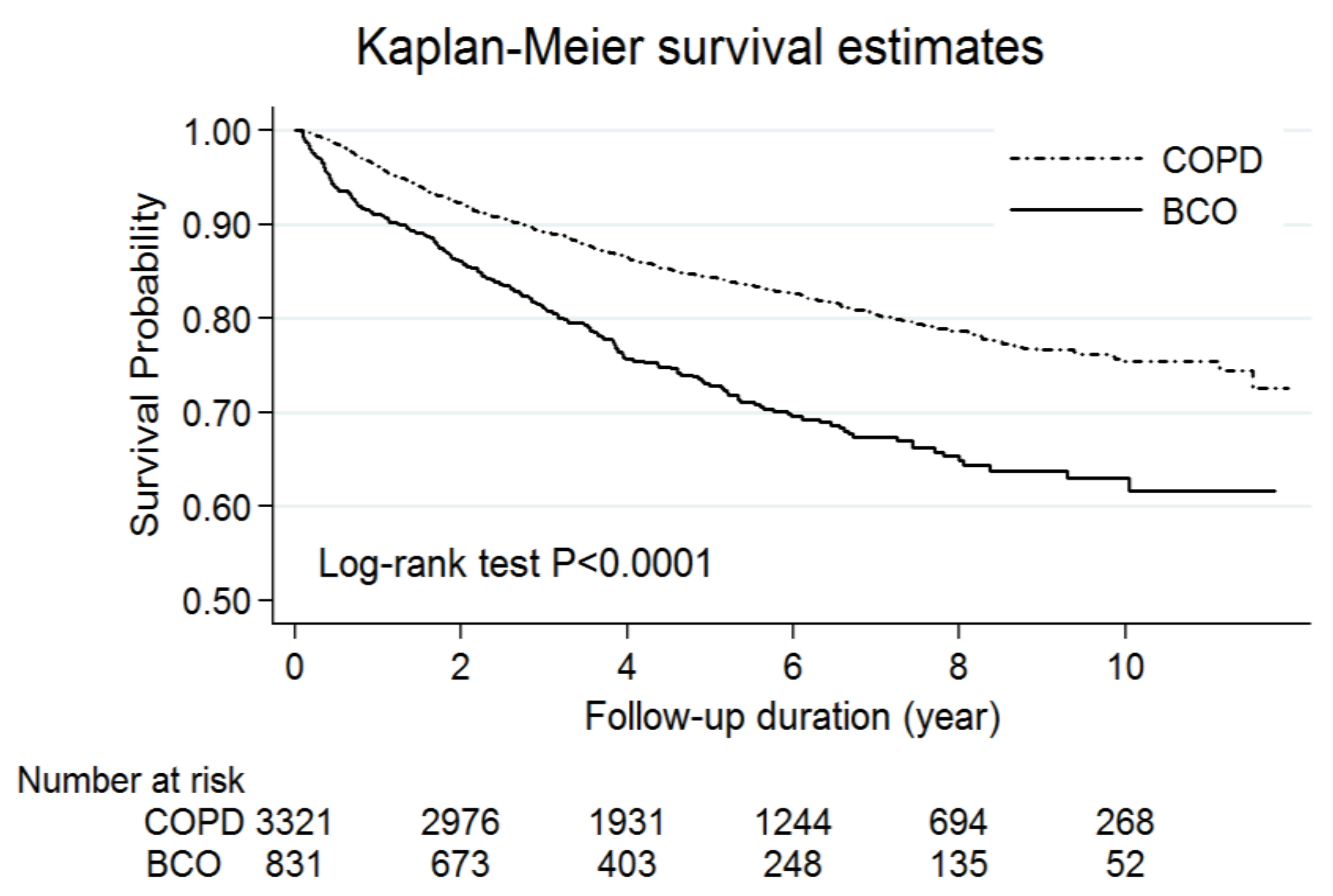
3.2. The Effect of Bronchiectasis on Acute Exacerbations and Mortality
3.3. Effects of Co-medications on Acute Exacerbations and Mortality
3.4. Sensitivity and Subgroup Analyses for the Effects of Co-Medications on Acute Exacerbations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgements
Conflict of interest
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2017. Available online: https://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/ (accessed on 15 May 2017).
- World Health Organization. The Top 10 Causes of Death. 2018. Available online: http://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 21 October 2018).
- Hurst, J.R.; Elborn, J.S.; de Soyza, A.; Consortium, B.-U. COPD-bronchiectasis overlap syndrome. Eur. Respir. J. 2015, 45, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Canton, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.S.; Vlahos, I.; Wilkinson, T.M.; Lloyd-Owen, S.J.; Donaldson, G.C.; Wilks, M.; Reznek, R.H.; Wedzicha, J.A. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2004, 170, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; de la Rosa Carrillo, D.; Soler-Cataluna, J.J.; Donat-Sanz, Y.; Serra, P.C.; Lerma, M.A.; Ballestin, J.; Sanchez, I.V.; Selma Ferrer, M.J.; Dalfo, A.R.; et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 823–831. [Google Scholar] [CrossRef]
- Pasteur, M.C.; Bilton, D.; Hill, A.T. British Thoracic Society Bronchiectasis non-CF Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010, 65, 1–58. [Google Scholar] [CrossRef]
- Su, V.Y.; Liu, C.J.; Wang, H.K.; Wu, L.A.; Chang, S.C.; Perng, D.W.; Su, W.J.; Chen, Y.M.; Lin, E.Y.; Chen, T.J.; et al. Sleep apnea and risk of pneumonia: A nationwide population-based study. CMAJ 2014, 186, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Su, V.Y.; Yang, K.Y.; Yang, Y.H.; Tsai, Y.H.; Perng, D.W.; Su, W.J.; Chou, K.T.; Su, K.C.; Yen, Y.F.; Chen, P.C. Use of ICS/LABA combinations or LAMA is associated with a lower risk of acute exacerbation in patients with coexistent COPD and asthma. J. Allergy Clin. Immunol. Pract. 2018, 6, 1927–1935. [Google Scholar] [CrossRef]
- Su, V.Y.; Su, W.J.; Yen, Y.F.; Pan, S.W.; Chuang, P.H.; Feng, J.Y.; Chou, K.T.; Yang, K.Y.; Lee, Y.C.; Chen, T.J. Statin use is associated with a lower risk of tuberculosis. Chest 2017. [Google Scholar] [CrossRef]
- Su, V.Y.; Yen, Y.F.; Pan, S.W.; Chuang, P.H.; Feng, J.Y.; Chou, K.T.; Chen, Y.M.; Chen, T.J.; Su, W.J. Latent tuberculosis infection and the risk of subsequent cancer. Medicine (Baltimore) 2016, 95, e2352. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Soler-Cataluna, J.J.; Donat Sanz, Y.; Catalan Serra, P.; Agramunt Lerma, M.; Ballestin, V.J.; Perpina-Tordera, M. Factors associated with bronchiectasis in patients with COPD. Chest 2011, 140, 1130–1137. [Google Scholar] [CrossRef]
- Gursel, G. Does coexistence with bronchiectasis influence intensive care unit outcome in patients with chronic obstructive pulmonary disease? Heart Lung 2006, 35, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Gatheral, T.; Kumar, N.; Sansom, B.; Lai, D.; Nair, A.; Vlahos, I.; Baker, E.H. COPD-related bronchiectasis; independent impact on disease course and outcomes. COPD 2014, 11, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Poole, P.; Chong, J.; Cates, C.J. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2015, 7, CD001287. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Rogliani, P.; Calzetta, L.; Hanania, N.A.; Matera, M.G. Impact of mucolytic agents on COPD exacerbations: A pair-wise and network meta-analysis. COPD 2017, 14, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Welsh, E.J.; Evans, D.J.; Fowler, S.J.; Spencer, S. Interventions for bronchiectasis: An overview of cochrane systematic reviews. Cochrane Database Syst. Rev. 2015, 14, CD010337. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.; Sugumar, K.; Milan, S.J.; Hart, A.; Crockett, A.; Crossingham, I. Mucolytics for bronchiectasis. Cochrane Database Syst. Rev. 2014, 2, CD001289. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, D.; Ciaccia, A.; Marangio, E.; Marsico, S.; Todisco, T.; Del Vita, M. Role of bromhexine in exacerbations of bronchiectasis. Double-blind randomized multicenter study versus placebo. Respiration 1991, 58, 117–121. [Google Scholar] [CrossRef]
- Tateda, K.; Standiford, T.J.; Pechere, J.C.; Yamaguchi, K. Regulatory effects of macrolides on bacterial virulence: Potential role as quorum-sensing inhibitors. Curr. Pharm. Des. 2004, 10, 3055–3065. [Google Scholar] [CrossRef]
- Tsai, W.C.; Standiford, T.J. Immunomodulatory effects of macrolides in the lung: Lessons from in-vitro and in-vivo models. Curr. Pharm. Des. 2004, 10, 3081–3093. [Google Scholar] [CrossRef]
- Rogers, G.B.; van der Gast, C.J.; Cuthbertson, L.; Thomson, S.K.; Bruce, K.D.; Martin, M.L.; Serisier, D.J. Clinical measures of disease in adult non-CF bronchiectasis correlate with airway microbiota composition. Thorax 2013, 68, 731–737. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Smith, M.P.; McHugh, B.J.; Doherty, C.; Govan, J.R.; Hill, A.T. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am. J. Respir. Crit. Care Med. 2012, 186, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Tangney, C.C.; Casey, L.C. Inhibition of proinflammatory cytokine production by pravastatin. Lancet 1999, 353, 983–984. [Google Scholar] [CrossRef]
- Dunzendorfer, S.; Rothbucher, D.; Schratzberger, P.; Reinisch, N.; Kahler, C.M.; Wiedermann, C.J. Mevalonate-dependent inhibition of transendothelial migration and chemotaxis of human peripheral blood neutrophils by pravastatin. Circ. Res. 1997, 81, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wu, Y.; Xu, Z.; Lv, D.; Zhang, C.; Lai, T.; Li, W.; Shen, H. The effect of statins on chronic obstructive pulmonary disease exacerbation and mortality: A systematic review and meta-analysis of observational research. Sci. Rep. 2015, 5, 16461. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Chalmers, J.D.; Graham, C.; Harley, C.; Sidhu, M.K.; Doherty, C.; Govan, J.W.; Sethi, T.; Davidson, D.J.; Rossi, A.G.; et al. Atorvastatin as a stable treatment in bronchiectasis: A randomised controlled trial. Lancet 2014, 2, 455–463. [Google Scholar] [CrossRef]
| Characteristics | BCO Cohort | COPD Alone Cohort | p Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| n | 831 | 3321 | |||
| Age, years (mean ± SD) | 68.70 ± 12.14 | 68.61 ± 12.08 | 0.8433 | ||
| Age | 0.9822 | ||||
| 40–49 | 62 | 7.46% | 248 | 7.47% | |
| 50–59 | 136 | 16.37% | 564 | 16.98% | |
| 60–69 | 207 | 24.91% | 834 | 25.11% | |
| 70–79 | 254 | 30.57% | 1016 | 30.59% | |
| ≥80 | 172 | 20.70% | 659 | 19.84% | |
| Sex | 0.9741 | ||||
| Male | 549 | 66.06% | 2196 | 66.12% | |
| Female | 282 | 33.94% | 1125 | 33.88% | |
| Follow-up, years (mean ± SD) § | 4.53 ± 3.00 | 5.22 ± 2.95 | <0.0001 | ||
| COPD severity † | |||||
| COPD-related ED visits, n (%) | <0.0001 | ||||
| 0 | 751 | 90.37% | 3214 | 96.78% | |
| ≥1 | 80 | 9.63% | 107 | 3.22% | |
| COPD-related hospitalizations, n (%) | <0.0001 | ||||
| 0 | 657 | 79.06% | 2943 | 88.62% | |
| ≥1 | 174 | 20.94% | 378 | 11.38% | |
| COPD medications ‡, n (%) | <0.0001 | ||||
| 0–2 | 525 | 63.18% | 2830 | 85.22% | |
| ≥3 | 306 | 36.82% | 491 | 14.78% | |
| Comorbidities # | |||||
| Diabetes mellitus | 152 | 18.29% | 622 | 18.73% | 0.7718 |
| Cardiovascular disease | 476 | 57.28% | 1846 | 55.59% | 0.3788 |
| Stroke | 166 | 19.98% | 513 | 15.45% | 0.0016 |
| Chronic kidney disease | 32 | 3.85% | 119 | 3.58% | 0.7126 |
| Antecedent Pneumonia | 289 | 34.78% | 317 | 9.55% | <0.0001 |
| Malignancy | 181 | 21.78% | 361 | 10.87% | <0.0001 |
| Medications during the follow-up period * | |||||
| Statins | 145 | 17.45% | 851 | 25.62% | <0.0001 |
| Macrolides | 118 | 14.20% | 163 | 4.91% | <0.0001 |
| Mucolytic agents | 712 | 85.68% | 2255 | 67.90% | <0.0001 |
| Medications, before the first AE $ | |||||
| Statins | 113 | 13.60% | 805 | 24.24% | <0.0001 |
| Macrolides | 70 | 8.42% | 121 | 3.64% | <0.0001 |
| Mucolytic agents | 638 | 76.77% | 2091 | 62.96% | <0.0001 |
| BCO Cohort | COPD Alone Cohort | Rate Ratio | p Value * | |||
|---|---|---|---|---|---|---|
| n | Rate § | n | Rate § | |||
| Total exacerbations † | 1224 | 32.53 | 1651 | 9.52 | 3.42 (3.17–3.68) | <0.0001 |
| Moderate exacerbations † | ||||||
| OPD visits | 841 | 22.35 | 1080 | 6.23 | 3.59 (3.28–3.93) | <0.0001 |
| ED visits | 43 | 1.14 | 71 | 0.41 | 2.79 (1.87–4.13) | <0.0001 |
| Severe exacerbations † | ||||||
| Hospital admissions | 340 | 9.04 | 500 | 2.88 | 3.14 (2.72–3.61) | <0.0001 |
| ICU admissions | 65 | 1.73 | 69 | 0.40 | 4.34 (3.05–6.18) | <0.0001 |
| Hospitalization days | 6146 | 163.32 | 8395 | 48.39 | 3.38 (3.27–3.49) | <0.0001 |
| Drugs for exacerbations, (mean ± SD) | ||||||
| Steroids ‡ (mg) | 281.2 ± 342.9 | 208.0 ± 273.5 | <0.0001 | |||
| Antibiotics (days) | 14.90 ± 14.78 | 12.63 ± 11.92 | 0.0011 | |||
| Anti-pseudomonal FQs #, event (%) | 15.03% | 8.96% | <0.0001 | |||
| Mucolytic agents, event (%) | 60.78% | 56.75% | 0.0301 | |||
| Variables | BCO Cohort | COPD Alone Cohort | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Medications, before first AE | ||||
| Statins (total) | 0.44 (0.29–0.65) | <0.0001 | 0.35 (0.26–0.46) | <0.0001 |
| Statins, 28–90 days | 0.77 (0.38–1.57) | 0.4647 | 0.71 (0.46–1.08) | 0.1071 |
| Statins, >90 days | 0.37 (0.23–0.59) | <0.0001 | 0.26 (0.18–0.37) | <0.0001 |
| Macrolides (total) | 0.85 (0.54–1.32) | 0.4611 | 0.46 (0.25–0.83) | 0.0103 |
| Macrolides, 28–90 days | 0.94 (0.58–1.52) | 0.7947 | 0.41 (0.21–0.79) | 0.0077 |
| Macrolides, >90 days | 0.53 (0.17–1.67) | 0.2795 | 0.99 (0.25–3.98) | 0.9861 |
| Mucolytic agents (total) | 0.58 (0.45–0.75) | <0.0001 | 0.73 (0.61–0.86) | 0.0003 |
| Mucolytic agents, 28–90 days | 0.80 (0.60–1.08) | 0.1504 | 0.82 (0.66–1.01) | 0.0638 |
| Mucolytic agents, >90 days | 0.48 (0.36–0.63) | <0.0001 | 0.67 (0.55–0.82) | <0.0001 |
| Variables | BCO Cohort | COPD Cohort | ||
|---|---|---|---|---|
| Adjusted HR (95% CI) | p | Adjusted HR (95% CI) | p | |
| Medications, before first AE | ||||
| Statins | ||||
| Simvastatin | 0.38 (0.19–0.77) | 0.0066 | 0.26 (0.15–0.46) | <0.0001 |
| Lovastatin | 0.23 (0.07–0.72) | 0.0113 | 0.43 (0.23–0.78) | 0.0053 |
| Pravastatin | 0.68 (0.28–1.68) | 0.4067 | 0.58 (0.30–1.13) | 0.1078 |
| Fluvastatin | 0.57 (0.23–1.39) | 0.2144 | 0.31 (0.15–0.66) | 0.0024 |
| Atorvastatin | 0.28 (0.15–0.53) | <0.0001 | 0.34 (0.23–0.52) | <0.0001 |
| Rosuvastatin | 0.42 (0.20–0.89) | 0.0229 | 0.25 (0.13–0.46) | <0.0001 |
| Macrolides | ||||
| Erythromycin | 0.89 (0.45–1.74) | 0.7303 | 0.85 (0.35–2.05) | 0.7102 |
| Azithromycin | 0.30 (0.04–2.13) | 0.2278 | N/A | N/A |
| Clarithromycin | 1.03 (0.42–2.50) | 0.9564 | 0.77 (0.32–1.86) | 0.5595 |
| Mucolytic agents | ||||
| N-acetylcysteine | 0.75 (0.58–0.98) | 0.0359 | 0.84 (0.68–0.98) | 0.0346 |
| Carbocysteine | 0.57 (0.34–0.96) | 0.0349 | 0.79 (0.54–1.14) | 0.2033 |
| Ambroxol | 0.60 (0.48–0.76) | <0.0001 | 0.78 (0.66–0.93) | 0.0055 |
| Iodinated glycerol | 0.72 (0.41–1.26) | 0.2522 | 1.01 (0.68–1.51) | 0.9444 |
| Bromhexine | 0.60 (0.45–0.80) | 0.0004 | 0.84 (0.68–1.02) | 0.0843 |
| Mesna | 0.50 (0.07–3.62) | 0.4949 | 0.55 (0.08–3.96) | 0.5563 |
| Eprazinone | 0.63 (0.41–0.98) | 0.0413 | 0.50 (0.33–0.78) | 0.0022 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, V.Y.-F.; Perng, D.-W.; Chou, T.-C.; Chou, Y.-C.; Chang, Y.-L.; Hsu, C.-C.; Chou, C.-L.; Lee, H.-C.; Chen, T.-J.; Hu, P.-W. Mucolytic Agents and Statins Use is Associated with a Lower Risk of Acute Exacerbations in Patients with Bronchiectasis-Chronic Obstructive Pulmonary Disease Overlap. J. Clin. Med. 2018, 7, 517. https://doi.org/10.3390/jcm7120517
Su VY-F, Perng D-W, Chou T-C, Chou Y-C, Chang Y-L, Hsu C-C, Chou C-L, Lee H-C, Chen T-J, Hu P-W. Mucolytic Agents and Statins Use is Associated with a Lower Risk of Acute Exacerbations in Patients with Bronchiectasis-Chronic Obstructive Pulmonary Disease Overlap. Journal of Clinical Medicine. 2018; 7(12):517. https://doi.org/10.3390/jcm7120517
Chicago/Turabian StyleSu, Vincent Yi-Fong, Diahn-Warng Perng, Ting-Chun Chou, Yueh-Ching Chou, Yuh-Lih Chang, Chia-Chen Hsu, Chia-Lin Chou, Hsin-Chen Lee, Tzeng-Ji Chen, and Po-Wei Hu. 2018. "Mucolytic Agents and Statins Use is Associated with a Lower Risk of Acute Exacerbations in Patients with Bronchiectasis-Chronic Obstructive Pulmonary Disease Overlap" Journal of Clinical Medicine 7, no. 12: 517. https://doi.org/10.3390/jcm7120517
APA StyleSu, V. Y.-F., Perng, D.-W., Chou, T.-C., Chou, Y.-C., Chang, Y.-L., Hsu, C.-C., Chou, C.-L., Lee, H.-C., Chen, T.-J., & Hu, P.-W. (2018). Mucolytic Agents and Statins Use is Associated with a Lower Risk of Acute Exacerbations in Patients with Bronchiectasis-Chronic Obstructive Pulmonary Disease Overlap. Journal of Clinical Medicine, 7(12), 517. https://doi.org/10.3390/jcm7120517







