Non-Hepatic Alkaline Phosphatase, hs-CRP and Progression of Vertebral Fracture in Patients with Rheumatoid Arthritis: A Population-Based Longitudinal Study
Abstract
1. Introduction
2. Methods
2.1. Cohort
2.2. Assessment of VFs
2.3. Outcomes and Follow Up
2.4. Variables
2.5. Statistical Methods
3. Results
3.1. Characteristics of the Study Population
3.2. NHALP
3.3. hs-CRP
3.4. NHALP and hs-CRP
4. Discussion
4.1. VF Progression and NHALP in RA
4.2. VF Progression and hs-CRP in RA
4.3. Interaction and Joint Effect of NHALP and hs-CRP on VF Progression
4.4. Limitation
5. Conclusions
Supplementary Materials
Data Availability
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El Maghraoui, A.; Rezqi, A.; Mounach, A.; Achemlal, L.; Bezza, A.; Ghozlani, I. Prevalence and risk factors of vertebral fractures in women with rheumatoid arthritis using vertebral fracture assessment. Rheumatology 2010, 49, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Peel, N.F.; Moore, D.J.; Barrington, N.A.; Bax, D.E.; Eastell, R. Risk of vertebral fracture and relationship to bone mineral density in steroid treated rheumatoid arthritis. Ann. Rheum. Dis. 1995, 54, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Van Staa, T.P.; Geusens, P.; Bijlsma, J.W.; Leufkens, H.G.; Cooper, C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006, 54, 3104–3112. [Google Scholar] [CrossRef] [PubMed]
- Spector, T.D.; Hall, G.M.; McCloskey, E.V.; Kanis, J.A. Risk of vertebral fracture in women with rheumatoid arthritis. BMJ 1993, 306, 558. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.J.; Wick, M.C.; Ackermann, P.W.; Montgomery, S.M. Increased fracture risk in patients with rheumatic disorders and other inflammatory diseases—A case-control study with 53,108 patients with fracture. J. Rheumatol. 2010, 37, 2247–2250. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.C.; Lisse, J.R.; Walitt, B.T.; Eaton, C.B.; Chen, Z. Arthritis increases the risk for fractures—Results from the women’s health initiative. J. Rheumatol. 2011, 38, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Inui, K.; Tada, M.; Sugioka, Y.; Mamoto, K.; Wakitani, S.; Koike, T.; Nakamura, H. High frequency of vertebral fracture and low bone quality in patients with rheumatoid arthritis—Results from tomorrow study. Mod. Rheumatol. 2017, 27, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, M.; Kolta, S.; Briot, K.; Fechtenbaum, J.; Paternotte, S.; Roux, C. Prevalence of vertebral fractures in patients with rheumatoid arthritis: Revisiting the role of glucocorticoids. Osteoporos. Int. 2012, 23, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Cheng, G.; Wang, H.; Feng, Y. Increased risk of vertebral fracture in patients with rheumatoid arthritis: A meta-analysis. Medicine 2016, 95, e5262. [Google Scholar] [CrossRef] [PubMed]
- Jalava, T.; Sarna, S.; Pylkkanen, L.; Mawer, B.; Kanis, J.A.; Selby, P.; Davies, M.; Adams, J.; Francis, R.M.; Robinson, J.; et al. Association between vertebral fracture and increased mortality in osteoporotic patients. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2003, 18, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Naves, M.; Diaz-Lopez, J.B.; Gomez, C.; Rodriguez-Rebollar, A.; Rodriguez-Garcia, M.; Cannata-Andia, J.B. The effect of vertebral fracture as a risk factor for osteoporotic fracture and mortality in a Spanish population. Osteoporos. Int. 2003, 14, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Torres, M.; Reyes-Garcia, R.; Garcia-Martin, A.; Jimenez-Moleon, J.J.; Gonzalez-Ramirez, A.R.; Lara-Villoslada, M.J.; Moreno, P.R. Ischemic heart disease is associated with vertebral fractures in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2013, 4, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Puisto, V.; Rissanen, H.; Heliovaara, M.; Impivaara, O.; Jalanko, T.; Kroger, H.; Knekt, P.; Aromaa, A.; Helenius, I. Vertebral fracture and cause-specific mortality: A prospective population study of 3210 men and 3730 women with 30 years of follow-up. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2011, 20, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Tasso, M.; Lupoli, R.; Di Minno, A.; Baldassarre, D.; Tremoli, E.; Di Minno, M.N. Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: A systematic review and meta-analysis of literature studies. Ann. Med. 2015, 47, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-S.; Chan, D.-C.; Chen, J.-F.; Cheng, T.-T.; Wu, C.-H.; Soong, Y.-K.; Tsai, K.-S.; Yang, R.-S. Clinical practice guidelines for the prevention and treatment of osteoporosis in Taiwan: Summary. J. Bone Miner. Metab. 2014, 32, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. Kdigo clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int. Suppl. 2009, S1–S130. [Google Scholar] [CrossRef]
- Shipman, K.E.; Holt, A.D.; Gama, R. Interpreting an isolated raised serum alkaline phosphatase level in an asymptomatic patient. BMJ 2013, 346, f976. [Google Scholar] [CrossRef] [PubMed]
- Colombet, I.; Pouchot, J.; Kronz, V.; Hanras, X.; Capron, L.; Durieux, P.; Wyplosz, B. Agreement between erythrocyte sedimentation rate and c-reactive protein in hospital practice. Am. J. Med. 2010, 123, 863.e7–863.e13. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.L.; Moverare-Skrtic, S.; Ljunggren, O.; Karlsson, M.; Mellstrom, D.; Ohlsson, C. High-sensitivity CRP is an independent risk factor for all fractures and vertebral fractures in elderly men: The MrOS Sweden study. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2014, 29, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Oei, L.; Rivadeneira, F.; Ly, F.; Breda, S.J.; Zillikens, M.C.; Hofman, A.; Uitterlinden, A.G.; Krestin, G.P.; Oei, E.H. Review of radiological scoring methods of osteoporotic vertebral fractures for clinical and research settings. Eur. Radiol. 2013, 23, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Kaden, J.J.; Dempfle, C.-E.; Grobholz, R.; Fischer, C.S.; Vocke, D.C.; Kılıç, R.; Sarıkoç, A.; Piñol, R.; Hagl, S.; Lang, S. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc. Pathol. 2005, 14, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.F.; Yeh, J.C.; Chiu, Y.L.; Liou, J.C.; Hsiung, J.R.; Tung, T.H. Periodontal pocket depth, hyperglycemia, and progression of chronic kidney disease: A population-based longitudinal study. Am. J. Med. 2017, 130, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.F.; Feng, Y.F.; Peng, Y.S.; Hsu, S.P.; Pai, M.F.; Chen, H.Y.; Wu, H.Y.; Yang, J.Y. Combined alkaline phosphatase and phosphorus levels as a predictor of mortality in maintenance hemodialysis patients. Medicine 2014, 93, e106. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.D.; Van de Langerijt, L.; Watts, N.B.; Eastell, R.; Genant, H.; Grauer, A.; Cahall, D.L. Underdiagnosis of vertebral fractures is a worldwide problem: The impact study. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2005, 20, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Saag, K.G.; Bridges, S.L., Jr.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016, 68, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Pasco, J.A.; Seeman, E.; Henry, M.J.; Merriman, E.N.; Nicholson, G.C.; Kotowicz, M.A. The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos. Int. 2006, 17, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.; Johnell, O.; Wedel, H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996, 312, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, J.; Iki, M.; Kadowaki, E.; Sato, Y.; Chiba, Y.; Akiba, T.; Matsumoto, T.; Nishino, H.; Kagamimori, S.; Kagawa, Y.; et al. Biochemical markers for bone turnover predict risk of vertebral fractures in postmenopausal women over 10 years: The Japanese population-based osteoporosis (JPOS) cohort study. Osteoporos. Int. 2013, 24, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Vasikaran, S.; Eastell, R.; Bruyere, O.; Foldes, A.J.; Garnero, P.; Griesmacher, A.; McClung, M.; Morris, H.A.; Silverman, S.; Trenti, T.; et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos. Int. 2011, 22, 391–420. [Google Scholar] [CrossRef] [PubMed]
- Paul Tuck, S.; Layfield, R.; Walker, J.; Mekkayil, B.; Francis, R. Adult paget’s disease of bone: A review. Rheumatology 2017, 56, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.L.; Toomey, L.; Kotowicz, M.A.; Henry, M.J.; Griffiths, H.; Pasco, J.A. Rheumatoid arthritis and incident fracture in women: A case—Control study. BMC Musculoskelet. Disord. 2014, 15, 13. [Google Scholar] [CrossRef] [PubMed]
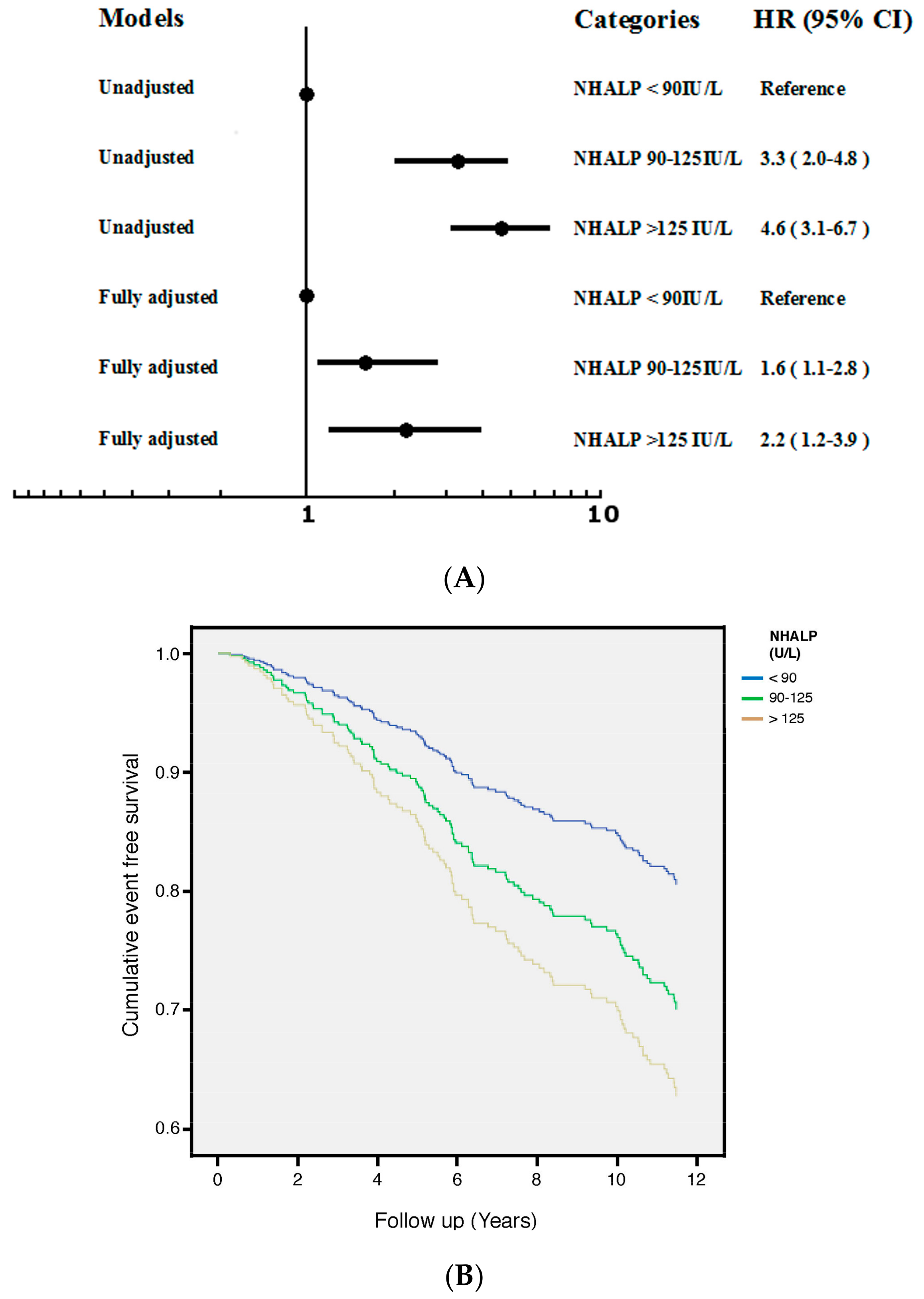
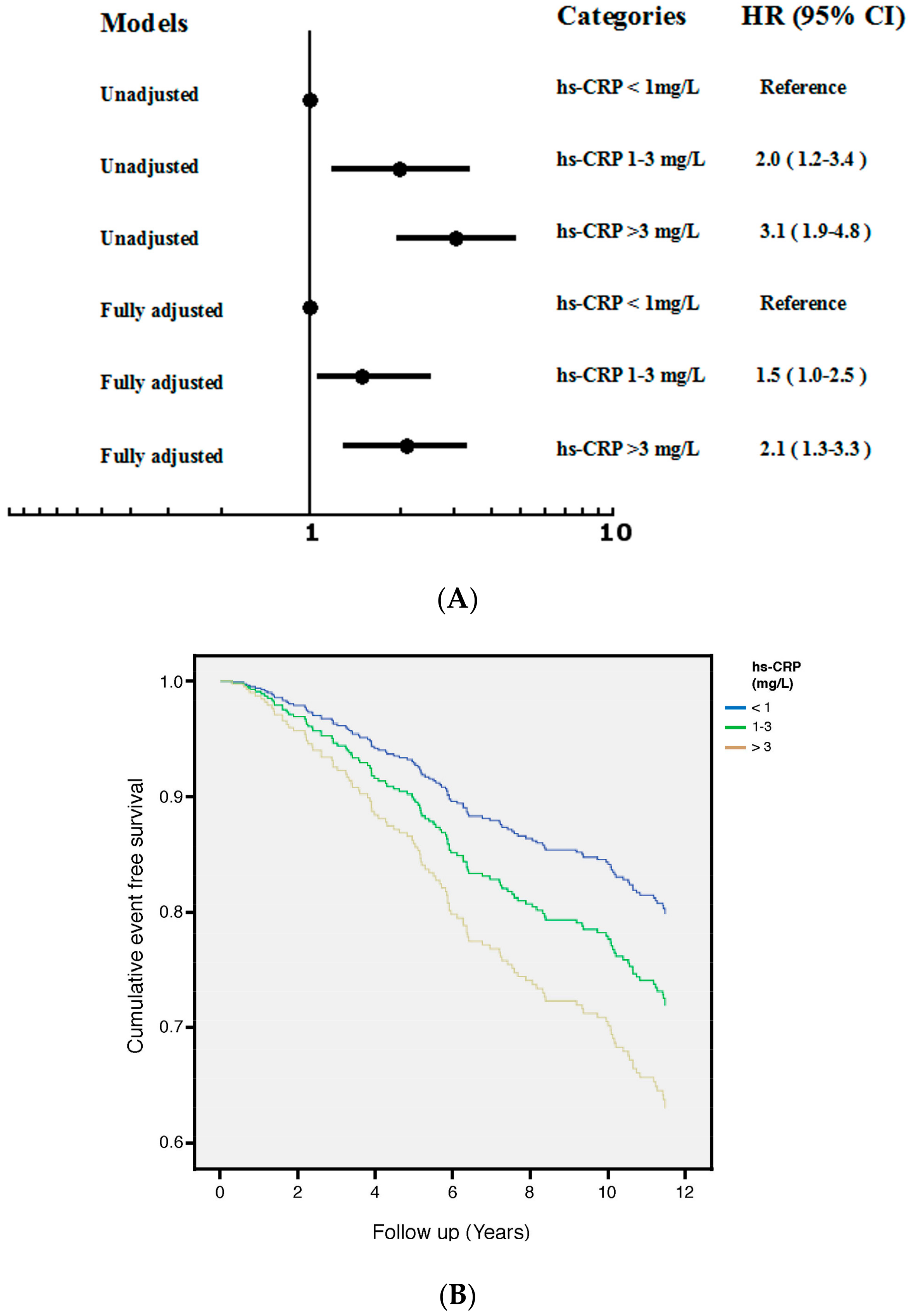

| Variable | Mean ± Standard Deviation or Median (Interquartile Range) |
|---|---|
| Age (years) | 57.5 ± 12.7 |
| Male (n, %) | 130 (25.2%) |
| Time (years) 4322 (45.4) | 8.7 (5.5–11.9) |
| Osteoporosis (n, %) | 123 (23.8%) |
| Hypertension (n, %) | 161 (31.2%) |
| Diabetes (n, %) | 119 (22.6%) |
| Hyperlipidemia (n, %) | 85 (16.5%) |
| Smoking (n, %) | 70 (13.6%) |
| Albumin (g/dL) | 4.1 ± 0.5 |
| ALT (IU/L) | 26.3 ± 5.7. |
| BUN (mg/dL) | 17.1 ± 13.4 |
| Cr (mg/dL) | 0.9 ± 0.8 |
| eGFR (mL/min) | 65.7 ± 41.0 |
| NHALP (IU/L) | 101.9 ± 70.3 |
| Potassium (mmol/L) | 4.0 ± 0.6 |
| Sodium (mmol/L) | 138.6 ± 4.2 |
| HbA1c (% of Hb) | 6.9 ± 2.3 |
| Fasting glucose (mg/dL) | 122.1 ± 76.5 |
| Total cholesterol (mg/dL) | 196 (168–220) |
| Triglyceride (mg/dL) | 97 (66–140) |
| hs-CRP (mg/L) | 1.5 (0.8–3.5) |
| Hb (g/dL) | 12.8 ± 1.9 |
| WBC (103/L) | 8.2 ± 3.3 |
| ESR (mm/h) | 43.7 ± 30.6 |
| RF (IU/mL) | 70.5 (18.3–236.0) |
| Variable | hs-CRP | NHALP |
|---|---|---|
| Age | 0.12 * | 0.20 * |
| Male | −0.21 * | −0.02 |
| Smoking history | 0.13 | −0.05 |
| Hypertension | 0.18 | 0.13 |
| Hyperlipidemia | 0.15 | 0.10 |
| Osteoporosis | 0.25 * | 0.22 * |
| hs-CRP | 1 | 0.26 * |
| NHALP | 0.26 * | 1 |
| Albumin | −0.23 * | −0.21 |
| BUN | −0.05 | 0.08 |
| Cr | 0.09 | 0.16 * |
| eGFR | −0.11 | −0.18 * |
| RF | 0.06 | 0.05 |
| ESR | 0.31 * | 0.11 |
| Potassium | 0.08 | 0.09 |
| Sodium | −0.01 | −0.15 |
| Uric acid | 0.19 | 0.19 * |
| ALT | 0.05 | 0.17 |
| Total cholesterol | 0.14 | 0.21 |
| Triglyceride | 0.19 | 0.23 |
| Hb | 0.12 | 0.08 |
| WBC | 0.16 * | 0.13 |
| Variable | Univariate HR for Vertebral Fracture (95% CI) |
|---|---|
| Age (years) | 1.016 (1.009–1.023) |
| Male | 0.705 (0.562–0.976) |
| Smoke history | 0.952 (0.872–1.031) |
| Hypertension | 1.651 (0.933–2.369) |
| Osteoporosis | 2.026 (1.538–3.689) |
| BUN (mg/dL) | 1.004 (0.989–1.020) |
| Cr (mg/dL) | 1.008 (1.000–1.017) |
| eGFR (mL/min/1.73m2) | 0.992 (0.980–1.003) |
| Sodium (mmol/L) | 0.931 (0.866–1.001) |
| Potassium (mmol/L) | 1.105 (0.698–1.748) |
| ALT (IU/L) | 1.006 (0.998–1.012) |
| NHALP (IU/L) | 1.005 (1.003–1.007) |
| HbA1c (%) | 1.022 (0.894–1.168) |
| Fasting glucose (mg/dL) | 1.000 (0.996–1.004) |
| Albumin (g/dL) | 0.748 (0.501–1.035) |
| Total cholesterol (mg/dL) | 0.998 (0.991–1.004) |
| Triglyceride (mg/dL) | 1.001 (0.999–1.002) |
| Uric acid (mg/dL) | 1.115 (0.990–1.256) |
| RF (IU/mL) | 1.000 (0.999–1.001) |
| ESR (mm/h) | 1.012 (1.005–1.019) |
| hs-CRP (mg/L) | 1.126 (1.050–1.220) |
| WBC count (1000 cells/uL) | 1.117 (0.997–1.238) |
| Hb (g/dL) | 0.993 (0.844–1.169) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, J.-C.; Wu, C.-C.; Choy, C.-S.; Chang, S.-W.; Liou, J.-C.; Chen, K.-S.; Tung, T.-H.; Lin, W.-N.; Hsieh, C.-Y.; Ho, C.-T.; et al. Non-Hepatic Alkaline Phosphatase, hs-CRP and Progression of Vertebral Fracture in Patients with Rheumatoid Arthritis: A Population-Based Longitudinal Study. J. Clin. Med. 2018, 7, 439. https://doi.org/10.3390/jcm7110439
Yeh J-C, Wu C-C, Choy C-S, Chang S-W, Liou J-C, Chen K-S, Tung T-H, Lin W-N, Hsieh C-Y, Ho C-T, et al. Non-Hepatic Alkaline Phosphatase, hs-CRP and Progression of Vertebral Fracture in Patients with Rheumatoid Arthritis: A Population-Based Longitudinal Study. Journal of Clinical Medicine. 2018; 7(11):439. https://doi.org/10.3390/jcm7110439
Chicago/Turabian StyleYeh, Jih-Chen, Chang-Chin Wu, Cheuk-Sing Choy, Shu-Wei Chang, Jian-Chiun Liou, Kuo-Shu Chen, Tao-Hsin Tung, Wei-Ning Lin, Chih-Yu Hsieh, Chun-Ta Ho, and et al. 2018. "Non-Hepatic Alkaline Phosphatase, hs-CRP and Progression of Vertebral Fracture in Patients with Rheumatoid Arthritis: A Population-Based Longitudinal Study" Journal of Clinical Medicine 7, no. 11: 439. https://doi.org/10.3390/jcm7110439
APA StyleYeh, J.-C., Wu, C.-C., Choy, C.-S., Chang, S.-W., Liou, J.-C., Chen, K.-S., Tung, T.-H., Lin, W.-N., Hsieh, C.-Y., Ho, C.-T., Wang, T.-M., & Chang, J.-F. (2018). Non-Hepatic Alkaline Phosphatase, hs-CRP and Progression of Vertebral Fracture in Patients with Rheumatoid Arthritis: A Population-Based Longitudinal Study. Journal of Clinical Medicine, 7(11), 439. https://doi.org/10.3390/jcm7110439






