Trends of Medication Usage and Associated Outcomes for Taiwanese Patients with Inflammatory Bowel Disease from 2001 to 2015
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analyses
3. Results
3.1. Patient Characteristics
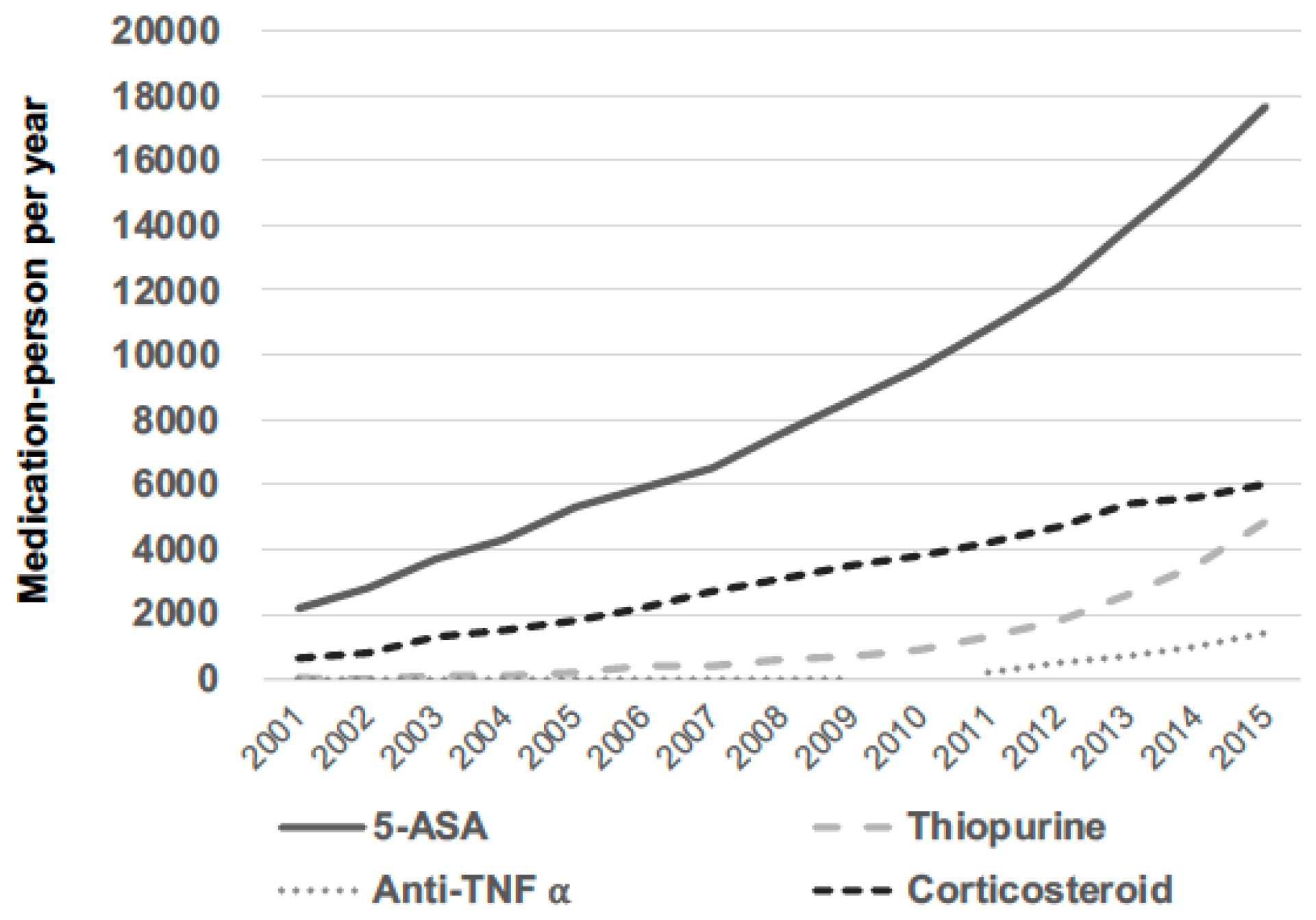
3.2. Medication Trends among Patients with IBD
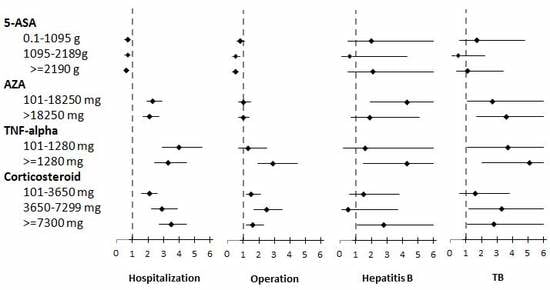
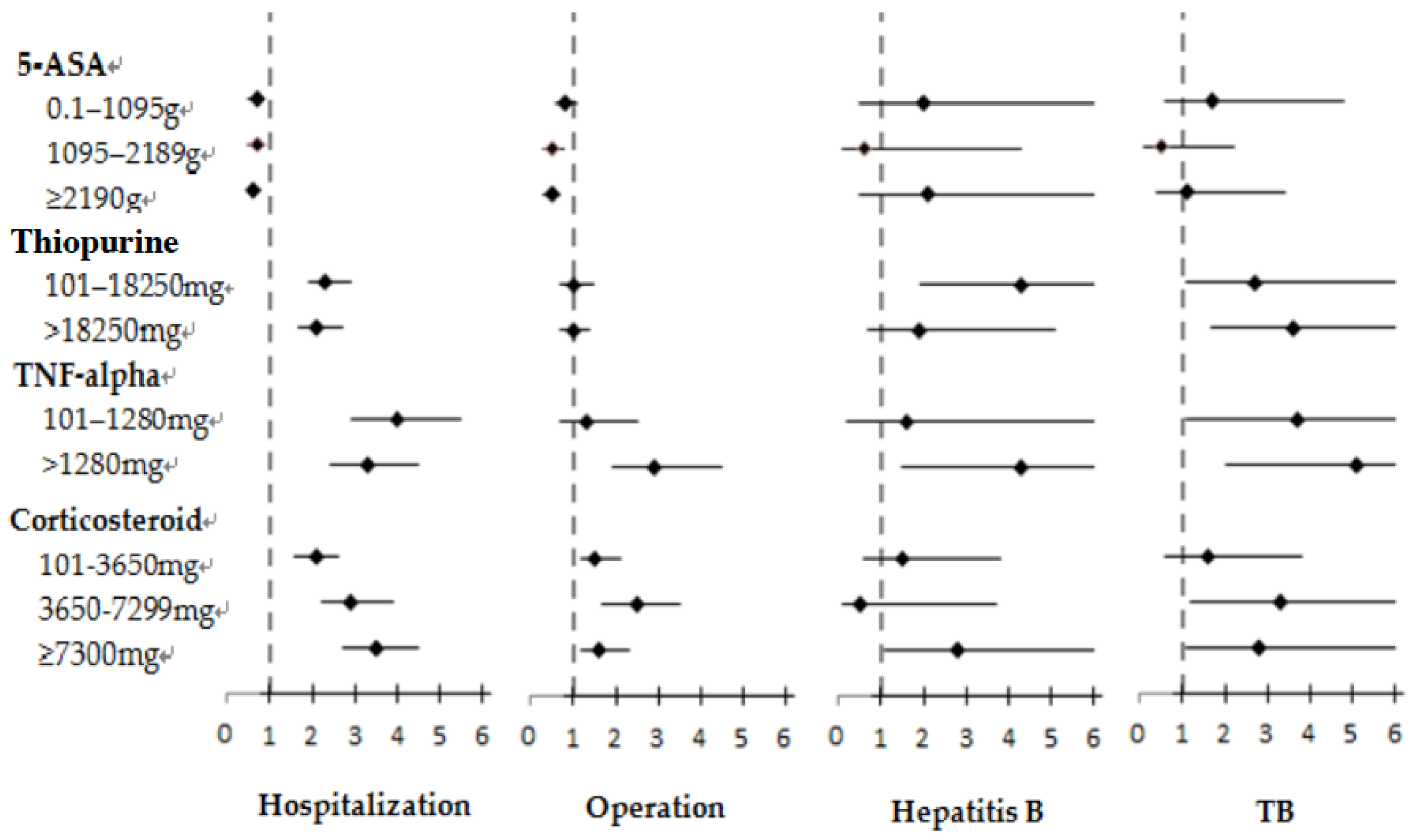
3.3. Risks of Hospitalization, Operation, and Infection Associated with 5-ASA Treatment
3.4. Risks of Hospitalization, Operation, and Infection Associated with Thiopurine Treatment
3.5. Risks of Hospitalization, Operation, and Infection Associated with Anti-TNF-α Treatment
3.6. Risks of Hospitalization, Operation, and Infection Associated with Corticosteroid Treatment
3.7. Risks of Tumor Incidence Associated with Various IBD Medications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Darfeuille-Michaud, A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Blanchard, J.F.; Rawsthorne, P.; Yu, N. The prevalence of extraintestinal diseases in inflammatory bowel disease: A population-based study. Am. J. Gastroenterol. 2001, 96, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Canavan, C.; Abrams, K.R.; Mayberry, J. Meta-analysis: Colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2006, 23, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, G.; Dignass, A.; Bokemeyer, B.; Danese, S.; Gionchetti, P.; Moser, G.; Beaugerie, L.; Gomollon, F.; Hauser, W.; Herrlinger, K.; et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: Special situations. J. Crohns Colitis 2013, 7, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Gomollon, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohns. Colitis. 2017, 11, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnar, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohns Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.; Van Assche, G.; Lindsay, J.O.; Lemann, M.; Soderholm, J.; Colombel, J.F.; Danese, S.; D’Hoore, A.; Gassull, M.; Gomollon, F.; et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J. Crohns Colitis 2010, 4, 28–62. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, K.; D’Rozario, J.; Pavli, P. Lymphoma and other lymphoproliferative disorders in inflammatory bowel disease: A review. J. Gastroenterol. Hepatol. 2013, 28, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, M.; Kirchgesner, J.; Rudnichi, A.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Association Between Use of Thiopurines or Tumor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients With Inflammatory Bowel Disease. JAMA 2017, 318, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Vuitton, L.; Peyrin-Biroulet, L. Biologic agents for IBD: Practical insights. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Mao, E.J.; Hazlewood, G.S.; Kaplan, G.G.; Peyrin-Biroulet, L.; Ananthakrishnan, A.N. Systematic review with meta-analysis: Comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 45, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Peyrin-Biroulet, L.; Sokol, H.; Aldeger, X.; Costa, A.; Higgins, P.D.; Joyce, J.C.; Katsanos, K.H.; Lopez, A.; de Xaxars, T.M.; et al. Extra-intestinal malignancies in inflammatory bowel disease: Results of the 3rd ECCO Pathogenesis Scientific Workshop (III). J. Crohns Colitis 2014, 8, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Thia, K.T.; Loftus, E.V., Jr.; Sandborn, W.J.; Yang, S.K. An update on the epidemiology of inflammatory bowel disease in Asia. Am. J. Gastroenterol. 2008, 103, 3167–3182. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Yun, S.; Kim, J.H.; Park, J.Y.; Kim, H.Y.; Kim, Y.H.; Chang, D.K.; Kim, J.S.; Song, I.S.; Park, J.B.; et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2005: A KASID study. Inflamm. Bowel. Dis. 2008, 14, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Asakura, K.; Nishiwaki, Y.; Inoue, N.; Hibi, T.; Watanabe, M.; Takebayashi, T. Prevalence of ulcerative colitis and Crohn’s disease in Japan. J Gastroenterol 2009, 44, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Chow, D.K.; Leong, R.W.; Tsoi, K.K.; Ng, S.S.; Leung, W.K.; Wu, J.C.; Wong, V.W.; Chan, F.K.; Sung, J.J. Long-term follow-up of ulcerative colitis in the Chinese population. Am. J. Gastroenterol. 2009, 104, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Shieh, M.J.; Chang, M.C.; Chang, Y.T.; Wang, C.Y.; Wong, J.M. Long-term follow-up of ulcerative colitis in Taiwan. J. Chin. Med. Assoc. 2012, 75, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Calip, G.S.; Patel, P.R.; Adimadhyam, S.; Xing, S.; Wu, Z.; Sweiss, K.; Schumock, G.T.; Lee, T.A.; Chiu, B.C. Tumor necrosis factor-alpha inhibitors and risk of non-Hodgkin lymphoma in a cohort of adults with rheumatologic conditions. Int. J. Cancer 2018, 10, 31407. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, L.; MacDonald, J.K. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2006, 2, CD000543. [Google Scholar] [CrossRef]
- Rousseaux, C.; El-Jamal, N.; Fumery, M.; Dubuquoy, C.; Romano, O.; Chatelain, D.; Langlois, A.; Bertin, B.; Buob, D.; Colombel, J.F.; et al. The 5-aminosalicylic acid antineoplastic effect in the intestine is mediated by PPARgamma. Carcinogenesis 2013, 34, 2580–2586. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Furusho, J.K.; Jacintez-Cazares, M.; Furuzawa-Carballeda, J.; Fonseca-Camarillo, G. Peroxisome proliferator-activated receptors family is involved in the response to treatment and mild clinical course in patients with ulcerative colitis. Dis. Markers 2014, 2014, 932530. [Google Scholar] [CrossRef] [PubMed]
- Dammann, K.; Khare, V.; Lang, M.; Claudel, T.; Harpain, F.; Granofszky, N.; Evstatiev, R.; Williams, J.M.; Pritchard, D.M.; Watson, A.; et al. PAK1 modulates a PPARgamma/NF-kappaB cascade in intestinal inflammation. Biochim. Biophys. Acta 2015, 1853, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Barbie, D.A.; Frank, D.A. Pharmacology of cancer: Genome synthesis, stability and maintenance. In Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy, 3rd ed; Golan D.E. Tashjian, A.H., Armstrong, E.J., Armstrong, A.W., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2012; pp. 2674–2693. [Google Scholar]
- Tiede, I.; Fritz, G.; Strand, S.; Poppe, D.; Dvorsky, R.; Strand, D.; Lehr, H.A.; Wirtz, S.; Becker, C.; Atreya, R.; et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J. Clin. Invest. 2003, 111, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Mok, M.Y.; Ng, W.L.; Yuen, M.F.; Wong, R.W.; Lau, C.S. Safety of disease modifying anti-rheumatic agents in rheumatoid arthritis patients with chronic viral hepatitis. Clin. Exp. Rheumatol. 2000, 18, 363–368. [Google Scholar] [PubMed]
- Manzano-Alonso, M.L.; Castellano-Tortajada, G. Reactivation of hepatitis B virus infection after cytotoxic chemotherapy or immunosuppressive therapy. World J. Gastroenterol. 2011, 17, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Guirao-Arrabal, E.; Santos, F.; Redel-Montero, J.; Vaquero, J.M.; Cantisan, S.; Vidal, E.; Torre-Gimenez, A.; Rivero, A.; Torre-Cisneros, J. Risk of tuberculosis after lung transplantation: The value of pretransplant chest computed tomography and the impact of mTOR inhibitors and azathioprine use. Transpl. Infect. Dis. 2016, 18, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, G.; Lewis, J.D.; Lichtenstein, G.R.; Loftus, E.V.; Ouyang, Q.; Panes, J.; Siegel, C.A.; Sandborn, W.J.; Travis, S.P.; Colombel, J.F. The London position statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organisation: Safety. Am. J. Gastroenterol. 2011, 106, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Alexakis, C.; Saxena, S.; Chhaya, V.; Cecil, E.; Curcin, V.; Pollok, R. Do Thiopurines Reduce the Risk of Surgery in Elderly Onset Inflammatory Bowel Disease? A 20-Year National Population-Based Cohort Study. Inflamm. Bowel. Dis. 2017, 23, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Lee, T.A.; Calip, G.S.; Suda, K.J.; Briars, L.; Schumock, G.T. Risk of Serious Bacterial Infection Associated With Tumor Necrosis Factor-Alpha Inhibitors in Children and Young Adults With Inflammatory Bowel Disease. Inflamm Bowel Dis 2018, 24, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.N.; Kim, H.J.; Kim, K.H.; Han, S.J.; Ahn, I.M.; Ahn, H.S. Risk of incident Mycobacterium tuberculosis infection in patients with inflammatory bowel disease: A nationwide population-based study in South Korea. Aliment. Pharmacol. Ther. 2017, 45, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.; Gershon, S.; Wise, R.P.; Mirabile-Levens, E.; Kasznica, J.; Schwieterman, W.D.; Siegel, J.N.; Braun, M.M. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 2001, 345, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fan, W.; Yang, G.; Xu, Z.; Wang, J.; Cheng, Q.; Yu, M. Risk of tuberculosis in patients treated with TNF-alpha antagonists: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2017, 7, e012567. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Spano, F.; Contatore, M.; Guastalla, A.; Penza, E.; Magnani, O.; Puppo, F. Infection risk associated with anti-TNF-alpha agents: A review. Expert. Opin. Drug Saf. 2015, 14, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Poetker, D.M.; Reh, D.D. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryngol. Clin. North Am. 2010, 43, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.H.; Sneller, M.C. Infectious complications of immunosuppressive therapy in patients with rheumatic diseases. Rheum. Dis. Clin. North Am. 1997, 23, 219–237. [Google Scholar] [CrossRef]
- Su, H.J.; Chiu, Y.T.; Chiu, C.T.; Lin, Y.C.; Wang, C.Y.; Hsieh, J.Y.; Wei, S.C. Inflammatory bowel disease and its treatment in 2018: Global and Taiwanese status updates. J. Formos. Med. Assoc. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, A.; Horn, J.; Mikolajczyk, R.T.; Krause, G.; Ott, J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet 2015, 386, 1546–1555. [Google Scholar] [CrossRef]
- Siroka, A. Global Tuberculosis Report 2017; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
| Variables | Crohn’s Disease, n = 919 | Ulcerative Colitis, n = 2887 |
|---|---|---|
| Age of diagnosis, year | ||
| Mean (sd) | 38.1 (18.5) | 45.0 (15.6) |
| median (Q1–Q3) | 35 (24–51) | 44 (34–55) |
| Age of diagnosis, n (%) | ||
| <20 | 128 (13.9) | 115 (4.0) |
| 20–39 | 415 (45.2) | 1003 (34.7) |
| 40–59 | 241 (26.2) | 1251 (43.3) |
| >60 | 135 (14.7) | 518 (17.9) |
| Male, n (%) | 631 (68.7) | 1787 (61.9) |
| Follow-up time, years | ||
| mean(sd) | 5.1 (4.2) | 6.7 (4.2) |
| median (Q1–Q3) | 3.9 (1.5–8.2) | 6.5 (3.0–10.2) |
| 5-aminosalicylic acid, n (%) | 754 (82.1) | 2636 (91.3) |
| Only | 78 | 659 |
| Others | 676 | 1977 |
| Thiopurine, n (%) | 424 (46.1) | 462 (16.0) |
| Only | 8 | 0 |
| Others | 416 | 462 |
| Anti-TNF a, n (%) | 249 (27.1) | 16 (0.6) |
| Only | 0 | 16 |
| Others | 249 | |
| Corticosteroid, n (%) | 726 (79.0) | 2105 (72.9) |
| Only | 0 | 0 |
| Others | 726 | 2105 |
| Outcome | 5–Aminosalicylic Acid (g) | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IBD | CD | UC | |||||||||||||||||||||||||
| <0.1 | 0.1–1095 | 1095–2189 | ≥2190 | <0.1 | 0.1–1095 | 1095–2189 | ≥2190 | <0.1 | 0.1–1095 | 1095–2189 | ≥2190 | ||||||||||||||||
| Follow person | 417 | 1715 | 636 | 1038 | 166 | 402 | 143 | 208 | 251 | 1313 | 493 | 830 | |||||||||||||||
| 4 causes of hospitalization‡ | |||||||||||||||||||||||||||
| IR | 413.4 | 256.2 | 235.4 | 208.3 | 514.7 | 626.8 | 501.7 | 475.2 | 368.5 | 193.0 | 176.0 | 154.4 | |||||||||||||||
| HR (95% CI) | 1.0 | 0.7 * | (0.5–0.8) | 0.7 * | (0.5–0.9) | 0.6 * | (0.5–0.8) | 1.0 | 1.1 | (0.8–1.6) | 1.1 | (0.7–1.6) | 1.1 | (0.8–1.7) | 1.0 | 0.6 * | (0.5–0.9) | 0.6 * | (0.4–0.9) | 0.6 * | (0.4–0.8) | ||||||
| Pneumonia | IR | 154.6 | 88.7 | 86.3 | 50.1 | 196.6 | 141.0 | 108.9 | 93.0 | 135.6 | 78.9 | 80.7 | 40.1 | ||||||||||||||
| HR | 1.0 | 0.7 | (0.5–1.0) | 0.8 | (0.5–1.2) | 0.5 * | (0.3–0.7) | 1.0 | 0.7 | (0.4–1.4) | 0.7 | (0.3–1.5) | 0.6 | (0.3–1.3) | 1.0 | 0.8 | (0.5–1.3) | 0.9 | (0.6–1.6) | 0.5 * | (0.3–0.8) | ||||||
| Urinary tract infection | IR | 160.9 | 86.8 | 83.8 | 67.0 | 178.5 | 160.0 | 141.2 | 112.2 | 152.6 | 73.2 | 69.7 | 56.6 | ||||||||||||||
| HR | 1.0 | 0.7 * | (0.5–0.9) | 0.7 | (0.5–1.2) | 0.6 * | (0.4–0.8) | 1.0 | 0.9 | (0.5–1.6) | 0.9 | (0.4–1.9) | 0.8 | (0.4–1.6) | 1.0 | 0.7 | (0.4–1.1) | 0.8 | (0.4–1.3) | 0.6 * | (0.4–0.9) | ||||||
| Sepsis | IR | 199.3 | 144.3 | 106.5 | 94.6 | 253.0 | 370.3 | 233.6 | 161.2 | 174.6 | 104.6 | 75.6 | 79.2 | ||||||||||||||
| HR | 1.0 | 0.8 | (0.6–1.1) | 0.6 * | (0.4–0.9) | 0.6 * | (0.4–0.8) | 1.0 | 1.3 | (0.8–2.1) | 1.0 | (0.5–1.8) | 0.8 | (0.4–1.4) | 1.0 | 0.8 | (0.5–1.2) | 0.6 | (0.4–1.0) | 0.6 * | (0.4–0.9) | ||||||
| Abdominal abscess | IR | 23.9 | 37.7 | 42.7 | 54.2 | 31.4 | 105.1 | 132.2 | 177.9 | 20.3 | 25.1 | 21.2 | 26.9 | ||||||||||||||
| HR | 1.0 | 1.5 | (0.7–3.4) | 1.7 | (0.7–3.9) | 2.4 * | (1.1–5.3) | 1.0 | 2.9 | (0.9–10.0) | 3.9 * | (1.1–13.9) | 6.4 * | (1.9–20.9) | 1.0 | 1.1 | (0.4–3.2) | 0.9 | (0.3–2.9) | 1.2 | (0.4–3.5) | ||||||
| 4 types of operation§ | |||||||||||||||||||||||||||
| IR | 189.2 | 159.6 | 88.0 | 65.8 | 222.2 | 366.3 | 136.7 | 146.7 | 172.6 | 121.9 | 76.4 | 48.1 | |||||||||||||||
| HR (95% CI) | 1.0 | 0.8 | (0.6–1.1) | 0.5 * | (0.3–0.8) | 0.5 * | (0.3–0.7) | 1.0 | 1.3 | (0.8–2.2) | 0.7 | (0.3–1.5) | 1.0 | (0.6–1.8) | 1.0 | 0.7 | (0.5–1.0) | 0.5 * | (0.3–0.8) | 0.4 * | (0.2–0.6) | ||||||
| Colectomy | IR | 10.1 | 5.4 | 8.4 | 1.9 | 10.2 | 17.3 | 20.9 | 0.0 | 10.0 | 3.2 | 5.3 | 2.4 | ||||||||||||||
| HR | 1.0 | 0.6 | (0.1–2.4) | 1.1 | (0.2–5.2) | 0.3 | (0.1–1.9) | 1.0 | 1.4 | (0.1–13.9) | 3.6 | (0.3–43.2) | – | 1.0 | 0.4 | (0.1–2.4) | 0.8 | (0.1–5.8) | 0.4 | (0.1–2.7) | |||||||
| Colostomy | IR | 105.9 | 105.7 | 45.2 | 33.5 | 127.1 | 260.7 | 53.5 | 73.3 | 95.3 | 77.3 | 43.1 | 24.3 | ||||||||||||||
| HR | 1.0 | 1.0 | (0.6–1.4) | 0.5 * | (0.3–0.8) | 0.4 * | (0.3–0.7) | 1.0 | 1.6 | (0.9–3.1) | 0.5 | (0.2–1.4) | 0.9 | (0.4–1.9) | 1.0 | 0.8 | (0.5–1.4) | 0.5 * | (0.2–0.9) | 0.3 * | (0.2–0.6) | ||||||
| Exploratory laparotomy | IR | 27.1 | 16.4 | 4.2 | 9.7 | 74.0 | 58.2 | 10.4 | 36.2 | 5.0 | 8.6 | 2.6 | 3.6 | ||||||||||||||
| HR | 1.0 | 0.6 | (0.2–1.3) | 0.2 * | (0.0–0.7) | 0.4 | (0.2–1.1) | 1.0 | 0.6 | (0.2–1.7) | 0.2 | (0.0–1.3) | 0.7 | (0.3–2.1) | 1.0 | 1.6 | (0.2–13.1) | 0.5 | (0.0–8.0) | 0.8 | (0.1–7.5) | ||||||
| Ileostomy | IR | 52.0 | 43.8 | 40.8 | 23.6 | 41.9 | 63.2 | 77.2 | 25.7 | 57.0 | 40.0 | 32.0 | 23.2 | ||||||||||||||
| HR | 1.0 | 0.8 | (0.5–1.5) | 0.8 | (0.4–1.7) | 0.6 | (0.3–1.1) | 1.0 | 1.2 | (0.4–3.8) | 1.9 | (0.6–6.7) | 1.0 | (0.3–3.6) | 1.0 | 0.7 | (0.3–1.4) | 0.6 | (0.3–1.3) | 0.5 | (0.2–1.0) | ||||||
| 2 types of disease¶ | |||||||||||||||||||||||||||
| Hepatitis B | IR | 6.7 | 14.6 | 4.2 | 13.6 | 0.0 | 40.3 | 10.5 | 5.0 | 10.0 | 9.7 | 2.6 | 15.6 | ||||||||||||||
| HR | 1.0 | 2.0 | (0.5–8.8) | 0.6 | (0.1–4.3) | 2.1 | (0.5–9.5) | – | – | . | – | – | 1.0 | 0.9 | (0.2–4.3) | 0.2 | (0.0–2.8) | 1.6 | (0.4–7.0) | ||||||||
| TB | IR | 13.5 | 21.9 | 6.3 | 12.6 | 31.3 | 76.4 | 20.9 | 35.5 | 5.0 | 11.9 | 2.6 | 7.2 | ||||||||||||||
| HR | 1.0 | 1.7 | (0.6–4.8) | 0.5 | (0.1–2.2) | 1.1 | (0.4–3.4) | 1.0 | 1.9 | (0.5–6.8) | 0.6 | (0.1–3.8) | 1.3 | (0.3–5.1) | 1.0 | 3.3 | (0.4–25.6) | 0.7 | (0.0–11.5) | 2.1 | (0.2–17.5) | ||||||
| Outcome | Thiopurine (mg) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IBD | CD | UC | ||||||||||||||||||||||
| <100 | 101–18,250 | >18,250 | <100 | 101–18,250 | 1,095,000–2,189,999 | <100 | 101–18,250 | >18,250 | ||||||||||||||||
| Follow person | 2928 | 437 | 441 | 498 | 177 | 244 | 2430 | 260 | 197 | |||||||||||||||
| 4 causes of hospitalization‡ | ||||||||||||||||||||||||
| IR | 210.9 | 496.6 | 378.1 | 518.4 | 796.0 | 457.9 | 166.4 | 384.4 | 302.8 | |||||||||||||||
| HR (95% CI) | 1.0 | 2.3 * | (1.9–2.9) | 2.1 * | (1.7–2.7) | 1.0 | 1.2 | (0.9–1.7) | 1.0 | (0.7–1.3) | 1.0 | 2.6 * | (2.0–3.5) | 2.3 * | (1.7–3.2) | |||||||||
| Pneumonia | IR | 80.5 | 95.2 | 73.5 | 152.6 | 113.3 | 82.9 | 68.9 | 87.8 | 64.1 | ||||||||||||||
| HR | 1.0 | 1.5 | (1.0–2.4) | 1.4 | (0.9–2.2) | 1.0 | 0.8 | (0.4–1.6) | 0.7 | (0.4–1.3) | 1.0 | 1.8 * | (1.0–3.0) | 1.3 | (0.7–2.6) | |||||||||
| Urinary tract infection | IR | 78.2 | 167.4 | 87.9 | 155.9 | 208.3 | 89.7 | 65.8 | 150.8 | 86.1 | ||||||||||||||
| HR | 1.0 | 2.5 * | (1.8–3.6) | 1.9 * | (1.2–2.8) | 1.0 | 1.1 | (0.6–2.1) | 0.8 | (0.4–1.4) | 1.0 | 3.1 * | (2.0–4.7) | 2.2 * | (1.2–3.9) | |||||||||
| Sepsis | IR | 109.4 | 233.2 | 167.9 | 256.8 | 392.6 | 182.0 | 86.2 | 169.4 | 153.8 | ||||||||||||||
| HR | 1.0 | 2.2 * | (1.6–3.0) | 1.9 * | (1.4–2.6) | 1.0 | 1.3 | (0.8–2.0) | 0.8 | (0.5–1.3) | 1.0 | 2.3 * | (1.6–3.5) | 2.3 * | (1.5–3.5) | |||||||||
| Abdominal abscess | IR | 28.2 | 116.4 | 98.3 | 84.3 | 243.2 | 145.4 | 19.1 | 66.2 | 52.0 | ||||||||||||||
| HR | 1.0 | 3.2 * | (2.0–5.0) | 2.6 * | (1.7–4.0) | 1.0 | 2.2 * | (1.2–4.2) | 1.5 | (0.8–2.6) | 1.0 | 3.0 * | (1.5–6.0) | 2.3 * | (1.1–5.0) | |||||||||
| 4 types of operation§ | ||||||||||||||||||||||||
| IR | 114.1 | 144.5 | 113.4 | 247.2 | 298.0 | 149.2 | 93.0 | 85.1 | 78.8 | |||||||||||||||
| HR (95% CI) | 1.0 | 1.0 | (0.7–1.5) | 1.0 | (0.7–1.4) | 1.0 | 0.8 | (0.5–1.3) | 0.7 | (0.4–1.1) | 1.0 | 0.8 | (0.5–1.5) | 0.9 | (0.5–1.6) | |||||||||
| Colectomy | IR | 5.6 | 4.0 | 3.1 | 15.0 | 0.0 | 6.2 | 4.0 | 5.7 | 0.0 | ||||||||||||||
| HR | 1.0 | 0.6 | (0.1–4.3) | 0.7 | (0.1–5.3) | 1.0 | – | 0.6 | (0.1–5.2) | 1.0 | 1.2 | (0.1–9.3) | – | |||||||||||
| Colostomy | IR | 68.3 | 88.9 | 64.1 | 145.8 | 206.4 | 83.1 | 55.7 | 41.6 | 45.0 | ||||||||||||||
| HR | 1.0 | 1.1 | (0.7–1.7) | 1.0 | (0.6–1.6) | 1.0 | 1.0 | (0.5–1.8) | 0.6 | (0.3–1.2) | 1.0 | 0.7 | (0.3–1.5) | 0.9 | (0.4–1.9) | |||||||||
| Exploratory laparotomy | IR | 12.4 | 16.3 | 15.8 | 51.8 | 42.7 | 31.8 | 6.0 | 5.7 | 0.0 | ||||||||||||||
| HR | 1.0 | 0.9 | (0.3–2.6) | 1.0 | (0.4–2.7) | 1.0 | 0.5 | (0.1–1.7) | 0.6 | (0.2–1.6) | 1.0 | 0.8 | (0.1–6.6) | – | ||||||||||
| Ileostomy | IR | 38.0 | 37.2 | 28.6 | 61.6 | 56.7 | 18.8 | 34.1 | 29.2 | 38.6 | ||||||||||||||
| HR | 1.0 | 0.8 | (0.4–1.6) | 0.8 | (0.4–1.5) | 1.0 | 0.6 | (0.2–1.7) | 0.3 | (0.1–1.1) | 1.0 | 0.8 | (0.3–2.0) | 1.2 | (0.5–2.7) | |||||||||
| 2 types of disease¶ | ||||||||||||||||||||||||
| Hepatitis B | IR | 8.5 | 36.9 | 15.6 | 9.0 | 70.4 | 6.2 | 8.5 | 23.1 | 25.3 | ||||||||||||||
| HR | 1.0 | 4.3 * | (1.9–9.7) | 1.9 | (0.7–5.1) | 1.0 | 9.7 * | (2.1–44.1) | 0.8 | (0.1–8.0) | 1.0 | 2.5 | (0.8–7.6) | 2.8 | (0.9–8.4) | |||||||||
| TB | IR | 11.1 | 28.4 | 34.7 | 42.7 | 41.6 | 50.3 | 6.0 | 23.0 | 19.0 | ||||||||||||||
| HR | 1.0 | 2.7 * | (1.1–6.2) | 3.6 * | (1.7–7.3) | 1.0 | 0.8 | (0.2–2.7) | 1.3 | (0.5–3.1) | 1.0 | 4.8 * | (1.5–15.2) | 4.1 * | (1.1–14.7) | |||||||||
| Outcome | Anti-TNF-α Agent (mg) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IBD | CD | UC | |||||||||||||||||||||||
| <100 | 101–1280 | >1280 | <100 | 101–1280 | >1280 | <100 | 101–1280 | 1280 | |||||||||||||||||
| Follow person | 3545 | 134 | 127 | 673 | 125 | 121 | 2872 | 9 | 6 | ||||||||||||||||
| 4 causes of hospitalization‡ | |||||||||||||||||||||||||
| IR | 228.1 | 1024 | 565.7 | 479.8 | 970.0 | 566.4 | 187.6 | 1782 | 551.3 | ||||||||||||||||
| HR (95% CI) | 1.0 | 4.0 * | (2.9– | 5.5) | 3.3 * | (2.4– | 4.5) | 1.0 | 1.6 * | (1.2– | 2.4) | 1.4 | (1.0– | 2.0) | 1.0 | 5.9 * | (2.4– | 14.4) | 4.9 * | (1.2– | 19.8) | ||||
| Pneumonia | IR | 80.6 | 147.2 | 52.2 | 142.2 | 139.4 | 55.0 | 69.9 | 242.0 | 0.0 | |||||||||||||||
| HR | 1.0 | 2.2 * | (1.1– | 4.5) | 1.3 | (0.5– | 3.1) | 1.0 | 1.0 | (0.4– | 2.2) | 0.5 | (0.2– | 1.3) | 1.0 | 2.2 | (0.3– | 16.3) | |||||||
| Urinary tract infection | IR | 83.6 | 182.4 | 119.9 | 150.2 | 159.8 | 102.1 | 71.8 | 419.6 | 551.3 | |||||||||||||||
| HR | 1.0 | 2.4 * | (1.3– | 4.5) | 3.2 * | (1.7– | 6.0) | 1.0 | 0.8 | (0.4– | 1.8) | 1.1 | (0.5– | 2.3) | 1.0 | 6.0 * | (1.5– | 24.6) | 28.6 * | (6.8– | 119.4) | ||||
| Sepsis | IR | 117.0 | 486.3 | 188.7 | 239.7 | 468.9 | 199.4 | 95.8 | 657.4 | 0.0 | |||||||||||||||
| HR | 1.0 | 4.0 * | (2.6– | 6.1) | 2.3 * | (1.4– | 3.9) | 1.0 | 1.7 * | (1.0– | 2.7) | 1.1 | (0.6– | 1.8) | 1.0 | 5.1 * | (1.6– | 16.2) | |||||||
| Abdominal abscess | IR | 31.7 | 302.1 | 241.4 | 78.2 | 282.5 | 243.2 | 23.5 | 551.1 | 210.3 | |||||||||||||||
| HR | 1.0 | 6.0 * | (3.4– | 10.5) | 5.4 * | (3.3– | 8.8) | 1.0 | 2.9 * | (1.5– | 5.6) | 2.9 * | (1.7– | 5.1) | 1.0 | 15.1 * | (3.6– | 63.8) | 5.8 | (0.8– | 42.5) | ||||
| 4 types of operation§ | |||||||||||||||||||||||||
| IR | 108.6 | 210.4 | 308.7 | 210.0 | 233.1 | 295.8 | 90.9 | 0.0 | 619.7 | ||||||||||||||||
| HR (95% CI) | 1.0 | 1.3 | (0.7– | 2.5) | 2.9 * | (1.9– | 4.5) | 1.0 | 0.8 | (0.4– | 1.6) | 1.8 * | (1.1– | 2.8) | 1.0 | 0.0 | 6.5 * | (1.6– | 26.5) | ||||||
| Colectomy | IR | 5.1 | 17.9 | 0.0 | 11.8 | 19.7 | 0.0 | 3.9 | 0.0 | 0.0 | |||||||||||||||
| HR | 1.0 | 2.5 | (0.3– | 19.6) | – | 1.0 | 1.6 | (0.2– | 14.1) | – | 1.0 | – | – | ||||||||||||
| Colostomy | IR | 63.9 | 108.5 | 218.5 | 123.2 | 119.6 | 203.1 | 53.2 | 0.0 | 619.7 | |||||||||||||||
| HR | 1.0 | 1.3 | (0.6– | 2.9) | 3.9 * | (2.4– | 6.4) | 1.0 | 0.8 | (0.3– | 1.8) | 2.1 * | (1.2– | 3.7) | 1.0 | – | 12.4 * | (3.0– | 51.3) | ||||||
| Exploratory laparotomy | IR | 12.4 | 55.8 | 10.3 | 50.6 | 61.7 | 10.8 | 5.6 | 0.0 | 0.0 | |||||||||||||||
| HR | 1.0 | 2.3 | (0.7– | 7.6) | 0.7 | (0.1– | 5.0) | 1.0 | 0.8 | (0.2– | 2.7) | 0.2 | (0.0– | 1.9) | 1.0 | – | – | ||||||||
| Ileostomy | IR | 36.0 | 35.9 | 65.4 | 45.5 | 39.5 | 69.0 | 34.2 | 0.0 | 0.0 | |||||||||||||||
| HR | 1.0 | 0.7 | (0.2– | 2.7) | 1.9 | (0.8– | 4.4) | 1.0 | 0.6 | (0.1– | 2.6) | 1.9 | (0.8– | 5.0) | 1.0 | – | – | ||||||||
| 2 types of disease¶ | |||||||||||||||||||||||||
| Hepatitis B | IR | 10.5 | 17.8 | 41.5 | 9.4 | 19.6 | 43.7 | 10.7 | 0.0 | 0.0 | |||||||||||||||
| HR | 1.0 | 1.6 | (0.2– | 11.9) | 4.3 * | (1.5– | 12.7) | 1.0 | 2.6 | (0.3– | 25.3) | 8.3 * | (1.8– | 38.9) | 1.0 | – | – | ||||||||
| TB | IR | 13.1 | 54.0 | 52.7 | 40.6 | 59.5 | 55.6 | 8.2 | 0.0 | 0.0 | |||||||||||||||
| HR | 1.0 | 3.7 * | (1.1– | 12.0) | 5.1 * | (2.0– | 13.6) | 1.0 | 1.1 | (0.3– | 3.9) | 1.6 | (0.6– | 4.3) | 1.0 | – | – | ||||||||
| Outcome | Corticosteroid (mg) | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IBD | CD | UC | ||||||||||||||||||||||||||||||
| <100 | 101–3650 | 3650–7299 | ≥7300 | <100 | 101–3650 | 3650–7299 | ≥7300 | <100 | 101–3650 | 3650–7299 | ≥7300 | |||||||||||||||||||||
| Follow person | 1257 | 1571 | 357 | 621 | 246 | 389 | 97 | 187 | 1011 | 1182 | 260 | 434 | ||||||||||||||||||||
| 4 causes of hospitalization‡ | ||||||||||||||||||||||||||||||||
| IR | 120.3 | 261.8 | 355.8 | 383.6 | 383.1 | 577.2 | 800.7 | 511.3 | 82.3 | 201.1 | 257.5 | 334.7 | ||||||||||||||||||||
| HR (95% CI) | 1.0 | 2.1 * | (1.6– | 2.6) | 2.9 * | (2.2– | 3.9) | 3.5 * | (2.7– | 4.5) | 1.0 | 1.4 * | (1.0– | 2.0) | 2.2 * | (1.4– | 3.4) | 1.7 * | (1.1– | 2.5) | 1.0 | 2.3 * | (1.7– | 3.1) | 2.9 * | (2.0– | 4.2) | 4.2 * | (3.1– | 5.7) | ||
| Pneumonia | IR | 31.0 | 93.3 | 103.3 | 117.8 | 61.2 | 155.7 | 139.5 | 134.4 | 26.3 | 80.2 | 93.6 | 111.0 | |||||||||||||||||||
| HR | 1.0 | 2.7 * | (1.8– | 4.2) | 3.0 * | (1.8– | 5.2) | 4.2 * | (2.7– | 6.6) | 1.0 | 2.3 * | (1.0– | 5.3) | 2.7 | (1.0– | 7.4) | 3.1 * | (1.3– | 7.3) | 1.0 | 2.7 * | (1.6– | 4.4) | 2.8 * | (1.5– | 5.3) | 4.1 * | (2.4– | 7.0) | ||
| Urinary tract infection | IR | 43.1 | 97.3 | 150.3 | 100.0 | 107.6 | 154.6 | 266.4 | 115.6 | 33.2 | 85.0 | 120.8 | 93.4 | |||||||||||||||||||
| HR | 1.0 | 2.1 * | (1.4– | 3.0) | 3.6 * | (2.3– | 5.7) | 2.8 * | (1.9– | 4.3) | 1.0 | 1.2 | (0.6– | 2.3) | 2.6 * | (1.2– | 5.6) | 1.4 | (0.7– | 2.8) | 1.0 | 2.3 * | (1.5– | 3.6) | 3.4 * | (1.9– | 6.0) | 3.1 * | (1.9– | 5.2) | ||
| Sepsis | IR | 56.5 | 130.9 | 184.3 | 190.8 | 180.3 | 288.7 | 321.6 | 237.1 | 37.4 | 98.5 | 149.3 | 171.9 | |||||||||||||||||||
| HR | 1.0 | 2.2 * | (1.6– | 3.1) | 3.3 * | (2.2– | 4.9) | 3.7 * | (2.7– | 5.3) | 1.0 | 1.5 | (0.9– | 2.5) | 2.0 * | (1.1– | 3.9) | 1.8 * | (1.0– | 3.1) | 1.0 | 2.4 * | (1.6– | 3.7) | 3.7 * | (2.2– | 6.2) | 4.6 * | (2.9– | 7.2) | ||
| Abdominal abscess | IR | 22.7 | 33.4 | 67.9 | 79.4 | 99.1 | 101.8 | 253.0 | 118.4 | 11.0 | 18.8 | 23.4 | 63.3 | |||||||||||||||||||
| HR | 1.0 | 1.6 | (0.9– | 2.7) | 2.8 * | (1.5– | 5.4) | 3.4 * | (2.0– | 5.9) | 1.0 | 1.1 | (0.5– | 2.2) | 2.5 * | (1.1– | 5.7) | 1.4 | (0.7– | 3.0) | 1.0 | 1.8 | (0.8– | 4.3) | 2.1 | (0.7– | 6.3) | 5.7 * | (2.6– | 12.7) | ||
| 4 types of operation§ | ||||||||||||||||||||||||||||||||
| IR | 85.3 | 125.4 | 203.6 | 107.1 | 269.4 | 234.5 | 249.8 | 176.0 | 57.1 | 102.3 | 190.9 | 80.3 | ||||||||||||||||||||
| HR (95% CI) | 1.0 | 1.5 * | (1.2– | 2.1) | 2.5 * | (1.7– | 3.5) | 1.6 * | (1.2– | 2.3) | 1.0 | 0.9 | (0.6– | 1.5) | 1.1 | (0.6– | 2.1) | 1.2 | (0.7– | 2.0) | 1.0 | 1.9 * | (1.3– | 2.8) | 3.4 * | (2.1– | 5.3) | 1.7 * | (1.1– | 2.8) | ||
| Colectomy | IR | 10.7 | 3.4 | 3.6 | 1.6 | 35.0 | 4.7 | 0.0 | 5.5 | 6.9 | 3.1 | 4.6 | 0.0 | |||||||||||||||||||
| HR | 1.0 | 0.3 | (0.1– | 1.0) | 0.4 | (0.0– | 3.0) | 0.2 | (0.0– | 1.7) | 1.0 | 0.2 | (0.0– | 1.4) | – | 0.4 | (0.0– | 4.0) | 1.0 | 0.4 | (0.1– | 1.9) | 0.8 | (0.1– | 6.6) | – | ||||||
| Colostomy | IR | 53.3 | 75.9 | 105.1 | 64.6 | 167.6 | 138.6 | 167.3 | 99.6 | 35.1 | 62.3 | 88.6 | 50.3 | |||||||||||||||||||
| HR | 1.0 | 1.5 * | (1.1– | 2.2) | 2.1 * | (1.3– | 3.4) | 1.6 * | (1.0– | 2.5) | 1.0 | 0.9 | (0.5– | 1.6) | 1.2 | (0.5– | 2.6) | 1.1 | (0.5– | 2.1) | 1.0 | 1.9 * | (1.2– | 3.0) | 2.6 * | (1.4– | 4.8) | 1.8 * | (1.0– | 3.2) | ||
| Exploratory laparotomy | IR | 10.7 | 11.9 | 25.7 | 13.3 | 34.9 | 47.6 | 70.8 | 40.1 | 6.9 | 4.1 | 13.9 | 2.3 | |||||||||||||||||||
| HR | 1.0 | 1.2 | (0.5– | 2.7) | 2.4 | (0.9– | 6.5) | 1.4 | (0.6– | 3.8) | 1.0 | 1.4 | (0.4– | 4.6) | 2.2 | (0.5– | 8.9) | 1.9 | (0.5– | 6.5) | 1.0 | 0.6 | (0.2– | 2.4) | 1.9 | (0.5– | 8.1) | 0.4 | (0.0– | 3.1) | ||
| Ileostomy | IR | 18.0 | 46.8 | 65.7 | 31.8 | 35.3 | 87.3 | 0.0 | 28.2 | 15.2 | 38.0 | 84.8 | 33.3 | |||||||||||||||||||
| HR | 1.0 | 2.8 * | (1.6– | 4.9) | 3.9 * | (1.9– | 7.8) | 2.3 * | (1.2– | 4.6) | 1.0 | 2.7 | (0.9– | 8.0) | – | 1.4 | (0.4– | 5.3) | 1.0 | 2.7 * | (1.4– | 5.3) | 5.7 * | (2.7– | 12.3) | 2.7 * | (1.2– | 5.9) | ||||
| 2 types of disease¶ | ||||||||||||||||||||||||||||||||
| Hepatitis B | IR | 8.3 | 11.8 | 3.7 | 19.8 | 17.3 | 18.8 | 17.4 | 11.0 | 6.9 | 10.3 | 0.0 | 23.5 | |||||||||||||||||||
| HR | 1.0 | 1.5 | (0.6– | 3.8) | 0.5 | (0.1– | 3.7) | 2.8 * | (1.1– | 7.2) | 1.0 | 1.5 | (0.3– | 8.4) | 1.5 | (0.1– | 16.7) | 1.1 | (0.2– | 8.4) | 1.0 | 1.6 | (0.5– | 4.7) | – | 3.6 * | (1.2– | 10.5) | ||||
| TB | IR | 8.3 | 13.6 | 29.3 | 21.5 | 34.6 | 53.1 | 69.6 | 33.6 | 4.1 | 5.2 | 18.6 | 16.5 | |||||||||||||||||||
| HR | 1.0 | 1.6 | (0.6– | 3.8) | 3.3 * | (1.2– | 9.2) | 2.8 * | (1.1– | 7.1) | 1.0 | 1.5 | (0.5– | 4.7) | 2.5 | (0.6– | 10.2) | 1.4 | (0.4– | 5.0) | 1.0 | 1.1 | (0.3– | 4.6) | 3.6 | (0.8– | 16.3) | 3.7 | (0.9– | 14.4) | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, M.-T.; Tung, C.-C.; Chang, Y.-T.; Leong, Y.-L.; Wang, Y.-T.; Wong, J.-M.; Wei, S.-C. Trends of Medication Usage and Associated Outcomes for Taiwanese Patients with Inflammatory Bowel Disease from 2001 to 2015. J. Clin. Med. 2018, 7, 394. https://doi.org/10.3390/jcm7110394
Weng M-T, Tung C-C, Chang Y-T, Leong Y-L, Wang Y-T, Wong J-M, Wei S-C. Trends of Medication Usage and Associated Outcomes for Taiwanese Patients with Inflammatory Bowel Disease from 2001 to 2015. Journal of Clinical Medicine. 2018; 7(11):394. https://doi.org/10.3390/jcm7110394
Chicago/Turabian StyleWeng, Meng-Tzu, Chien-Chih Tung, Yuan-Ting Chang, Yew-Loong Leong, Yu-Ting Wang, Jau-Min Wong, and Shu-Chen Wei. 2018. "Trends of Medication Usage and Associated Outcomes for Taiwanese Patients with Inflammatory Bowel Disease from 2001 to 2015" Journal of Clinical Medicine 7, no. 11: 394. https://doi.org/10.3390/jcm7110394
APA StyleWeng, M.-T., Tung, C.-C., Chang, Y.-T., Leong, Y.-L., Wang, Y.-T., Wong, J.-M., & Wei, S.-C. (2018). Trends of Medication Usage and Associated Outcomes for Taiwanese Patients with Inflammatory Bowel Disease from 2001 to 2015. Journal of Clinical Medicine, 7(11), 394. https://doi.org/10.3390/jcm7110394