Low Blood ALT Activity and High FRAIL Questionnaire Scores Correlate with Increased Mortality and with Each Other. A Prospective Study in the Internal Medicine Department
Abstract
1. Introduction
2. Methods
Statistical Methods
3. Results
3.1. Patients’ Characteristics
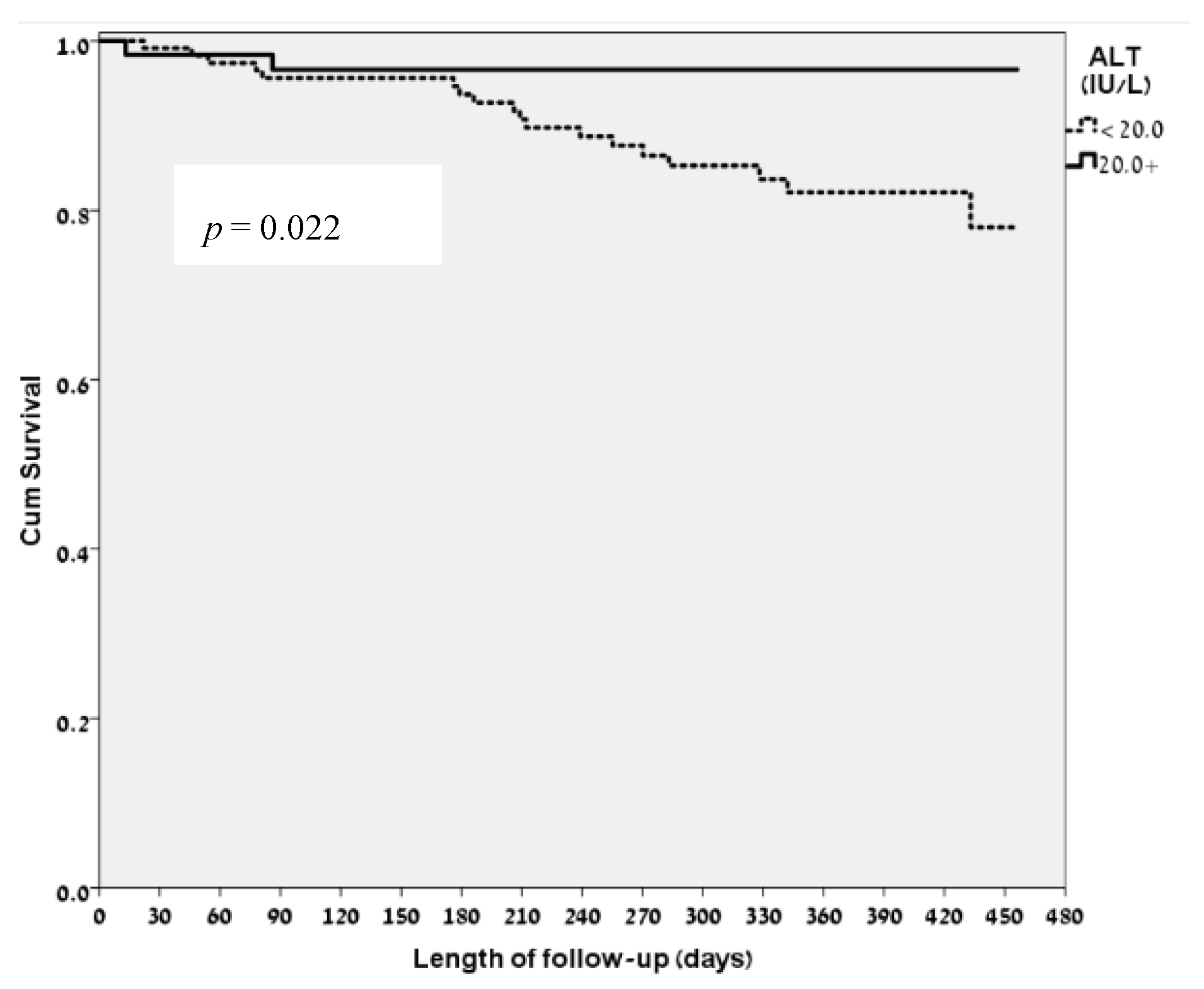
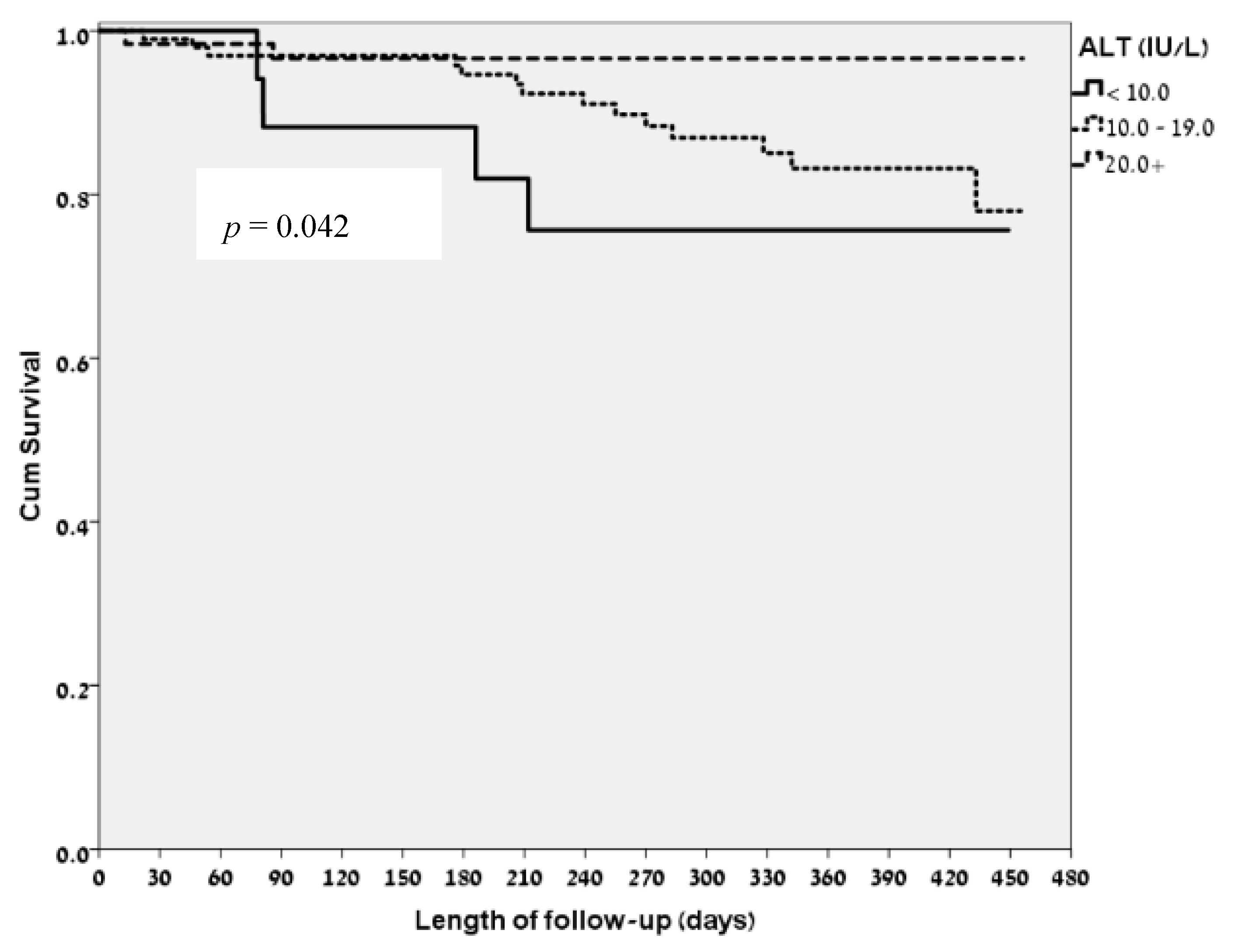
3.2. ALT Levels and Clinical Correlates
3.3. FRAIL Questionnaire Classifications and Clinical Correlates
3.4. Correlation of Low ALT Levels and the FRAIL Questionnaire Ggroups
4. Discussion
4.1. The FRAIL Questionnaire, Validation and Applications
4.2. Low ALT, Sarcopenia, Frailty and Survival
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018. [Google Scholar] [CrossRef] [PubMed]
- Mudge, A.M.; Hubbard, R.E. Frailty: Mind the gap. Age Ageing 2017, 47, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Uchmanowicz, I.; Chudiak, A.; Jankowska-Polańska, B.; Gobbens, R. Hypertension and Frailty Syndrome in Old Age: Current Perspectives. Card. Fail. Rev. 2017, 3, 102. [Google Scholar] [CrossRef]
- Abdel-Kader, K.; Girard, T.D.; Brummel, N.E.; Saunders, C.T.; Blume, J.D.; Clark, A.J.; Vincz, A.J.; Ely, E.W.; Jackson, J.C.; Bell, S.P.; et al. Acute Kidney Injury and Subsequent Frailty Status in Survivors of Critical Illness. Crit. Care Med. 2018, 46, e380–e388. [Google Scholar] [CrossRef] [PubMed]
- Abel, G.A.; Klepin, H.D. Frailty and the management of hematologic malignancies. Blood 2018, 131, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Abellan van Kan, G.; Rolland, Y.; Bergman, H.; Morley, J.E.; Kritchevsky, S.B.; Vellas, B. The I.A.N.A. Task Force on frailty assessment of older people in clinical practice. J. Nutr. Health Aging 2008, 12, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Abellan van Kan, G.; Rolland, Y.M.; Morley, J.E.; Vellas, B. Frailty: Toward a clinical definition. J. Am. Med. Dir. Assoc. 2008, 9, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Hyde, Z.; Flicker, L.; Almeida, O.P.; Hankey, G.J.; McCaul, K.A.; Chubb, S.A.P.; Yeap, B.B. Low free testosterone predicts frailty in older men: The health in men study. J. Clin. Endocrinol. Metab. 2010, 95, 3165–3172. [Google Scholar] [CrossRef] [PubMed]
- Tarrant, J.; Meyer, D.; Katavolos, P. Use of optimized aminotransferase methods in regulated preclinical studies. Vet. Clin. Pathol. 2013, 42, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; Blyth, F.M.; Creasey, H.M.; Handelsman, D.J.; Naganathan, V.; Sambrook, P.N.; Seibel, M.J.; Waite, L.M.; Cumming, R.G. The Association of Alanine Transaminase with Aging, Frailty, and Mortality. J. Gerontol. Ser. A 2010, 65A, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Ramaty, E.; Maor, E.; Peltz-Sinvani, N.; Brom, A.; Grinfeld, A.; Kivity, S.; Segev, S.; Sidi, Y.; Kessler, T.; Sela, B.A.; et al. Low ALT blood levels predict long-term all-cause mortality among adults. A historical prospective cohort study. Eur. J. Intern. Med. 2014, 25, 919–921. [Google Scholar] [CrossRef] [PubMed]
- Ramati, E.; Israel, A.; Tal Kessler Petz-Sinuani, N.; Sela, B.A.; Goren, I.; Grinfeld, A.; Lavi, B.; Segal, G. Low ALT activity amongst patients hospitalized in internal medicine wards is a widespread phenomenon associated with low vitamin B6 levels in their blood. Eur. PMC 2015, 154, 89–93. [Google Scholar]
- Peltz-Sinvani, N.; Klempfner, R.; Ramaty, E.; Sela, B.A.; Goldenberg, I.; Segal, G. Low ALT levels independently associated with 22-year all-cause mortality among coronary heart disease patients. J. Gen. Intern. Med. 2015, 31, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Gringauz, I.; Weismann, J.; Justo, D.; Adunsky, A.; Segal, G. Alanine aminotransferase blood levels and rehabilitation outcome in older adults following hip fracture surgery. Int. J. Rehabil. Res. 2017, 41, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Vespasiani-Gentilucci, U.; de Vincentis, A.; Ferrucci, L.; Bandinelli, S.; Antonelli Incalzi, R.; Picardi, A. Low Alanine Aminotransferase Levels in the Elderly Population: Frailty, Disability, Sarcopenia, and Reduced Survival. J. Gerontol. Ser. A 2018, 73, 925–930. [Google Scholar] [CrossRef] [PubMed]


| Patients’ Demographics | |
|---|---|
| Age (years, Median (IQR)) | 72 (65–79) |
| Female gender (%) | 46.4 |
| Norton score (Median (IQR)) | 19 (18–20) |
| Body weight (Kg, Median (IQR)) | 75 (65–84.5) |
| BMI (Median (IQR)) | 26.7 (23.6–29.8) |
| Comorbidities (%) | |
| Cardiovascular disease | 43.2 |
| Diabetes mellitus | 33.1 |
| Respiratory disease | 12.7 |
| Infectious disease | 12.2 |
| Background malignancy | 12.2 |
| Laboratory Data | |
| Creatinine (mg/dL, (Median (IQR)) | 0.86 (0.68–1.15) |
| Urea (mg/dL, (Median (IQR)) | 38 (29–52) |
| Albumin (mg/dL, (Median (IQR)) | 3.7 (3.3–4.0) |
| Hemoglobin (gr/dL, (mean ± SD) | 11.8 ± 2.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irina, G.; Refaela, C.; Adi, B.; Avia, D.; Liron, H.; Chen, A.; Gad, S. Low Blood ALT Activity and High FRAIL Questionnaire Scores Correlate with Increased Mortality and with Each Other. A Prospective Study in the Internal Medicine Department. J. Clin. Med. 2018, 7, 386. https://doi.org/10.3390/jcm7110386
Irina G, Refaela C, Adi B, Avia D, Liron H, Chen A, Gad S. Low Blood ALT Activity and High FRAIL Questionnaire Scores Correlate with Increased Mortality and with Each Other. A Prospective Study in the Internal Medicine Department. Journal of Clinical Medicine. 2018; 7(11):386. https://doi.org/10.3390/jcm7110386
Chicago/Turabian StyleIrina, Gringauz, Cohen Refaela, Brom Adi, Davidi Avia, Hofstetter Liron, Avaki Chen, and Segal Gad. 2018. "Low Blood ALT Activity and High FRAIL Questionnaire Scores Correlate with Increased Mortality and with Each Other. A Prospective Study in the Internal Medicine Department" Journal of Clinical Medicine 7, no. 11: 386. https://doi.org/10.3390/jcm7110386
APA StyleIrina, G., Refaela, C., Adi, B., Avia, D., Liron, H., Chen, A., & Gad, S. (2018). Low Blood ALT Activity and High FRAIL Questionnaire Scores Correlate with Increased Mortality and with Each Other. A Prospective Study in the Internal Medicine Department. Journal of Clinical Medicine, 7(11), 386. https://doi.org/10.3390/jcm7110386





