Grip Strength Moderates the Association between Anthropometric and Body Composition Indicators and Liver Fat in Youth with an Excess of Adiposity
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design, Setting, and Participants
2.2. Physical Fitness Parameters
2.3. Anthropometric and Body Composition Measures
2.4. Controlled Attenuation Parameter
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sayiner, M.; Koenig, A.; Henry, L.; Younossi, Z.M. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin. Liver Dis. 2016, 20, 205–214. [Google Scholar] [CrossRef] [PubMed]
- López-Velázquez, J.A.; Silva-Vidal, K.V.; Ponciano-Rodríguez, G.; Chávez-Tapia, N.C.; Arrese, M.; Uribe, M.; Méndez-Sánchez, N. The prevalence of nonalcoholic fatty liver disease in the Americas. Ann. Hepatol. 2014, 13, 166–178. [Google Scholar]
- Schwimmer, J.B.; Deutsch, R.; Kahen, T.; Lavine, J.E.; Stanley, C.; Behling, C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006, 118, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hermoso, A.; Correa-Bautista, J.E.; Olloquequi, J.; Ramírez-Vélez, R. Health-related physical fitness and weight status in 13- to 15-year-old Latino adolescents. A pooled analysis. J. Pediatr. (Rio. J.) 2018. [Google Scholar] [CrossRef] [PubMed]
- García-Hermoso, A.; Cavero-Redondo, I.; Ramírez-Vélez, R.; Ruiz, J.R.; Ortega, F.B.; Lee, D.C.; Martínez-Vizcaíno, V. Muscular strength as a predictor of all-cause mortality in an apparently healthy population: A systematic review and meta-analysis of data from approximately 2 million men and women. Arch. Phys. Med. Rehabil. 2018, 99, 2100–2113. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, S.M. Association of muscle mass and fat mass with insulin resistance and the prevalence of metabolic syndrome in Korean adults: A cross-sectional study. Sci. Rep. 2018, 8, 2703. [Google Scholar] [CrossRef] [PubMed]
- Grontved, A.; Ried-Larsen, M.; Moller, N.C.; Kristensen, P.L.; Froberg, K.; Brage, S.; Andersen, L.B. Muscle strength in youth and cardiovascular risk in young adulthood (the European youth heart study). Br. J. Sports Med. 2015, 49, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Morelli, M.; Buzzigoli, E.; DeFronzo, R.A.; Bugianesi, E.; Gastaldelli, A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 2013, 5, 1544–1560. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Yoon, J.S.; Won, K.C.; Lee, H.W. The role of skeletal muscle in development of nonalcoholic fatty liver disease. Diabetes Metab. J. 2013, 37, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, S.U.; Song, K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, B.W.; Kang, E.S.; Cha, B.S.; Han, K.H. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008–2011). Hepatology 2016, 63, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, C.W.; Park C, H.; Choi, J.Y.; Han, K.; Merchant, A.T.; Park, Y.M. Low skeletal muscle mass is associated with non-alcoholic fatty liver disease in Korean adults: The fifth Korea national health and nutrition examination survey. Hepatobiliary Pancreat Dis. Int. 2016, 15, 39–47. [Google Scholar] [CrossRef]
- Faigenbaum, A.D.; MacDonald, J.P. Dynapenia: It’s not just for grown-ups anymore. Acta Paediatr. 2017, 106, 696–697. [Google Scholar] [CrossRef] [PubMed]
- Faigenbaum, A.D.; Rebullido, T.R.; MacDonald, J.P. Pediatric inactivity triad. Curr. Sports Med. Rep. 2018, 17, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Faigenbaum, A.D.; Bruno, L.E. A fundamental approach for treating pediatric dynapenia in kids. ACSMs Health Fit. J. 2017, 21, 18–24. [Google Scholar]
- Silverman, I.W. The secular trend for grip strength in Canada and the United States. J. Sports Sci. 2011, 29, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Laurson, K.R.; Saint-Maurice, P.F.; Welk, G.J.; Eisenmann, J.C. Reference curves for field tests of musculoskeletal fitness in U.S. children and adolescents. J. Strength Cond. Res. 2017, 31, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Voss, C.; Taylor, M.; Delextrat, A.; Ogunleye, A.A.; Sandercock, G.R. Ten-year secular changes in muscular fitness in English children. Acta Paediatr. 2011, 100, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Moliner-Urdiales, D.; Ruiz, J.R.; Ortega, F.B.; Jiménez-Pavón, D.; Vicente-Rodriguez, G.; Rey-López, J.P.; Martínez-Gómez, D.; Casajús, J.A.; Mesana, M.I.; Marcos, A.; et al. Secular trends in health-related physical fitness in Spanish adolescents: The AVENA and HELENA Studies. J. Sci. Med. Sport 2010, 13, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.; Ramírez-Campillo, R.; Cano-Montoya, J.; Ramírez-Vélez, R.; Harridge, S.D.R.; Alonso-Martínez, A.M.; Izquierdo, M. Exercise and glucose control in children with insulin resistance: prevalence of non-responders. Pediatr. Obes. 2018. [Google Scholar] [CrossRef]
- Bae, J.C.; Suh, S.; Park, S.E.; Rhee, E.J.; Park, C.Y.; Oh, K.W.; Park, S.W.; Kim, S.W.; Hur, K.Y.; Kim, J.H.; et al. Regular exercise is associated with a reduction in the risk of NAFLD and decreased liver enzymes in individuals with NAFLD independent of obesity in Korean adults. PLoS ONE 2012, e46819. [Google Scholar] [CrossRef] [PubMed]
- Perseghin, G.; Lattuada, G.; De Cobelli, F.; Ragogna, F.; Ntali, G.; Esposito, A.; Belloni, E.; Canu, T.; Terruzzi, I.; Scifo, P.; et al. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care 2007, 30, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Hallsworth, K.; Fattakhova, G.; Hollingsworth, K.G.; Thoma, C.; Moore, S.; Taylor, R.; Day, C.P.; Trenell, M.I. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011, 60, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Buch, A.; Yeshua, H.; Vaisman, N.; Webb, M.; Harari, G.; Kis, O.; Fliss-Isakov, N.; Izkhakov, E.; Halpern, Z.; et al. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J. Gastroenterol. 2014, 20, 4382–4392. [Google Scholar] [CrossRef] [PubMed]
- González-Ruiz, K.; Ramírez-Vélez, R.; Correa-Bautista, J.E.; Peterson, M.D.; García-Hermoso, A. The effects of exercise on abdominal fat and liver enzymes in pediatric obesity: A systematic review and meta-analysis. Child Obes. 2017, 13, 272–282. [Google Scholar] [CrossRef] [PubMed]
- García-Hermoso, A.; Ramírez-Vélez, R.; Saavedra, J.M. Exercise, health outcomes, and pædiatric obesity: A systematic review of meta-analyses. J. Sci. Med. Sport 2018. [Google Scholar] [CrossRef] [PubMed]
- Mizgier, M.L.; Casas, M.; Contreras-Ferrat, A.; Llanos, P.; Galgani, J.E. Potential role of skeletal muscle glucose metabolism on the regulation of insulin secretion. Obes. Rev. 2014, 15, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Suglia, S.F.; Koenen, K.C.; Boynton-Jarrett, R.; Chan, P.S.; Clark, C.J.; Danese, A.; Faith, M.S.; Goldstein, B.I.; Hayman, L.L.; Isasi, C.R.; et al. Childhood and adolescent adversity and cardiometabolic outcomes: A scientific statement from the American Heart Association. Circulation 2018, 137, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Peña-Ibagon, J.C.; Martínez-Torres, J.; Tordecilla-Sanders, A.; Correa-Bautista, J.E.; Lobelo, F.; García-Hermoso, A. Handgrip strength cutoff for cardiometabolic risk index among Colombian children and adolescents: The FUPRECOL study. Sci. Rep. 2017, 7, 42622. [Google Scholar] [CrossRef] [PubMed]
- González-Ruíz, K.; Correa-Bautista, J.E.; Izquierdo, M.; García-Hermoso, A.; Dominguez-Sanchez, M.A.; Bustos-Cruz, R.H.; García-Prieto, J.C.; Martínez-Vizcaíno, V.; Lobelo, F.; González-Jiménez, E.; et al. Effects of an exercise program on hepatic metabolism, hepatic fat, and cardiovascular health in overweight/obese adolescents from Bogotá, Colombia (the HEPAFIT study): Study protocol for a randomized controlled trial. Trials 2018, 19, 330. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Rodrigues-Bezerra, D.; Correa-Bautista, J.E.; Izquierdo, M.; Lobelo, F. Reliability of health-related physical fitness tests among Colombian children and adolescents: The FUPRECOL study. PLoS ONE. 2015, 10, e0140875. [Google Scholar] [CrossRef] [PubMed]
- Léger, L.A.; Mercier, D.; Gadoury, C.; Lambert, J. The multistage 20 metre shuttle run test for aerobic fitness. J. Sports Sci. 1988, 6, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Mirwald, R.L.; Baxter-Jones, A.D.G.; Bailey, D.A.; Beunen, G.P. An assessment of maturity from anthropometric measurements. Med. Sci. Sports Exerc. 2002, 34, 689–694. [Google Scholar] [PubMed]
- Tokuhara, D.; Cho, Y.; Shintaku, H. Transient elastography-based liver stiffness age-dependently increases in children. PLoS ONE 2016, 11, e0166683. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Zarski, J.-P.; De Ledinghen, V.; Rousselet, M.C.; Sturm, N.; Lebail, B.; Fouchard-Hubert, I.; Gallois, Y.; Oberti, F.; Bertrais, S.; et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013, 57, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.K.; Harney, S.; Raza, R.; Al-Ibraheemi, A.; Shillingford, N.; Mitchell, P.D.; Jonas, M.M. Comparison of controlled attenuation parameter and liver biopsy to assess hepatic steatosis in pediatric patients. J. Pediatr. 2016, 173, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Preacher, K.J.; Hayes, A.F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 2008, 40, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Neyman, J. Tests of certian linear hypotheses and their applications to some educational problems. Stat. Res. Memoirs 1936, 1, 57–93. [Google Scholar]
- Meng, G.; Wu, H.; Fang, L.; Li, C.; Yu, F.; Zhang, Q.; Liu, L.; Du, H.; Shi, H.; Xia, Y.; et al. Relationship between grip strength and newly diagnosed nonalcoholic fatty liver disease in a large-scale adult population. Sci. Rep. 2016, 6, 33255. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Boys (n = 42) | Girls (n = 85) | p-Value |
|---|---|---|---|
| Chronological age, years | 12.9 (1.2) | 13.7 (1.7) | 0.003 |
| Age of PHV, years | 12.3 (0.6) | 14.4 (0.6) | 0.001 |
| Anthropometric parameters | |||
| Body mass index, kg/m2 | 24.2 (2.5) | 23.5 (4.1) | 0.359 |
| Body mass index, z-score | 1.73 (0.64) | 1.39 (0.85) | 0.013 |
| Overweight + obese prevalence (%) * | 41.1 | 55.9 | 0.066 |
| BF > 30% by DXA prevalence (%) * | 97.6 | 100 | 0.997 |
| Waist circumference, cm | 79.4 (6.8) | 74.6 (8.4) | 0.009 |
| Waist-to-height ratio | 0.505 (0.039) | 0.480 (0.052) | 0.009 |
| Body composition parameter | |||
| BF% by DXA | 40.8 (4.1) | 38.0 (4.6) | 0.001 |
| Visceral adipose tissue (cm3) | 382.9 (82.4) | 323.1 (108.0) | 0.003 |
| Vibration controlled transient elastography | |||
| Controlled attenuation parameter, dB/m | 245.8 (41.9) | 216.2 (40.9) | <0.001 |
| Liver stiffness, kPa | 3.9 (0.7) | 4.0 (3.1) | 0.850 |
| NAFLD prevalence, n (%) | 25 (59.5) | 43 (50.5) | 0.010 |
| Physical fitness parameters | |||
| Handgrip strength (kg) | 21.6 (6.4) | 20.7 (4.7) | 0.376 |
| Handgrip strength, (kg)/Weight, (kg) | 0.37 (0.07) | 0.36 (0.07) | 0.485 |
| VO2max (mL/kg/min) | 39.4 (3.8) | 37.2 (3.1) | 0.001 |
| Shuttles (total count) | 21.3 (15.9) | 18.1 (9.0) | 0.268 |
| Stage (last completed) | 3.2 (1.9) | 2.9 (1.1) | 0.161 |
| Running speed at last completed shuttle (km∙h−1) | 9.6 (0.9) | 9.4 (0.6) | 0.291 |
| Parameter | β (Standardized) | p-Value |
|---|---|---|
| Waist circumference (cm) | ||
| Model 1 | 0.564 | <0.001 |
| Model 2 | 0.574 | <0.001 |
| Model 3 | 0.510 | <0.001 |
| Model 4 | 0.526 | <0.001 |
| Waist-to-height ratio | ||
| Model 1 | 0.550 | <0.001 |
| Model 2 | 0.558 | <0.001 |
| Model 3 | 0.484 | <0.001 |
| Model 4 | 0.500 | <0.001 |
| Fat mass (kg) | ||
| Model 1 | 0.478 | <0.001 |
| Model 2 | 0.481 | <0.001 |
| Model 3 | 0.423 | <0.001 |
| Model 4 | 0.435 | <0.001 |
| Visceral adipose tissue (cm3) | ||
| Model 1 | 0.580 | <0.001 |
| Model 2 | 0.576 | <0.001 |
| Model 3 | 0.535 | <0.001 |
| Model 4 | 0.537 | <0.001 |
| Physical Fitness Parameter | WC | WHtR | Fat Mass | Visceral Adipose Tissue | ||||
|---|---|---|---|---|---|---|---|---|
| Moderator # | p-Value | Moderator # | p-Value | Moderator # | p-Value | Moderator # | p-Value | |
| VO2max, mL/kg/min | No interaction | 0.812 | No interaction | 0.485 | No interaction | 0.291 | No interaction | 0.760 |
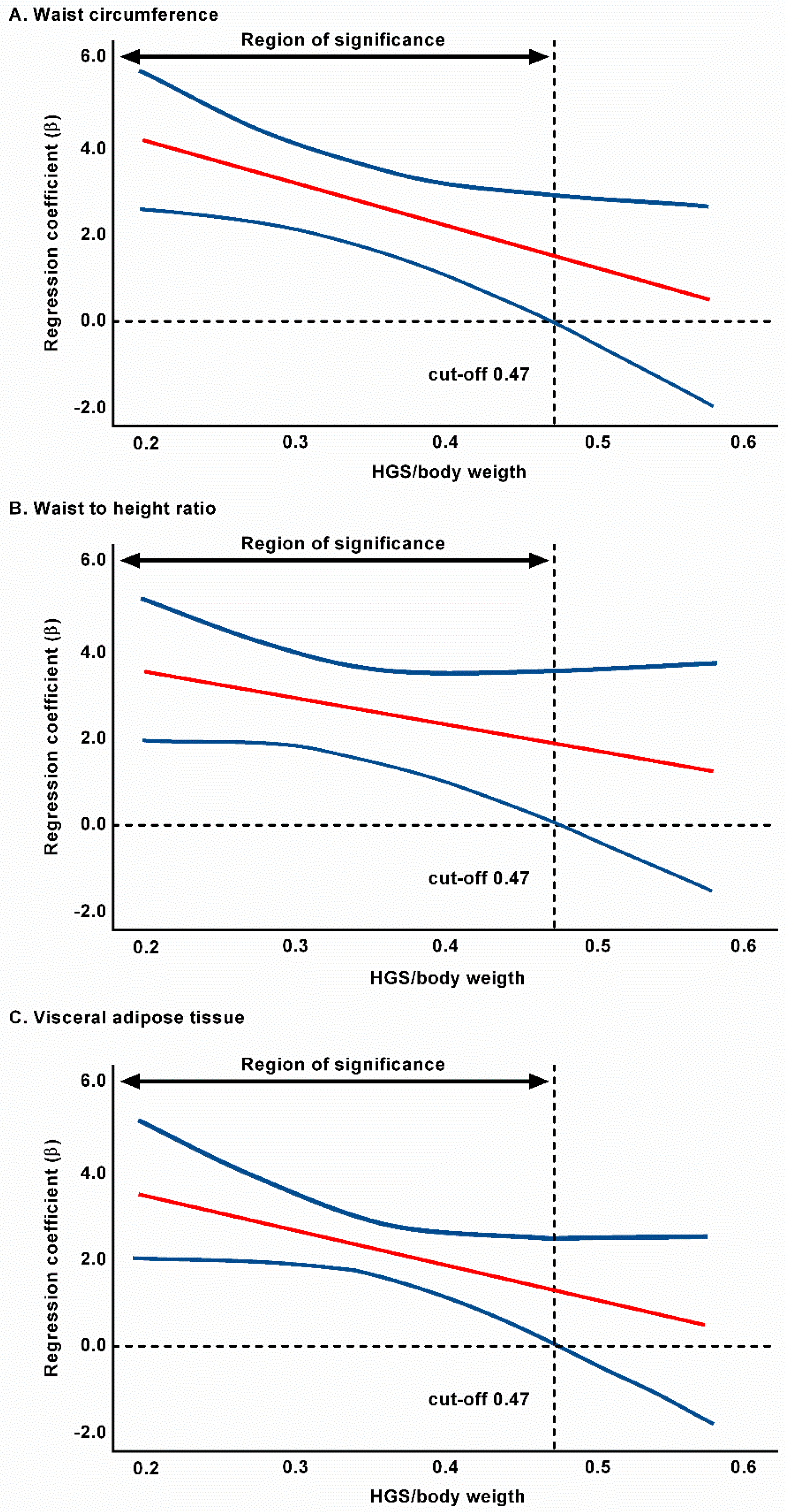
| Grip strength/weight | 0.475 * | 0.027 | 0.469 * | 0.037 | No interaction | 0.318 | 0.470* | 0.019 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Vélez, R.; Izquierdo, M.; Correa-Bautista, J.E.; Tordecilla-Sanders, A.; Correa-Rodríguez, M.; Schmidt Rio-Valle, J.; González-Jiménez, E.; González-Ruíz, K. Grip Strength Moderates the Association between Anthropometric and Body Composition Indicators and Liver Fat in Youth with an Excess of Adiposity. J. Clin. Med. 2018, 7, 347. https://doi.org/10.3390/jcm7100347
Ramírez-Vélez R, Izquierdo M, Correa-Bautista JE, Tordecilla-Sanders A, Correa-Rodríguez M, Schmidt Rio-Valle J, González-Jiménez E, González-Ruíz K. Grip Strength Moderates the Association between Anthropometric and Body Composition Indicators and Liver Fat in Youth with an Excess of Adiposity. Journal of Clinical Medicine. 2018; 7(10):347. https://doi.org/10.3390/jcm7100347
Chicago/Turabian StyleRamírez-Vélez, Robinson, Mikel Izquierdo, Jorge Enrique Correa-Bautista, Alejandra Tordecilla-Sanders, María Correa-Rodríguez, Jacqueline Schmidt Rio-Valle, Emilio González-Jiménez, and Katherine González-Ruíz. 2018. "Grip Strength Moderates the Association between Anthropometric and Body Composition Indicators and Liver Fat in Youth with an Excess of Adiposity" Journal of Clinical Medicine 7, no. 10: 347. https://doi.org/10.3390/jcm7100347
APA StyleRamírez-Vélez, R., Izquierdo, M., Correa-Bautista, J. E., Tordecilla-Sanders, A., Correa-Rodríguez, M., Schmidt Rio-Valle, J., González-Jiménez, E., & González-Ruíz, K. (2018). Grip Strength Moderates the Association between Anthropometric and Body Composition Indicators and Liver Fat in Youth with an Excess of Adiposity. Journal of Clinical Medicine, 7(10), 347. https://doi.org/10.3390/jcm7100347








