Effect of Resveratrol on Reactive Oxygen Species-Induced Cognitive Impairment in Rats with Angiotensin II-Induced Early Alzheimer’s Disease †
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Chemicals
2.2. Animals
2.3. Intracerebroventricular Injection Procedure
2.4. Blood Pressure Measurement
2.5. Morris Water Maze
2.6. Measurement of Aβ40 and Aβ42 in the Hippocampus and Post-Hypothalamus
2.7. Immunoblotting Analysis
2.8. Immunohistochemistry Analysis
2.9. Statistical Analyses
3. Results
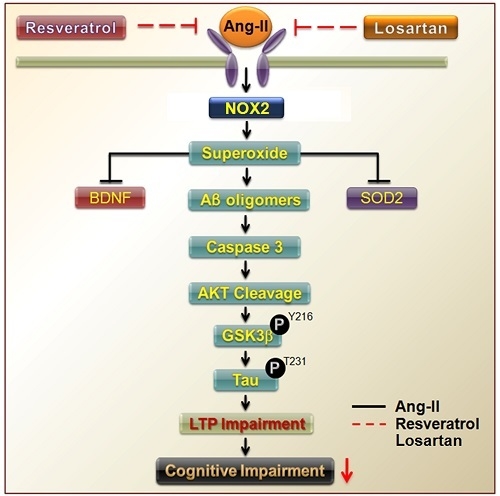
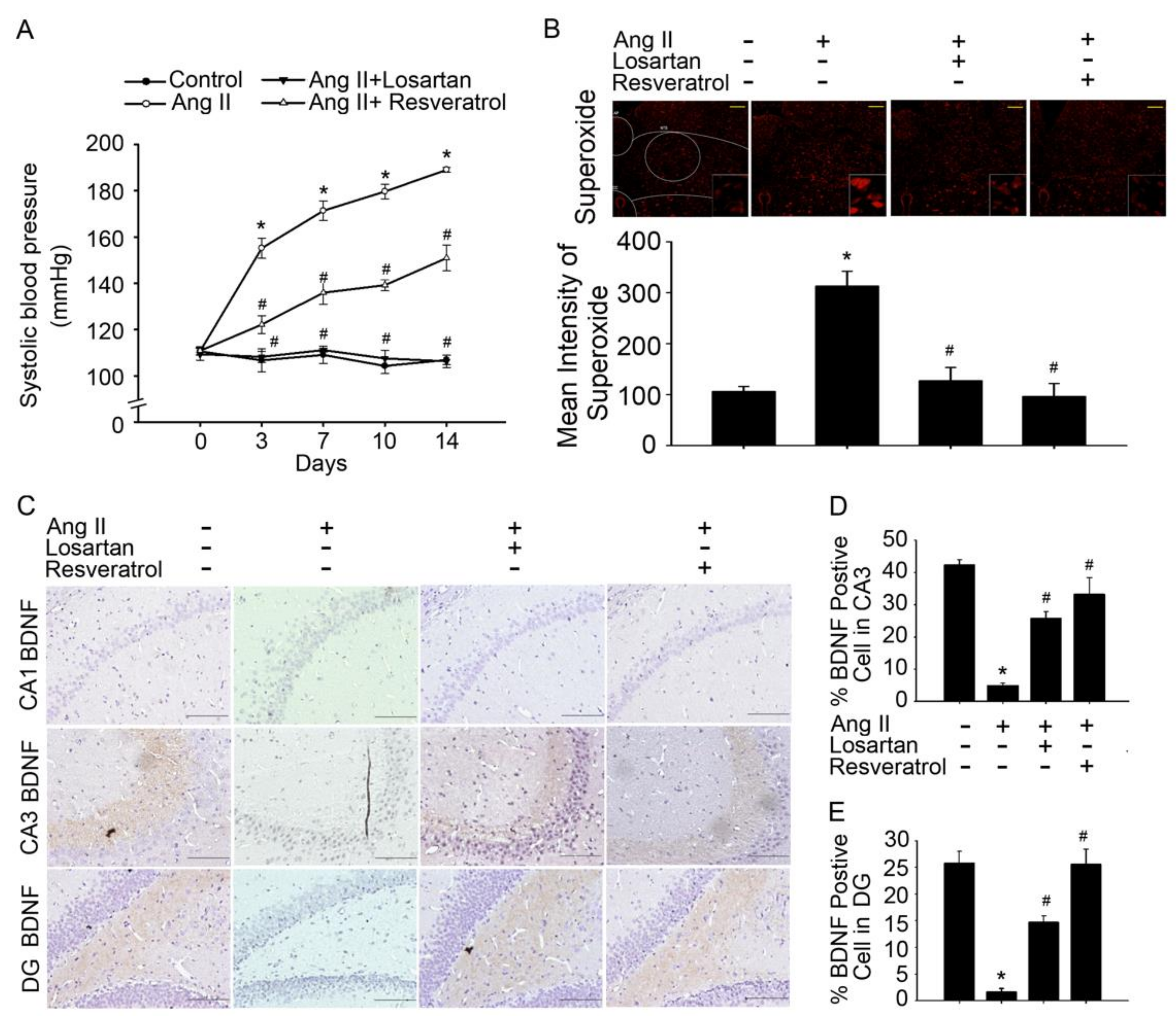
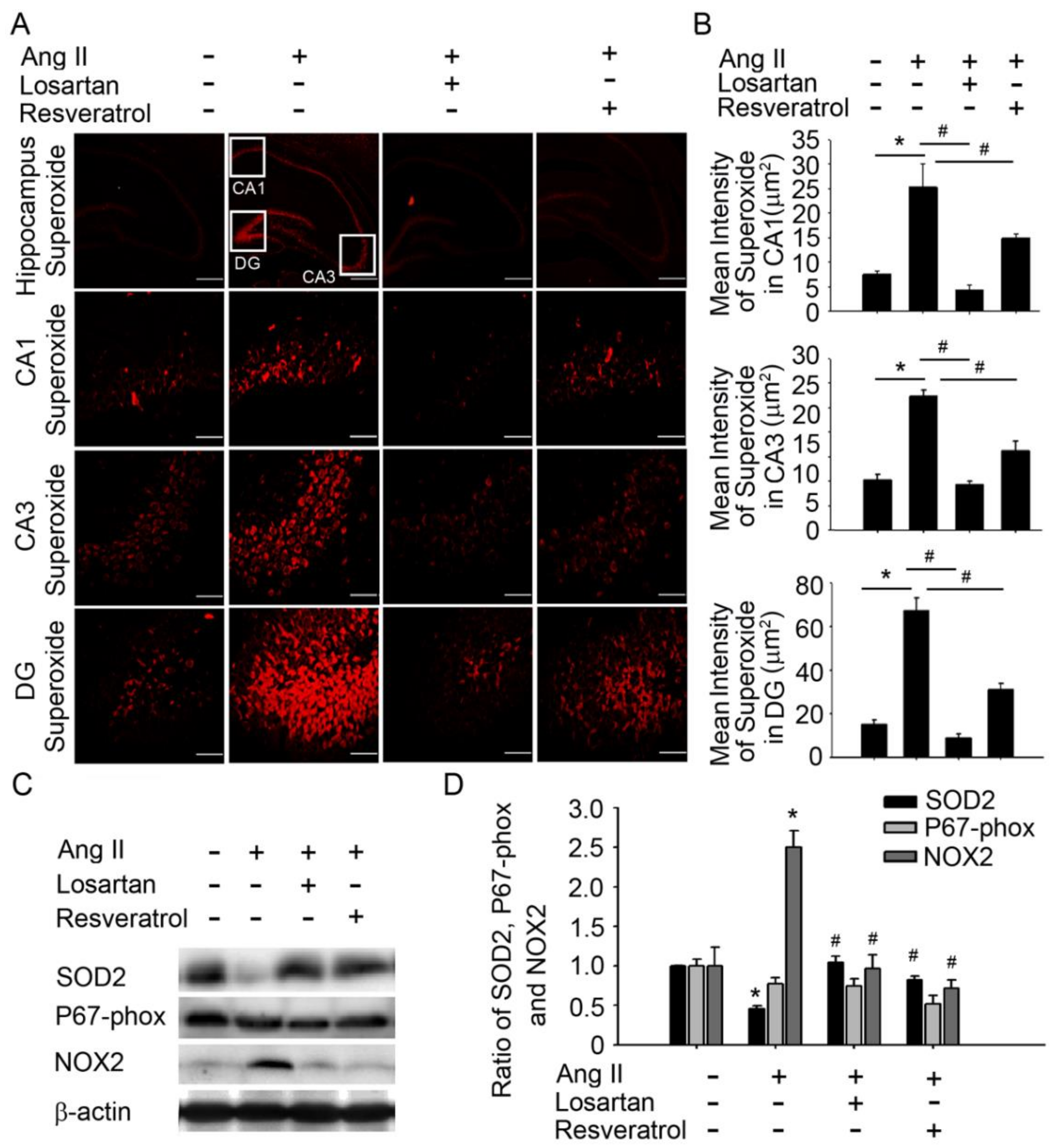
3.1. Brain-Derived Neurotrophic Factor Levels and Superoxide Imbalance Contributed to Hypertension and Early AD
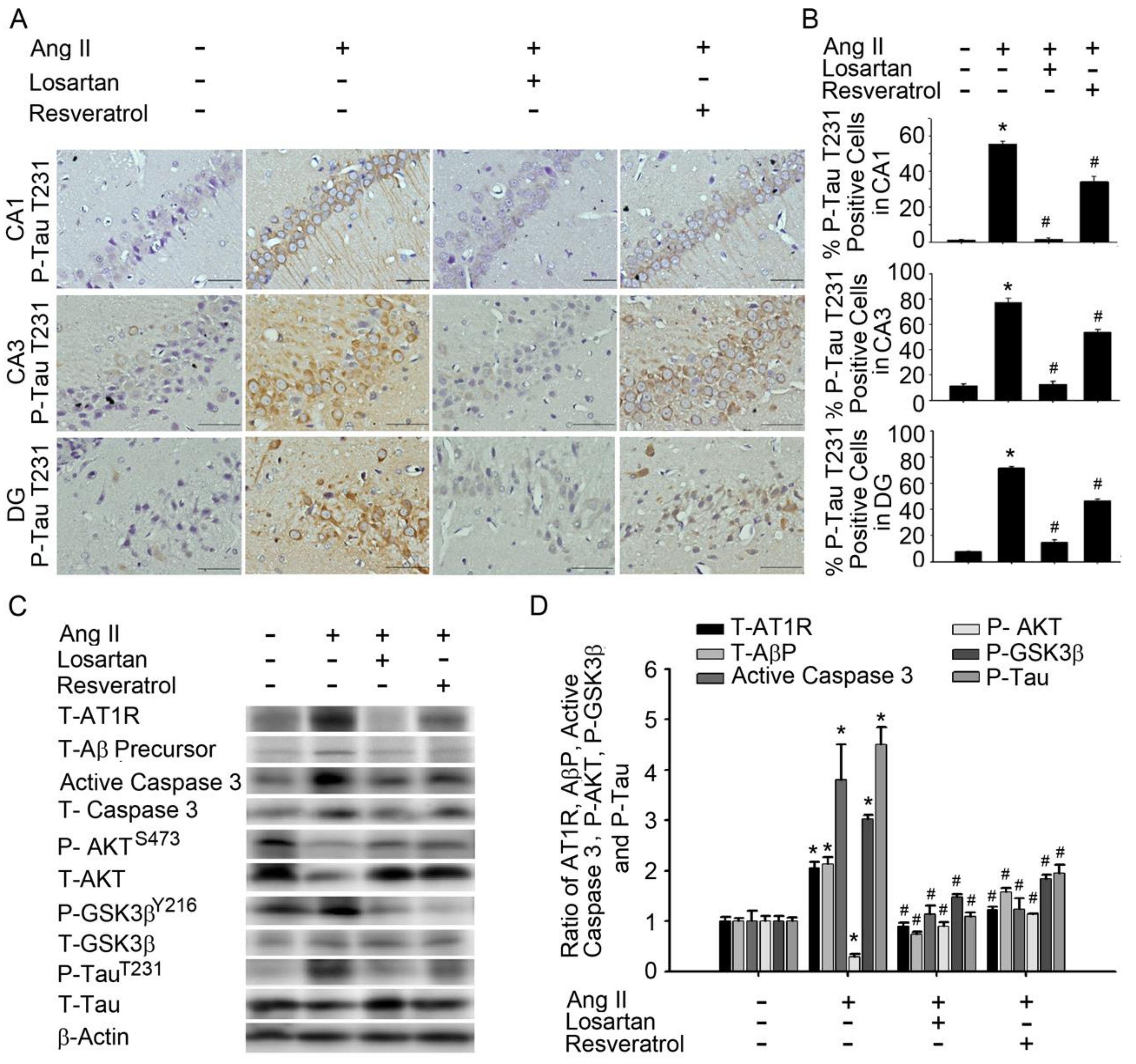
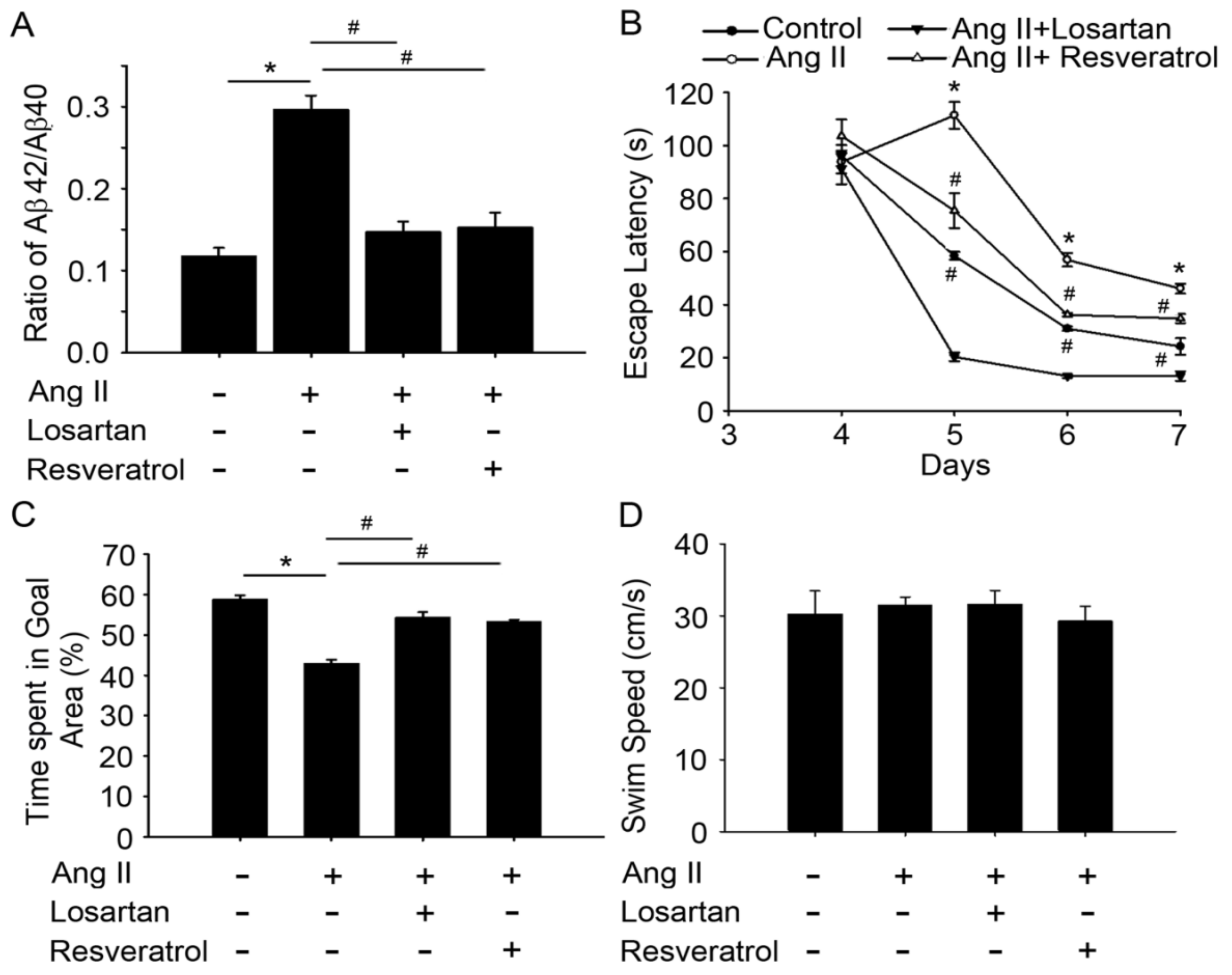
3.2. Resveratrol Impaired the Activity of Aβ Precursors, Active Caspase 3, and GSK-3-Tau by Normalising Renal AT1R Signalling in the Hippocampus of Rats with Ang-II-Induced Early AD
3.3. Resveratrol Treatment Improved Spatial Learning and Memory in Rats with Ang-II-Induced Early AD
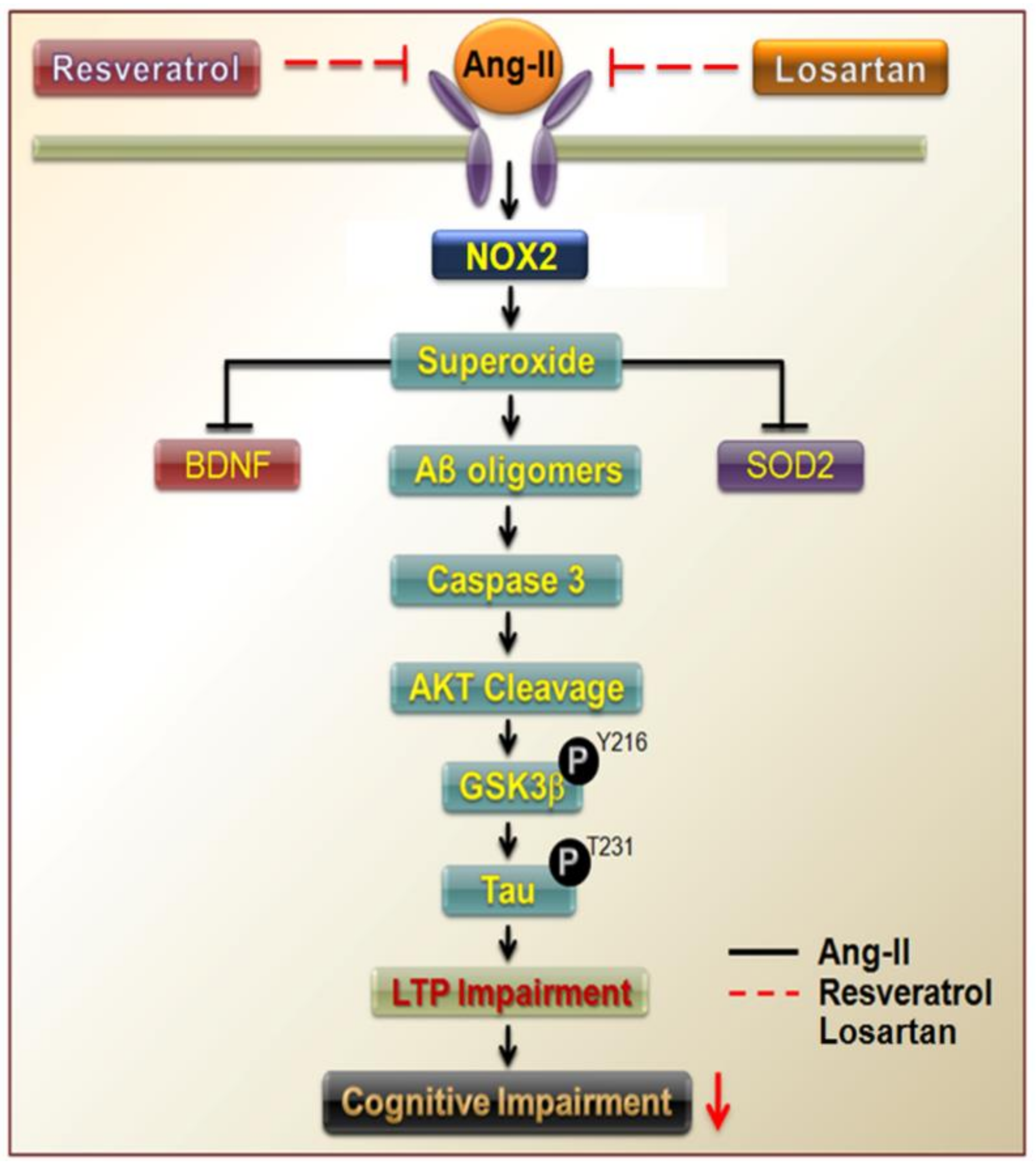
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yin, F.; Sancheti, H.; Patil, I.; Cadenas, E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic. Biol. Med. 2016, 100, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Haapasalo, A.; Kauppinen, A.; Kaarniranta, K.; Soininen, H.; Hiltunen, M. Impaired mitochondrial energy metabolism in Alzheimer’s disease: Impact on pathogenesis via disturbed epigenetic regulation of chromatin landscape. Prog. Neurobiol. 2015, 131, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Arendt, T.; Stieler, J.T.; Holzer, M. Tau and tauopathies. Brain Res. Bull. 2016, 126, 238–292. [Google Scholar] [CrossRef] [PubMed]
- Salloway, S.; Correia, S. Alzheimer disease: Time to improve its diagnosis and treatment. Clevel. Clin. J. Med. 2009, 76, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Opazo, F.A.; Vergara-Pulgar, K.; Perez, M.J.; Jara, C.; Osorio-Fuentealba, C.; Quintanilla, R.A. Mitochondrial dysfunction contributes to the pathogenesis of Alzheimer’s disease. Oxidative Med. Cell. Longev. 2015, 2015, 509654. [Google Scholar] [CrossRef] [PubMed]
- Ashby, E.L.; Kehoe, P.G. Current status of renin-aldosterone angiotensin system-targeting anti-hypertensive drugs as therapeutic options for Alzheimer’s disease. Expert Opin. Investig. Drugs 2013, 22, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Durango, N.; Fuentes, C.A.; Castillo, A.E.; Gonzalez-Gomez, L.M.; Vecchiola, A.; Fardella, C.E.; Kalegris, A.M. Role of the renin-angiotensin-aldosterone system beyond blood pressure regulation: Molecular and cellular mechanisms involved in end-organ damage during arterial hypertension. Int. J. Mol. Sci. 2016, 17, 797. [Google Scholar] [CrossRef] [PubMed]
- Paglieri, C.; Bisbocci, D.; Caserta, M.; Rabbia, F.; Bertello, C.; Canade, A.; Veglio, F. Hypertension and cognitive function. Clin. Exp. Hypertens. 2008, 30, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, P.G. The coming of age of the angiotensin hypothesis in Alzheimer’s disease: progress toward disease prevention and treatment? J. Alzheimer’s Dis. 2018, 62, 1443–1466. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, P.G.; Wong, S.; Al Mulhim, N.; Palmer, L.E.; Miners, J.S. Angiotensin-converting enzyme 2 is reduced in Alzheimer’s disease in association with increasing amyloid-beta and tau pathology. Alzheimers Res. Ther. 2016, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Faraco, G.; Park, L.; Zhou, P.; Luo, W.; Paul, S.M.; Anrather, J.; Ladecola, C. Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J. Cereb. Blood Flow Metab. 2016, 36, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.L.; Hu, W.Y.; Jiang, L.H.; Li, L.; Gaung, X.F.; Xiao, Z.C. p38 MAPK inhibition improves synaptic plasticity and memory in angiotensin II-dependent hypertensive mice. Sci. Rep. 2016, 6, 27600. [Google Scholar] [CrossRef] [PubMed]
- Bloch, S.; Obari, D.; Girouard, H. Angiotensin and neurovascular coupling: Beyond hypertension. Microcirculation 2015, 22, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.; Abo-Youssef, A.M.; Messiha, B.A.; Khattab, M.M. Tempol and perindopril protect against lipopolysaccharide-induced cognition impairment and amyloidogenesis by modulating brain-derived neurotropic factor, neuroinflammation and oxido-nitrosative stress. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 637–656. [Google Scholar] [CrossRef] [PubMed]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Richard, T.; Pawlus, A.D.; Iglesias, M.L.; Pedrot, E.; Waffo-Teguo, P.; Merillon, J.M.; Monti, J.-P. Neuroprotective properties of resveratrol and derivatives. Ann. N.Y. Acad. Sci. 2011, 1215, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Jugder, B.E.; Poljak, A.; Jayasena, T.; Mansour, H.; Nabavi, S.M.; Sachev, P.; Grant, R. Resveratrol as a potential therapeutic candidate for the treatment and management of Alzheimer’s disease. Curr. Top. Med. Chem. 2016, 16, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Francini, F.; Castro, M.C.; Schinella, G.; Garcia, M.E.; Maiztegui, B.; Raschia, M.A.; Gagliardino, J.J.; Massa, M.L. Changes induced by a fructose-rich diet on hepatic metabolism and the antioxidant system. Life Sci. 2010, 86, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.W.; Lee, H.C.; Lu, P.J.; Chen, H.H.; Lai, C.C.; Sun, G.C.; Yeh, T.-C.; Hsiao, M.; Lin, Y.-T.; Liu, C.-P.; et al. Resveratrol inhibition of rac1-derived reactive oxygen species by AMPK decreases blood pressure in a fructose-induced rat model of hypertension. Sci. Rep. 2016, 6, 25342. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Sellers, K.W.; Sumners, C.; Raizada, M.K. NAD(P)H oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II. Circ. Res. 2005, 96, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.H.; Lu, P.J.; Ho, W.Y.; Tung, C.S.; Cheng, P.W.; Hsiao, M.; Tseng, C.-J. Angiotensin II inhibits neuronal nitric oxide synthase activation through the ERK1/2-RSK signaling pathway to modulate central control of blood pressure. Circ. Res. 2010, 106, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Kocisova, E.; Petr, M.; Sipova, H.; Kylian, O.; Prochazka, M. Drop coating deposition of a liposome suspension on surfaces with different wettabilities: “Coffee ring” formation and suspension preconcentration. Phys. Chem. Chem. Phys. 2016, 19, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.S.; McCormack, M.; Semprum-Prieto, L.C.; Shenouda, S.; Majid, D.S.; Kobori, H.; Navar, L.G.; Prieto, M.C. AT1 receptor-mediated augmentation of angiotensinogen, oxidative stress, and inflammation in ANG II-salt hypertension. Am. J. Physiol. Renal. Physiol. 2012, 302, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Giil, L.M.; Kristoffersen, E.K.; Vedeler, C.A.; Aarsland, D.; Nordrehaug, J.E.; Winblad, B.; Cedazo-Minguez, A.; Lind, A.; Reksten, T.R. Autoantibodies toward the angiotensin 2 type 1 receptor: A novel autoantibody in Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 47, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Zhu, D.; Xie, W.; Shi, J. Central angiotensin II-induced Alzheimer-like tau phosphorylation in normal rat brains. FEBS Lett. 2012, 586, 3737–3745. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Chen, Y.R. The coexistence of an equal amount of Alzheimer’s amyloid-beta 40 and 42 forms structurally stable and toxic oligomers through a distinct pathway. FEBS J. 2014, 281, 2674–2687. [Google Scholar] [CrossRef] [PubMed]
- Trojanowski, J.Q.; Arnold, S.E.; Karlawish, J.H.; Naylor, M.; Brunden, K.R.; Lee, V.M. A model for improving the treatment and care of Alzheimer’s disease patients through interdisciplinary research. Alzheimers Dement. 2012, 8, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Korf, E.S.; White, L.R.; Scheltens, P.; Launer, L.J. Midlife blood pressure and the risk of hippocampal atrophy: The Honolulu Asia aging study. Hypertension 2004, 44, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Kril, J.J.; Patel, S.; Harding, A.J.; Halliday, G.M. Patients with vascular dementia due to microvascular pathology have significant hippocampal neuronal loss. J. Neurol. Neurosurg. Psychiatry 2002, 72, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Hu, Y.; Yao, A.; Yang, Y.; Shi, Y.; Jiang, Y.; Yhang, J. Impact of renin-angiotensin system-targeting antihypertensive drugs on treatment of Alzheimer’s disease: A meta-analysis. Int. J. Clin. Pract. 2015, 69, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Petrovitch, H.; White, L.R.; Izmirilian, G.; Ross, G.W.; Havlik, R.J.; Markesbery, W.; Nelson, J.; Davis, D.G.; Hardman, J.; Foley, D.J.; et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: The HAAS. Honolulu-Asia aging study. Neurobiol. Aging 2000, 21, 57–62. [Google Scholar] [PubMed]
- Saavedra, J.M. Opportunities and limitations of genetic analysis of hypertensive rat strains. J. Hypertens. 2009, 27, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Pietranera, L.; Lima, A.; Roig, P.; De Nicola, A.F. Involvement of brain-derived neurotrophic factor and neurogenesis in oestradiol neuroprotection of the hippocampus of hypertensive rats. J. Neuroendocrinol. 2010, 22, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Jazbutyte, V.; Arias-Loza, P.A.; Hu, K.; Widder, J.; Govindaraj, V.; Von Poser-Klein, C.; Bauersachs, J.; Fritzemeier, K.-H.; Hegele-Hartung, C.; Neyes, L.; et al. Ligand-dependent activation of ERβ lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized spontaneously hypertensive rats. Cardiovasc. Res. 2008, 77, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Kregel, K.C.; Zhang, H.J. An integrated view of oxidative stress in aging: Basic mechanisms, functional effects, and pathological considerations. American journal of physiology. Regul. Integr. Comp. Physiol. 2007, 292, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Pattillo, C.B.; Bir, S.; Rajaram, V.; Kevil, C.G. Inorganic nitrite and chronic tissue ischaemia: A novel therapeutic modality for peripheral vascular diseases. Cardiovasc. Res. 2011, 89, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, A.C.; Aranke, M.; Bryan, N.S. Nitric oxide and geriatrics: Implications in diagnostics and treatment of the elderly. J. Geriat. Cardiol. JGC 2011, 8, 230–242. [Google Scholar]
- Yeh, T.C.; Shin, C.S.; Chen, H.H.; Lai, C.C.; Sun, G.C.; Tseng, C.J.; Cheng, P.-W. Resveratrol regulates blood pressure by enhancing AMPK signaling to downregulate a Rac1-derived NADPH oxidase in the central nervous system. J. Appl. Physiol. 2018, 125, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Kim, M.Y.; Lim, J.H.; Kim, Y.; Shin, S.J.; Park, C.W.; Kim, Y.-S.; Chang, Y.S; Yoon, H.E.; Shoi, B.S. The protective effect of resveratrol on vascular aging by modulation of the renin-angiotensin system. Atherosclerosis 2018, 270, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, R.; Ichiki, T.; Hashimoto, T.; Inanaga, K.; Imayama, I.; Sadoshima, J.; Sunagawa, K. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Te Riet, L.; van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, A.H. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lee, C.T.; Huang, L.T.; Tain, Y.L. Aliskiren in early postnatal life prevents hypertension and reduces asymmetric dimethylarginine in offspring exposed to maternal caloric restriction. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Culmsee, C.; Monnig, J.; Kemp, B.E.; Mattson, M.P. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J. Mol. Neurosci. 2001, 17, 45–58. [Google Scholar] [CrossRef]
- Vingtdeux, V.; Giliberto, L.; Zhao, H.; Chandakkar, P.; Wu, Q.; Simon, J.E.; Janle, E.L.; Lobo, J.; Ferruzzi, M.G.; Davies, P.; et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 2010, 285, 9100–9113. [Google Scholar] [CrossRef] [PubMed]
- Albani, D.; Polito, L.; Forloni, G. Sirtuins as novel targets for Alzheimer’s disease and other neurodegenerative disorders: Experimental and genetic evidence. J. Alzheimer’s Dis. 2010, 19, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, X.P.; Yang, S.G.; Wang, Y.J.; Zhang, X.; Du, X.T.; Sun, X.-X.; Zhao, M.; Huang, L.; Liu, R.-T. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology 2009, 30, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Ladiwala, A.R.; Lin, J.C.; Bale, S.S.; Marcelino-Cruz, A.M.; Bhattacharya, M.; Dordick, J.S.; Tessier, P.M. Resveratrol selectively remodels soluble oligomers and fibrils of amyloid Aβ into off-pathway conformers. J. Biol. Chem. 2010, 285, 24228–24237. [Google Scholar] [CrossRef] [PubMed]
- Frozza, R.L.; Bernardi, A.; Hoppe, J.B.; Meneghetti, A.B.; Matte, A.; Battastini, A.M.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C. Neuroprotective effects of resveratrol against Abeta administration in rats are improved by lipid-core nanocapsules. Mol. Neurobiol. 2013, 47, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Sindi, S.; Mangialasche, F.; Kivipelto, M. Advances in the prevention of Alzheimer’s disease. F1000Prime Rep. 2015, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Rimando, A.; Pallas, M.; Camins, A.; Porquet, D.; Reeves, J.; Shukitt-Hale, B.; Smith, M.A.; Joshep, J.A.; Casadesus, G. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol. Aging 2012, 33, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Asien, P.S.; et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.S.; Abdelkader, N.F.; Abd El-Rahman, S.S.; Emara, M.; Zaki, H.F.; Khattab, M.M. Stimulation of ACE2/ANG(1–7)/Mas axis by diminazene ameliorates Alzheimer’s disease in the D-galactose-ovariectomized rat model: Role of PI3K/Akt pathway. Mol. Neurobiol. 2018, 55, 8188–8202. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.H.; Baek, S.J.; Heo, S.; Park, H.J.; Kwon, H.; Lee, S.; Jung, J.; Park, S.J.; Kim, B.C.; Lee, Y.C.; et al. Direct pharmacological Akt activation rescues Alzheimer’s disease like memory impairments and aberrant synaptic plasticity. Neuropharmacology 2018, 128, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Quincozes-Santos, A.; Gottfried, C. Resveratrol modulates astroglial functions: Neuroprotective hypothesis. Ann. N.Y. Acad. Sci. 2011, 1215, 72–78. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; Wu, Y.-C.; Sun, G.-C.; Ho, C.-Y.; Wong, T.-Y.; Lin, C.-H.; Chen, H.-H.; Yeh, T.-C.; Li, C.-J.; Tseng, C.-J.; et al. Effect of Resveratrol on Reactive Oxygen Species-Induced Cognitive Impairment in Rats with Angiotensin II-Induced Early Alzheimer’s Disease †. J. Clin. Med. 2018, 7, 329. https://doi.org/10.3390/jcm7100329
Lin Y-T, Wu Y-C, Sun G-C, Ho C-Y, Wong T-Y, Lin C-H, Chen H-H, Yeh T-C, Li C-J, Tseng C-J, et al. Effect of Resveratrol on Reactive Oxygen Species-Induced Cognitive Impairment in Rats with Angiotensin II-Induced Early Alzheimer’s Disease †. Journal of Clinical Medicine. 2018; 7(10):329. https://doi.org/10.3390/jcm7100329
Chicago/Turabian StyleLin, Yu-Te, Yi-Chung Wu, Gwo-Ching Sun, Chiu-Yi Ho, Tzyy-Yue Wong, Ching-Huang Lin, Hsin-Hung Chen, Tung-Chen Yeh, Chia-Jung Li, Ching-Jiunn Tseng, and et al. 2018. "Effect of Resveratrol on Reactive Oxygen Species-Induced Cognitive Impairment in Rats with Angiotensin II-Induced Early Alzheimer’s Disease †" Journal of Clinical Medicine 7, no. 10: 329. https://doi.org/10.3390/jcm7100329
APA StyleLin, Y.-T., Wu, Y.-C., Sun, G.-C., Ho, C.-Y., Wong, T.-Y., Lin, C.-H., Chen, H.-H., Yeh, T.-C., Li, C.-J., Tseng, C.-J., & Cheng, P.-W. (2018). Effect of Resveratrol on Reactive Oxygen Species-Induced Cognitive Impairment in Rats with Angiotensin II-Induced Early Alzheimer’s Disease †. Journal of Clinical Medicine, 7(10), 329. https://doi.org/10.3390/jcm7100329