Predictive Value of the Pretreatment Neutrophil-to-Lymphocyte Ratio in Head and Neck Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Immunohistochemical (IHC) Staining
2.3. MDSC Isolation and Flow Cytometry Analysis
2.4. Statistical Analysis
3. Results
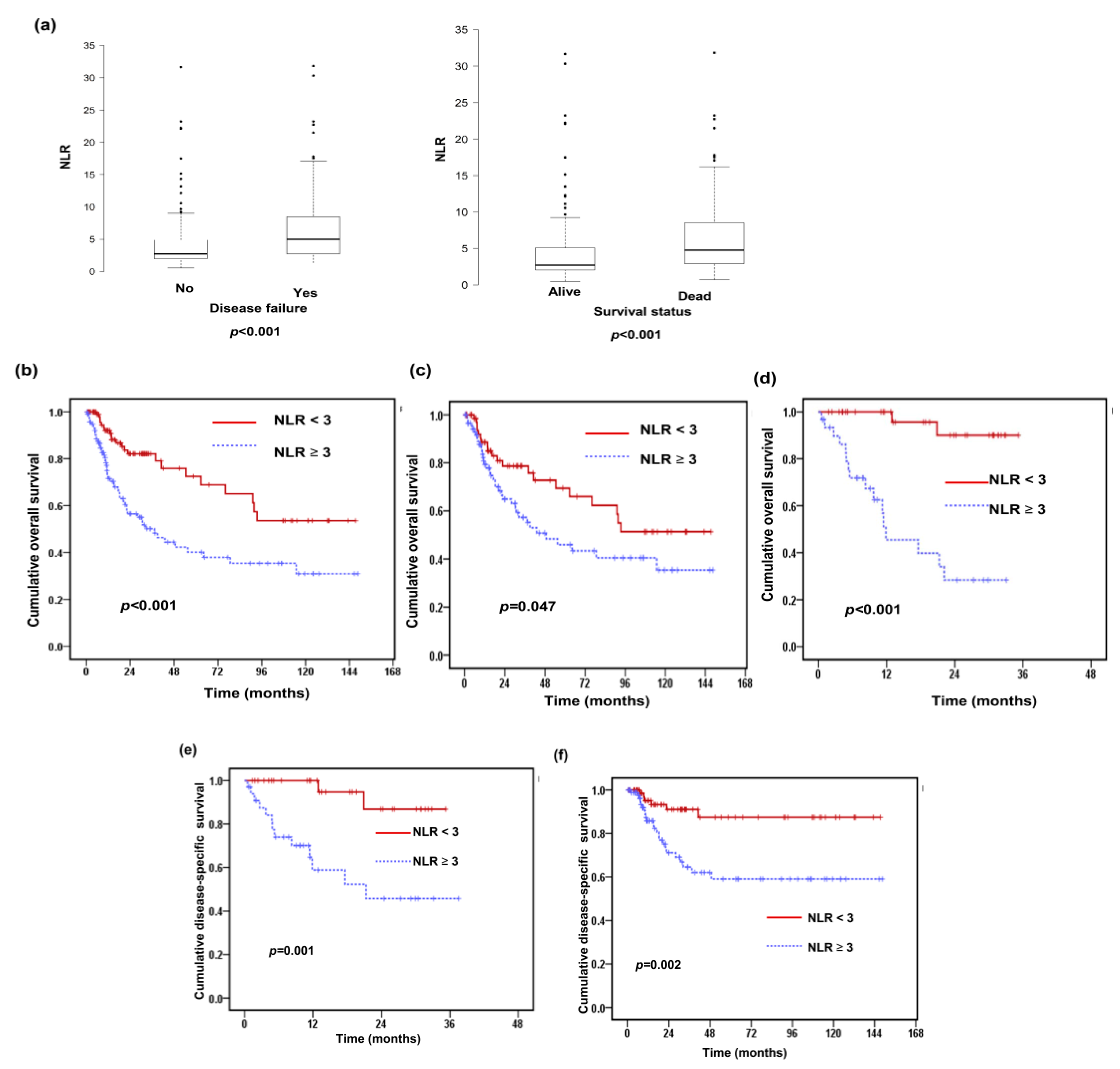
3.1. Correlations Between the Pretreatment NLR and Clinicopathological Characteristics of HNSCC Patients
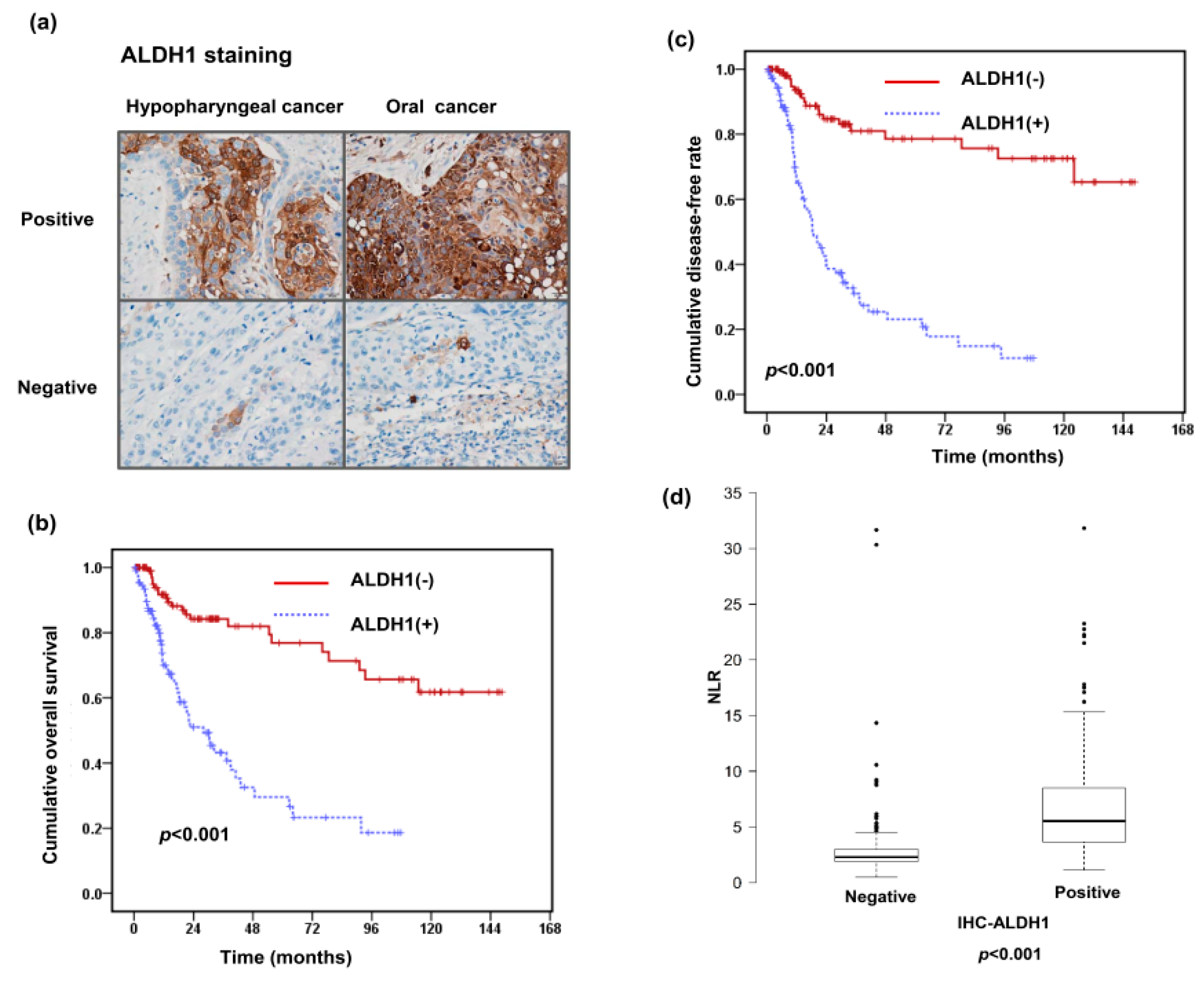
3.2. Relationships of ALDH1 Expression with the Pretreatment NLR and Clinical Outcome
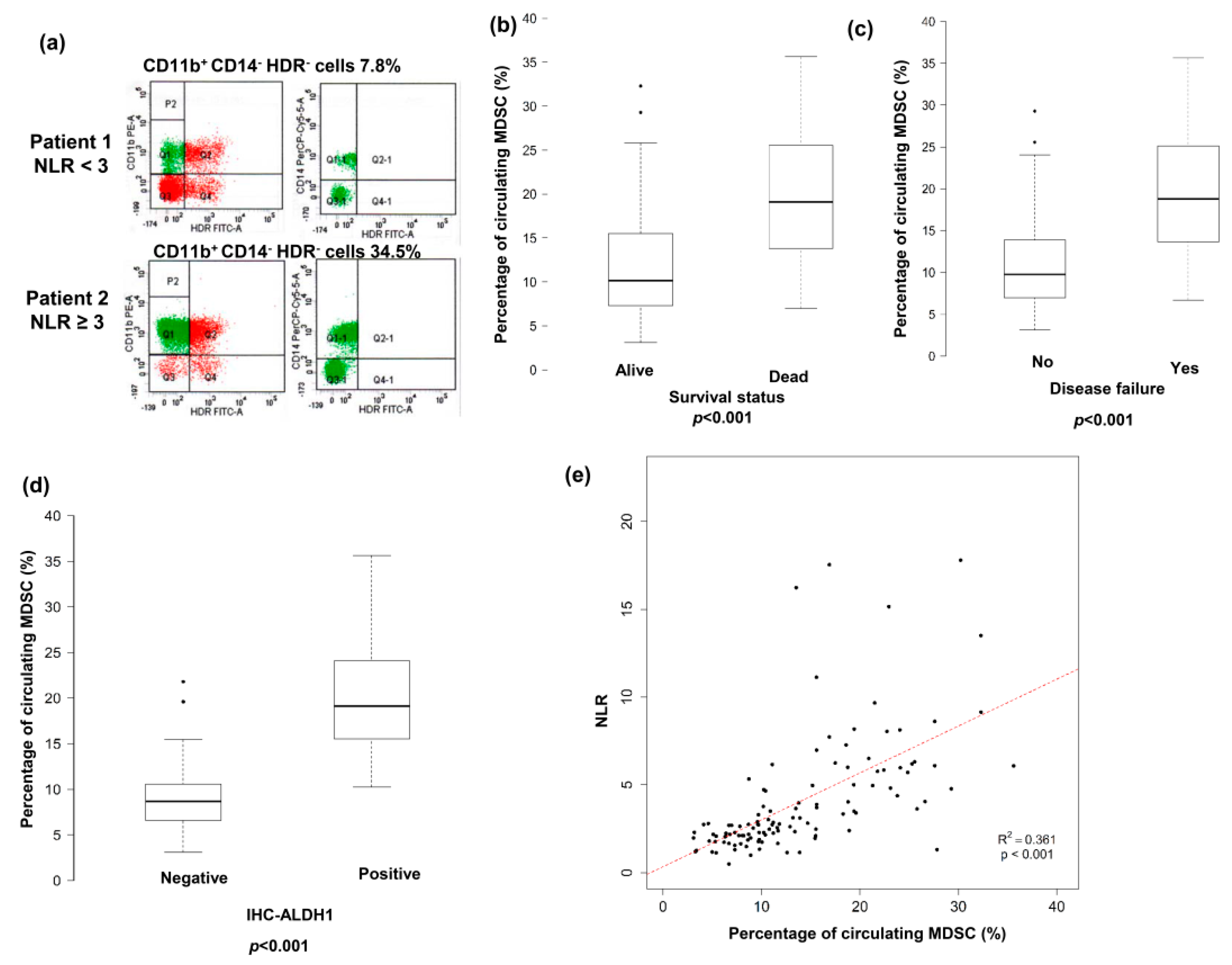
3.3. Predictive Role of Pretreatment NLR on Levels of CD11b+CD14−HLA-DR− Cells in Peripheral Circulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Gillison, M.L.; D’Souza, G.; Westra, W.; Sugar, E.; Xiao, W.; Begum, S.; Viscidi, R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J. Natl. Cancer Inst. 2008, 100, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Tiwari, R.; Nauta, J.J.; van der Waal, I.; Snow, G.B. Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer 1994, 73, 187–190. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.M. The paradox of tumor-associated neutrophils: Fueling tumor growth with cytotoxic substances. Cell Cycle 2010, 9, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.A.; Moses, K.; Trellakis, S.; Lang, S.; Brandau, S. Neutrophils and granulocytic myeloid-derived suppressor cells: Immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol. Immunother. 2012, 61, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Yodying, H.; Matsuda, A.; Miyashita, M.; Matsumoto, S.; Sakurazawa, N.; Yamada, M.; Uchida, E. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: A systematic review and meta-analysis. Ann. Surg. Oncol. 2016, 23, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Oya, R.; Kitamiura, T.; Ashida, N.; Shimizu, K.; Takemura, K.; Yamamoto, Y.; Uno, A. Prognostic role of neutrophil-to-lymphocyte ratio in head and neck cancer: A meta-analysis. Head Neck 2018, 40, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Bunt, S.K.; Sinha, P.; Clements, V.K.; Leips, J.; Ostrand-Rosenberg, S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J. Immunol. 2006, 176, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Cretney, E.; Kershaw, M.H.; Hayakawa, Y. Cytokines in cancer immunity and immunotherapy. Immunol. Rev. 2004, 202, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Lai, C.H.; Chuang, H.C.; Lin, P.Y.; Chen, M.F. Inflammation-induced myeloid-derived suppressor cells associated with squamous cell carcinoma of the head and neck. Head Neck 2017, 39, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Dubrovska, A.; Linge, A.; Baumann, M. Cancer stem cells: Radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv. Drug Deliv. Rev. 2017, 109, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.Y.; Schatton, T.; Frank, M.H. The therapeutic promise of the cancer stem cell concept. J. Clin. Investig. 2010, 120, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.J.; Sinyuk, M.; Vogelbaum, M.A.; Ahluwalia, M.S.; Lathia, J.D. The intersection of cancer, cancer stem cells, and the immune system: Therapeutic opportunities. Neuro. Oncol. 2016, 18, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, R.M.; Kong, F.M.; Li, H.; Yu, J.P.; Ren, X.B. How do tumor stem cells actively escape from host immunosurveillance? Biochem. Biophys. Res. Commun. 2012, 420, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Parsa, A.T.; Waldron, J.S.; Panner, A.; Crane, C.A.; Parney, I.F.; Barry, J.J.; Cachola, K.E.; Murray, J.C.; Tihan, T.; Jensen, M.C.; et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007, 13, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Wagner, S.; Ma, C.; Coordes, A.; Gekeler, J.; Klussmann, J.P.; Hummel, M.; Kaufmann, A.M.; Albers, A.E. Prognostic significance of ALDH1A1-positive cancer stem cells in patients with locally advanced, metastasized head and neck squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2014, 140, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Muller, S.; Nannapaneni, S.; Pan, L.; Wang, Y.; Peng, X.; Wang, D.; Tighiouart, M.; Chen, Z.; Saba, N.F.; et al. Comparison of quantum dot technology with conventional immunohistochemistry in examining aldehyde dehydrogenase 1A1 as a potential biomarker for lymph node metastasis of head and neck cancer. Eur. J. Cancer 2012, 48, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Chen, W.C.; Lai, C.H.; Chen, Y.Y.; Chen, M.F. Epigenetic therapy regulates the expression of ALDH1 and immunologic response: Relevance to the prognosis of oral cancer. Oral Oncol. 2017, 73, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Brandau, S.; Moses, K.; Lang, S. The kinship of neutrophils and granulocytic myeloid-derived suppressor cells in cancer: Cousins, siblings or twins? Semin. Cancer Biol. 2013, 23, 171–182. [Google Scholar] [CrossRef] [PubMed]
- De Vlaeminck, Y.; Gonzalez-Rascon, A.; Goyvaerts, C.; Breckpot, K. Cancer-Associated Myeloid Regulatory Cells. Front. Immunol. 2016, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N. Neutrophils, from marrow to microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Querol, E.; Rosales, C. Neutrophils in cancer: Two sides of the same coin. J. Immunol. Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Swierczak, A.; Mouchemore, K.A.; Hamilton, J.A.; Anderson, R.L. Neutrophils: Important contributors to tumor progression and metastasis. Cancer Metastasis Rev. 2015, 34, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Seruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocana, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, H.; Yan, A.; Wang, H.; Li, X.; Liu, J.; Li, W. Pretreatment neutrophil to lymphocyte ratio in determining the prognosis of head and neck cancer: A meta-analysis. BMC Cancer 2018, 18, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Zamarron, B.F.; Chen, W. Dual roles of immune cells and their factors in cancer development and progression. Int. J. Biol. Sci. 2011, 7, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017, 27, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Treffers, L.W.; Hiemstra, I.H.; Kuijpers, T.W.; van den Berg, T.K.; Matlung, H.L. Neutrophils in cancer. Immunol. Rev. 2016, 273, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Schupp, J.; Krebs, F.K.; Zimmer, N.; Trzeciak, E.; Schuppan, D.; Tuettenberg, A. Targeting myeloid cells in the tumor sustaining microenvironment. Cell Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Moses, K.; Brandau, S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin. Immunol. 2016, 28, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Najjar, Y.G.; Finke, J.H. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front. Oncol. 2013, 3, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Pillay, J.; Tak, T.; Kamp, V.M.; Koenderman, L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: Similarities and differences. Cell. Mol. Life Sci. 2013, 70, 3813–3827. [Google Scholar] [CrossRef] [PubMed]
- Koch, U.; Krause, M.; Baumann, M. Cancer stem cells at the crossroads of current cancer therapy failures—radiation oncology perspective. Semin. Cancer Biol. 2010, 20, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Gerweck, L.E.; Wakimoto, H. At the crossroads of cancer stem cells; radiation biology; and radiation oncology. Cancer Res. 2016, 76, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Clay, M.R.; Tabor, M.; Owen, J.H.; Carey, T.E.; Bradford, C.R.; Wolf, G.T.; Wicha, M.S.; Prince, M.E. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck 2010, 32, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cancer Res. 2007, 1, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Cho, R.W.; Clarke, M.F. Recent advances in cancer stem cells. Curr. Opin. Genet. Dev. 2008, 18, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Ghiso, J.A. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer 2007, 7, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Codony-Servat, J.; Rosell, R. Cancer stem cells and immunoresistance: Clinical implications and solutions. Transl. Lung Cancer Res. 2015, 4, 689–703. [Google Scholar] [PubMed]
- Sica, A.; Porta, C.; Amadori, A.; Pasto, A. Tumor-associated myeloid cells as guiding forces of cancer cell stemness. Cancer Immunol. Immunother. 2017, 66, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
| Number of Patients | |||
|---|---|---|---|
| NLR < 3 | NLR ≥ 3 | p-Value | |
| Patient | 108 | 119 | |
| Age | |||
| <55 (median) | 52 | 53 | 0.58 |
| ≥55 | 56 | 66 | |
| Differentiation | 0.38 | ||
| Well differentiated | 39 | 39 | |
| Moderately differentiated | 38 | 41 | |
| Poorly differentiated | 23 | 32 | |
| Unknown | 8 | 7 | |
| Tumor stage | 0.073 | ||
| ≤T2 | 61 | 53 | |
| T3–T4 | 47 | 66 | |
| Clinical LN involvement | 0.87 | ||
| Negative | 42 | 45 | |
| Positive | 66 | 74 | |
| Tx policy | 0.98 | ||
| Definite CCRT | 31 | 34 | |
| Surgery +/− neoadjuvant/adjuvant Tx | 77 | 85 | |
| Location | 0.23 | ||
| Oral cavity | 72 | 88 | |
| Pharynx (Oro-Hypo) | 36 | 31 | |
| Loco-regional recurrence | <0.001 * | ||
| Control | 88 | 65 | |
| Failure | 20 | 54 | |
| Distant metastasis | 0.146 | ||
| Negative | 102 | 108 | |
| Positive | 6 | 13 | |
| Status | <0.001 * | ||
| Alive | 86 | 66 | |
| Dead | 22 | 53 | |
| Number of Patients | |||
|---|---|---|---|
| ALDH1 (−) | ALDH1 (+) | p-Value | |
| Patients | 118 | 109 | |
| Age | |||
| <55 (median) | 56 | 49 | 0.71 |
| ≥55 | 62 | 60 | |
| Differentiation | 0.38 | ||
| Well differentiated | 44 | 34 | |
| Moderately differentiated | 39 | 40 | |
| Poorly differentiated | 27 | 28 | |
| Unknown | 8 | 7 | |
| Tumor stage | 0.13 | ||
| ≤T2 | 65 | 49 | |
| T3–T4 | 53 | 60 | |
| Clinical LN involvement | 0.016 * | ||
| Negative | 54 | 33 | |
| Positive | 64 | 76 | |
| Tx policy | 0.42 | ||
| Definite CCRT | 35 | 34 | |
| Surgery +/− neoadjuvant/adjuvant Tx | 83 | 75 | |
| Location | 0.96 | ||
| Oral cavity | 72 | 77 | |
| Pharynx (Oro-Hypo) | 36 | 32 | |
| NLR | <0.001 * | ||
| <3 | 89 | 19 | |
| ≥3 | 29 | 90 | |
| Loco-regional recurrence | <0.001 * | ||
| Control | 104 | 49 | |
| Failure | 14 | 60 | |
| Distant metastasis | 0.019 * | ||
| Negative | 113 | 95 | |
| Positive | 5 | 14 | |
| Status | <0.001 * | ||
| Alive | 96 | 56 | |
| Dead | 22 | 53 | |
| Number of Patients | |||
|---|---|---|---|
| MDSC (Low) | MDSC (High) | p-Value | |
| Patients | 59 | 59 | |
| Age | |||
| <55 (median) | 28 | 26 | 0.72 |
| ≥55 | 31 | 33 | |
| Differentiation | 0.15 | ||
| Well differentiated | 12 | 9 | |
| Moderately differentiated | 24 | 18 | |
| Poorly differentiated | 17 | 23 | |
| Unknown | 6 | 9 | |
| Tumor stage | 0.005 * | ||
| ≤T2 | 35 | 20 | |
| T3–T4 | 24 | 39 | |
| Clinical LN involvement | 0.018 * | ||
| Negative | 25 | 13 | |
| Positive | 34 | 46 | |
| Tx policy | 0.016* | ||
| Definite CCRT | 26 | 39 | |
| Surgery +/− neoadjuvant/adjuvant Tx | 33 | 20 | |
| Location | 0.19 | ||
| Oral cavity | 29 | 22 | |
| Pharynx (Oro-Hypo) | 30 | 37 | |
| NLR | <0.001 * | ||
| <3 | 51 | 13 | |
| ≥3 | 8 | 46 | |
| ALDH1 staining | <0.001 * | ||
| Negative | 55 | 9 | |
| Positive | 4 | 50 | |
| Disease failure | <0.001 * | ||
| Negative | 52 | 26 | |
| Positive | 7 | 33 | |
| Status | <0.001 * | ||
| Alive | 54 | 34 | |
| Dead | 5 | 25 | |
| Variable | HR | 95% CI | p-Value |
|---|---|---|---|
| Age | |||
| <50 | Ref | ||
| ≥50 | 1.51 | 0.92–2.47 | 0.1 |
| Differentiation | |||
| Well–Moderately differentiated | Ref | ||
| Poorly differentiated. | 0.77 | 0.43–1.36 | 0.36 |
| Clinical T stage | |||
| ≥T2 | Ref | ||
| T3–T4 | 0.85 | 0.54–1.36 | 0.5 |
| Clinical N stage | |||
| N 0 | Ref | ||
| N (+) | 1.49 | 0.9–2.47 | 0.12 |
| NLR | |||
| <3 | Ref | ||
| ≥3 | 2.69 | 1.62–4.46 | <0.001 * |
| Treatment | |||
| Definite CCRT | Ref | ||
| Surgery +/− neoadjuvant/adjuvant Tx | 0.44 | 0.24–0.79 | 0.006 * |
| Variable | HR | 95% CI | p-Value |
|---|---|---|---|
| Age | |||
| <50 | Ref | ||
| ≥50 | 1.26 | 0.8–1.99 | 0.314 |
| Differentiation | |||
| Well–Moderately differentiated | Ref | ||
| Poorly differentiated | 0.87 | 0.52–0.47 | 0.61 |
| Clinical T stage | |||
| ≤T2 | Ref | ||
| T3–T4 | 0.94 | 0.61–1.45 | 0.77 |
| Clinical N stage | |||
| N 0 | Ref | ||
| N (+) | 1.6 | 0.99–2.58 | 0.057 |
| NLR | |||
| < 3 | Ref | ||
| ≥ 3 | 2.71 | 1.69–4.38 | <0.001 * |
| Treatment | |||
| Definite CCRT | Ref | ||
| Surgery +/− neoadjuvant/adjuvant Tx | 0.37 | 0.22–0.63 | <0.001 * |
| Variable | HR | 95% CI | p-Value |
|---|---|---|---|
| Age | |||
| <50 | Ref | ||
| ≥50 | 1.36 | 0.84–2.12 | 0.21 |
| Differentiation | |||
| Well–Moderately differentiated | Ref | ||
| Poorly differentiated | 0.79 | 0.45–1.39 | 0.42 |
| Clinical T stage | |||
| ≤T2 | Ref | ||
| T3–T4 | 1.01 | 0.62–1.60 | 0.98 |
| Clinical N stage | |||
| N 0 | Ref | ||
| N (+) | 1.17 | 0.71–1.93 | 0.53 |
| ALDH1 | |||
| Negative | Ref | ||
| Positive | 4.1 | 2.43–6.92 | <0.001 * |
| Treatment | |||
| Definite CCRT | Ref | ||
| Surgery +/− neoadjuvant/adjuvant Tx | 0.48 | 0.27–0.87 | 0.015 * |
| Variable | HR | 95% CI | p-Value |
|---|---|---|---|
| Age | |||
| <50 | Ref | ||
| ≥50 | 1.12 | 0.71–1.77 | 0.62 |
| Differentiation | |||
| Well–Moderately differentiated | Ref | ||
| Poorly differentiated | 0.88 | 0.53–1.47 | 0.63 |
| Clinical T stage | |||
| ≤T2 | Ref | ||
| T3–T4 | 1.1 | 0.70–1.71 | 0.68 |
| Clinical N stage | |||
| N 0 | Ref | ||
| N (+) | 1.26 | 0.78–2.03 | 0.35 |
| ALDH1 | |||
| Negative | Ref | ||
| Positive | 5.92 | 3.46–10.11 | <0.001 * |
| Treatment | |||
| Definite CCRT | Ref | ||
| Surgery +/− neoadjuvant/adjuvant Tx | 0.42 | 0.25–0.71 | 0.001 * |
| Variable | HR | 95% CI | p-Value |
|---|---|---|---|
| Age | |||
| <50 | Ref | ||
| ≥50 | 0.71 | 0.32–1.55 | 0.39 |
| Differentiation | |||
| Well–Moderately differentiated | Ref | ||
| Poorly differentiated | 0.44 | 0.18–1.06 | 0.07 |
| Clinical T stage | |||
| ≤T2 | Ref | ||
| T3–T4 | 1.04 | 0.44–2.48 | 0.93 |
| Clinical N stage | |||
| N 0 | Ref | ||
| N (+) | 1.19 | 0.49–2.90 | 0.70 |
| MDSC | |||
| Low | Ref | ||
| High | 6.19 | 2.34–18.0 | <0.001 * |
| Treatment | |||
| Definite CCRT | Ref | ||
| Surgery +/− neoadjuvant/adjuvant Tx | 0.43 | 0.19–0.98 | 0.044 * |
| Variable | HR | 95% CI | p-Value |
|---|---|---|---|
| Age | |||
| <50 | Ref | ||
| ≥50 | 0.70 | 0.34–1.40 | 0.31 |
| Differentiation | |||
| Well–Moderately differentiated | Ref | ||
| Poorly differentiated | 0.61 | 0.29–1.25 | 0.18 |
| Clinical T stage | |||
| ≤T2 | Ref | ||
| T3–T4 | 1.28 | 0.60–2.76 | 0.52 |
| Clinical N stage | |||
| N 0 | Ref | ||
| N (+) | 1.59 | 0.68–3.75 | 0.29 |
| ALDH1 | |||
| Negative | Ref | ||
| Positive | 5.31 | 2.23–12.62 | <0.001 * |
| Treatment | |||
| Definite CCRT | Ref | ||
| Surgery +/− neoadjuvant/adjuvant Tx | 0.39 | 0.19–0.82 | 0.012 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.-F.; Tsai, M.-S.; Chen, W.-C.; Chen, P.-T. Predictive Value of the Pretreatment Neutrophil-to-Lymphocyte Ratio in Head and Neck Squamous Cell Carcinoma. J. Clin. Med. 2018, 7, 294. https://doi.org/10.3390/jcm7100294
Chen M-F, Tsai M-S, Chen W-C, Chen P-T. Predictive Value of the Pretreatment Neutrophil-to-Lymphocyte Ratio in Head and Neck Squamous Cell Carcinoma. Journal of Clinical Medicine. 2018; 7(10):294. https://doi.org/10.3390/jcm7100294
Chicago/Turabian StyleChen, Miao-Fen, Ming-Shao Tsai, Wen-Cheng Chen, and Ping-Tsung Chen. 2018. "Predictive Value of the Pretreatment Neutrophil-to-Lymphocyte Ratio in Head and Neck Squamous Cell Carcinoma" Journal of Clinical Medicine 7, no. 10: 294. https://doi.org/10.3390/jcm7100294
APA StyleChen, M.-F., Tsai, M.-S., Chen, W.-C., & Chen, P.-T. (2018). Predictive Value of the Pretreatment Neutrophil-to-Lymphocyte Ratio in Head and Neck Squamous Cell Carcinoma. Journal of Clinical Medicine, 7(10), 294. https://doi.org/10.3390/jcm7100294






