The Concept and the Epidemiology of Diabetic Nephropathy Have Changed in Recent Years
Abstract
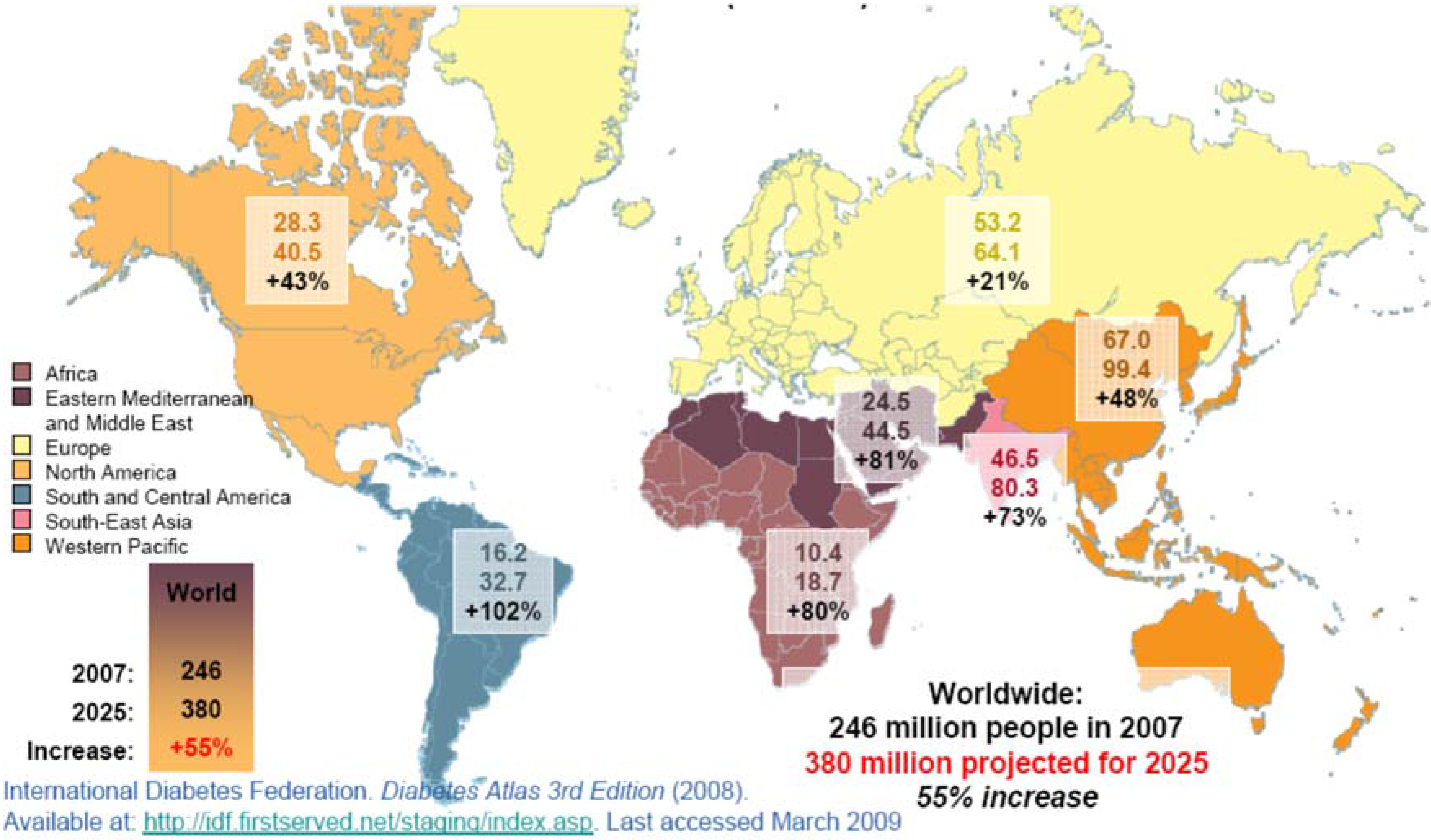
:1. Has the Diabetes Mellitus Epidemiology Changed in Recent Years?
2. A Changing Concept: From Diabetic Nephropathy to Diabetic Kidney Disease (DKD)
3. End-stage Renal Disease in Diabetic Patients: Has the Presence of DCKD Changed in Parallel to the DM Development?
| Classes of Glomerular Lesions | Description |
|---|---|
| Class I | Glomerular basement membrane thickening |
| Class II | Mesangial expansion, mild (IIa) or severe (IIb) |
| Class III | Nodular sclerosis (Kimmelstiel-Wilson lesions) |
| Class IV | Advanced diabetic glomerulosclerosis |
| Tubulointerstitial Lesions | Scores |
| IFTA | |
| No | 0 |
| <25% | 1 |
| 25%–50% | 2 |
| >50% | 3 |
| Interstitial inflammation | |
| No | 0 |
| Related to IFTA | 1 |
| Areas without IFTA | 2 |
| Vascular Lesions | |
| Arteriolar hyalinosis | |
| No | 0 |
| 1 area | 1 |
| >1 area | 2 |
| Presence of large vessels | |
| Arteriosclerosis | |
| No | 0 |
| Intimal thickening less than thickness of media | 1 |
| Intimal thickening greater than thickness of media | 2 |
| Nondiabetic Glomerular Lesions |
4. Conclusions and Implications
Author Contributions
Conflicts of Interest
References
- World Health Organization. The World Health Organization Report; WHO: Geneva, Switzerland, 1997. [Google Scholar]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.J.; Malay, D.S.; Hoffstad, O.J.; Leonard, C.E.; MaCurdy, T.; López de Nava, K.; Tan, Y.; Molina, T.; Siegel, K.L. Data Points Publication Series; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2011. [Google Scholar]
- Anonymous. The global challenge of diabetes. Lancet 2008, 371, 1723. [Google Scholar]
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Y.H.; Stevens, G.A.; et al. National, regional and global trends in fasting plasma glucose and diabetes since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef]
- Abraham, T.M.; Pencina, K.M.; Pencina, M.J.; Fox, C.S. Trends in diabetes incidence: The Framingham Heart Study. Diabetes Care 2015, 38, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Soriguer, F.; Goday, A.; Bosch-Comas, A.; Bordiú, E.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castaño, L.; Castell, C.; Catalá, M.; et al. Prevalencen of diabnetes mellitus and impaired glucose regulation in Spain: The Di@bet.es Study. Diabetologia 2012, 55, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, K.S. The past 200 years in Diabetes. N. Engl. J. Med. 2012, 367, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Living with Diabetes. Available online: http//:cdc.gov/diabetes/statistics/prevalence_national.html (accessed on 27 April 2015).
- Okrainec, K.; Booth, G.L.; Hollands, S.; Bell, C.M. Impact of language barriers on complications and mortality among immigrants with diabetes: A population-based cohort study. Diabetes Care 2015, 38, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Gregg, E.W.; Li, Y.; Wang, J.; Burrows, N.R.; Ali, M.K.; Rolka, D.; Williams, D.E.; Geiss, L. Changes in diabetes-related complications in the United States, 1990–2010. N. Engl. J. Med. 2014, 370, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castelao, A. Repercusiones clínicas y sociales de la la epidemia de diabetes mellitus (in Spanish). Nefrología 2008, 28, 245–248. [Google Scholar] [PubMed]
- Mata, M.; Antoñanzas, F.; Tafalla, M.; Sanz, P. The cost of type-2 diabetes in Spain: The CODE-2 study. Gac. Sanit. 2002, 16, 511–520. [Google Scholar] [CrossRef]
- Lorenzo, V.; Boronat, M. End stage renal disease associated with diabetes in the Canary Islands: A public health problem with significant human suffering and high economic costs. Nefrologia 2010, 30, 381–384. [Google Scholar] [PubMed]
- International Diabetes Federation 2008. Available online: http//:idf.first.net/staging/index.asp (accessed on 31 March 2013).
- American Diabetes Association. Available online: http//:www.diabetes.org (accessed on 13 March 2013).
- Breyer, J.A. Diabetic nephropathy in insulin-dependent patients. Am. J. Kidney Dis. 1992, 20, 533–547. [Google Scholar] [CrossRef]
- Mogensen, C.E.; Christiensen, C.K.; Vittinghus, E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 1983, 32, 64–78. [Google Scholar]
- Mogensen, C.E. The natural history of type 2 diabetic nephropathy. Am. J. Kidney Dis. 2001, 37, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Ruggenenti, P.; Gambara, V.; Perna, A.; Bertani, T.; Remuzzi, G. The nephropathy of non-insulin diabetes. Predictors of noutcome relative to diverse patterns of renal injury. J. Am. Soc. Nephrol. 1998, 9, 2336–2343. [Google Scholar]
- Dwyer, J.P.; Lewis, J.B. Nonproteinuric diabetic nephropathy: When diabetics don’t read the textbook. Med. Clin. North Am. 2013, 97, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Tervaert, T.W.; Mooyaart, A.L.; Amann, K.; Cohen, A.H.; Cook, H.T.; Drachenberg, C.B.; Ferrario, F.; Fogo, A.B.; Haas, M.; de Heer, E.; et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Abi, K.C.; Roussel, R.; Mohammedi, K.; Danchin, N.; Marre, M. Cause-specific mortality in diabetes: Recent changes in trend mortality. Eur. J. Prev. Cardiol. 2012, 19, 374–381. [Google Scholar]
- Martínez-Castelao, A.; De Alvaro, F.; Górriz, J.L. Epidemiology of diabetic nephropathy in Spain. Kidney Int. Suppl. 2005, 68, S20–S24. [Google Scholar] [CrossRef] [PubMed]
- Comas, J.; Arcos, E.; Casetll, C.; Cases, A.; Martínez-Castelao, A.; Doñate, T.; Esmatjes, E. Evolution of the incidence of chronic kidney disease stage 5 requiring renal replacement therapy in the diabetic population of Catalonia. Nephrol. Dial. Transplant. 2013, 28, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Dialysis and Transplantation annual report of the Spanish Society of Nephrology (S.E.N.)-ONT. In 44 Congress of the S.E.N., Barcelona, Spain, 5 October 2014.
- Friedman, E.A.; Friedman, A.L.; Eggers, P. End stage renal disease in diabetic persons: Is the pandemia subsiding? Kidney Int. Suppl. 2006, 70, S51–S54. [Google Scholar] [CrossRef] [PubMed]
- Burrows, N.R.; Li, Y.; Geiss, L.S. Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continuous to decline. Diabetes Care 2010, 33, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Grace, B.S.; Clayton, P.; McDonalds, S.P. Increases in renal replacement therapy in Australia and New Zealand: Understanding trends in diabetic nephropathy. Nephrology (Carlton) 2012, 17, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Llisterri, J.L.; Rodriguez-Roca, G.C.; Escobar, C.; Alonso-Moreno, F.J.; Prieto, M.A.; Barrios, V.; González-Alsina, D.; Divisón, J.A.; Pallarés, V.; Beato, P.; et al. Treatment and blood pressure control in Spain during 2002–2010. J. Hypertens. 2012, 30, 2425–2531. [Google Scholar] [CrossRef] [PubMed]
- Oluwatowoju, I.; Abu, E.; Wild, S.H.; Byrne, C.D. Improvements in glycaemic control and cholesterol concentrations associated with the Quality and Outcomes Framework: A regional 2-year audit of diabetes care in the UK. Diabet. Med. 2010, 27, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castelao, A.; Górriz, J.L.; Segura-de la Morena, J.; Cebollada, J.; Escalada, J.; Esmatjes, E.; Fácila, L.; Gamarra, J.; Gràcia, S.; Hernánd-Moreno, J.; et al. Consensus document for the detection and management of chronic kidney disease. Nefrologia 2014, 34, 243–262. [Google Scholar]
- Gómez-Huelgas, R.; Martínez-Castelao, A.; Artola, S.; Górriz, J.L.; Menéndez, E.; Grupo de Tabajo para el Documento de Consenso sobre el tratamiento de la diabetes tipo 2 en el paciente con enfermedad renal crónica. Consensus document on treatment of type 2 diabetes in patients with chronic kidney disease. Nefrología 2014, 34, 34–45. [Google Scholar]
- Couchoud, C.; Villar, E. End-stage renal disease epidemic in diabetes: Is there light at the end of the tunnel? Nephrol. Dial. Transplant. 2013, 28, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Kolko-Labadens, A.; de Cornelissen, F.; Couchoud, C. Charactéristics initials et indicateurs de prise en charge des nouveaux malades dialysés 2010 (in French). Nephrol. Ther. 2012, 8, S63–S69. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Castelao, A.; Navarro-González, J.F.; Górriz, J.L.; De Alvaro, F. The Concept and the Epidemiology of Diabetic Nephropathy Have Changed in Recent Years. J. Clin. Med. 2015, 4, 1207-1216. https://doi.org/10.3390/jcm4061207
Martínez-Castelao A, Navarro-González JF, Górriz JL, De Alvaro F. The Concept and the Epidemiology of Diabetic Nephropathy Have Changed in Recent Years. Journal of Clinical Medicine. 2015; 4(6):1207-1216. https://doi.org/10.3390/jcm4061207
Chicago/Turabian StyleMartínez-Castelao, Alberto, Juan F. Navarro-González, José Luis Górriz, and Fernando De Alvaro. 2015. "The Concept and the Epidemiology of Diabetic Nephropathy Have Changed in Recent Years" Journal of Clinical Medicine 4, no. 6: 1207-1216. https://doi.org/10.3390/jcm4061207
APA StyleMartínez-Castelao, A., Navarro-González, J. F., Górriz, J. L., & De Alvaro, F. (2015). The Concept and the Epidemiology of Diabetic Nephropathy Have Changed in Recent Years. Journal of Clinical Medicine, 4(6), 1207-1216. https://doi.org/10.3390/jcm4061207






