Bioengineering and Stem Cell Technology in the Treatment of Congenital Heart Disease
Abstract
:1. Clinical Consideration of Congenital Heart Disease
1.1. Structural Solutions
1.2. Stem Cells to Improve Cardiac Function
2. Induced Pluripotent Stem Cells to Study Causation in Congenital Heart Disease
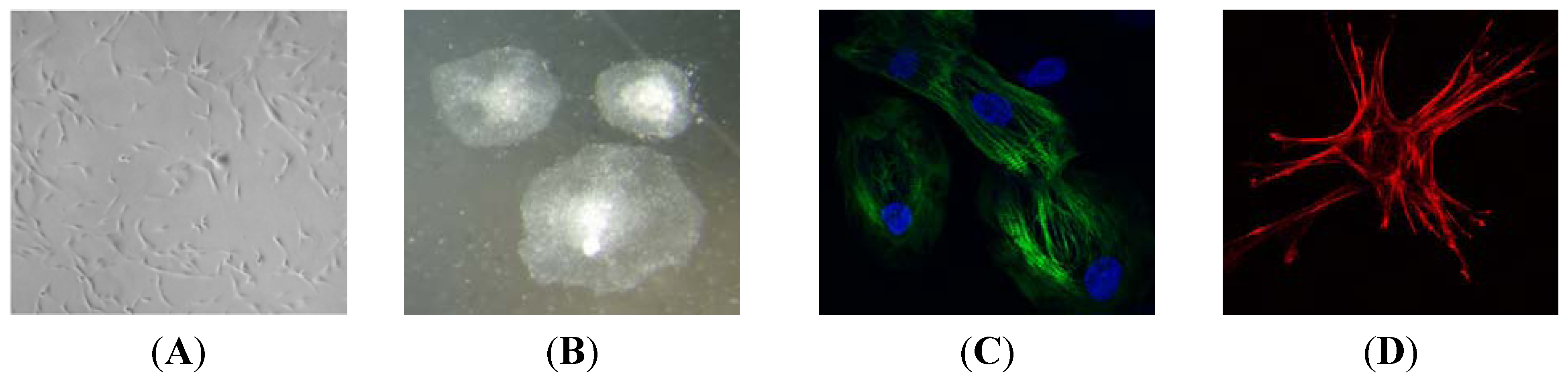
Bioengineering Heart Muscle Using iPS Cells
3. Conclusions
Author Contributions
Conflicts of Interest
References
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation 2014, 129, e28–e292. [Google Scholar] [CrossRef] [PubMed]
- D’Udekem, Y.; Iyengar, A.J.; Galati, J.C.; Forsdick, V.; Weintraub, R.G.; Wheaton, G.R.; Bullock, A.; Justo, R.N.; Grigg, L.E.; Sholler, G.F.; et al. Redefining expectations of long-term survival after the Fontan procedure: Twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation 2014, 130, S32–S38. [Google Scholar] [CrossRef]
- Leggat, S. Childhood Heart Disease in Australia: Current Practices and Future Needs; HeartKids Australia: Pennant Hills, NSW, Australia, 2011; pp. 1–52. [Google Scholar]
- Hoffman, J.I.E.; Kaplan, S. The incidence of congenital heart disease. JAC 2002, 39, 1890–1900. [Google Scholar]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef] [PubMed]
- Tutarel, O. Acquired heart conditions in adults with congenital heart disease: A growing problem. Heart 2014, 100, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, J.W. The encephalopathy of congenital heart disease. J. Thorac. Cardiovasc. Surg. 2014, 148, 1790–1791. [Google Scholar] [CrossRef] [PubMed]
- Masoller, N.; Martínez, J.M.; Gómez, O.; Bennasar, M.; Crispi, F.; Sanz-Cortés, M.; Egaña-Ugrinovic, G.; Bartrons, J.; Puerto, B.; Gratacós, E. Evidence of second-trimester changes in head biometry and brain perfusion in fetuses with congenital heart disease. Ultrasound Obstet. Gynecol. 2014, 44, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Kasparian, N.A.; Fidock, B.; Sholler, G.F.; Camphausen, C.; Murphy, D.N.; Cooper, S.G.; Kaul, R.; Jones, O.; Winlaw, D.S.; Kirk, E.P.E. Parents’ perceptions of genetics services for congenital heart disease: The role of demographic, clinical, and psychological factors in determining service attendance. Genet. Med. 2014, 16, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Blue, G.M.; Kirk, E.P.; Sholler, G.F.; Harvey, R.P.; Winlaw, D.S. Congenital heart disease: Current knowledge about causes and inheritance. Med. J. Aust. 2012, 197, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Cordell, H.J.; Bentham, J.; Topf, A.; Zelenika, D.; Heath, S.; Mamasoula, C.; Cosgrove, C.; Blue, G.; Granados-Riveron, J.; Setchfield, K.; et al. Genome-wide association study of multiple congenital heart disease phenotypes identifies a susceptibility locus for atrial septal defect at chromosome 4p16. Nat. Genet. 2013, 45, 822–824. [Google Scholar] [CrossRef]
- Zaidi, S.; Choi, M.; Wakimoto, H.; Ma, L.; Jiang, J.; Overton, J.D.; Romano-Adesman, A.; Bjornson, R.D.; Breitbart, R.E.; Brown, K.K.; et al. De novo mutations in histone-modifying genes in congenital heart disease. Nature 2013, 498, 220–223. [Google Scholar] [CrossRef]
- Blue, G.M.; Kirk, E.P.; Giannoulatou, E.; Dunwoodie, S.L.; Ho, J.W.K.; Hilton, D.C.K.; White, S.M.; Sholler, G.F.; Harvey, R.P.; Winlaw, D.S. Targeted next-generation sequencing identifies pathogenic variants in familial congenital heart disease. J. Am. Coll. Cardiol. 2014, 64, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Cebotari, S.; Lichtenberg, A.; Tudorache, I.; Hilfiker, A.; Mertsching, H.; Leyh, R.; Breymann, T.; Kallenbach, K.; Maniuc, L.; Batrinac, A.; et al. Clinical application of tissue engineered human heart valves using autologous progenitor cells. Circulation 2006, 114, I132–I137. [Google Scholar] [CrossRef] [PubMed]
- Dijkman, P.E.; Driessen-Mol, A.; Frese, L.; Hoerstrup, S.P.; Baaijens, F.P.T. Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials 2012, 33, 4545–4554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrée, B.; Bela, K.; Horvath, T.; Lux, M.; Ramm, R.; Venturini, L.; Ciubotaru, A.; Zweigerdt, R.; Haverich, A.; Hilfiker, A. Successful re-endothelialization of a perfusable biological vascularized matrix (BioVaM) for the generation of 3D artificial cardiac tissue. Basic Res. Cardiol. 2014, 109. [Google Scholar] [CrossRef]
- Udelsman, B.V.; Maxfield, M.W.; Breuer, C.K. Tissue engineering of blood vessels in cardiovascular disease: Moving towards clinical translation. Heart 2013, 99, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Kurobe, H.; Maxfield, M.W.; Breuer, C.K.; Shinoka, T. Concise review: Tissue-engineered vascular grafts for cardiac surgery: Past, present, and future. Stem Cells Transl. Med. 2012, 1, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, N.; Annabi, N.; Assmann, A.; Larson, B.L.; Hjortnaes, J.; Alemdar, N.; Kharaziha, M.; Manning, K.B.; Mayer, J.E.; Khademhosseini, A. Tri-layered elastomeric scaffolds for engineering heart valve leaflets. Biomaterials 2014, 35, 7774–7785. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Emmert, M.; Hoerstrup, S. Stem cells for heart valve regeneration. Swiss Med. Wkly. 2012, 142. [Google Scholar] [CrossRef] [Green Version]
- Simpson, D.L.; Wehman, B.; Galat, Y.; Sharma, S.; Mishra, R.; Galat, V.; Kaushal, S. Engineering patient-specific valves using stem cells generated from skin biopsy specimens. Ann. Thorac. Surg. 2014, 98, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.J.H.; Forte, E.; Harvey, R.P. Developmental origins and lineage descendants of endogenous adult cardiac progenitor cells. Stem Cell Res. 2014, 13, 592–614. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, K.; Makkar, R.R.; Smith, R.R.; Cheng, K.; Wu, E.; Bonow, R.O.; Marbán, L.; Mendizabal, A.; Cingolani, E.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells after myocardial infarction: Evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). JAC 2014, 63, 110–122. [Google Scholar]
- Piepoli, M.F.; Vallisa, D.; Arbasi, C.; Cavanna, L.; Cerri, L.; Mori, M.; Passerini, F.; Tommasi, L.; Rossi, A.; Capucci, A. Two year follow-up results of the CARDIAC (CARDIomyoplasty by Autologous intraCoronary bone marrow in acute myocardial infarction) randomised controlled trial. Int. J. Cardiol. 2013, 168, e132. [Google Scholar] [CrossRef] [PubMed]
- Vrtovec, B.; Poglajen, G.; Lezaic, L.; Sever, M.; Domanovic, D.; Cernelc, P.; Socan, A.; Schrepfer, S.; Torre-Amione, G.; Haddad, F.; et al. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ. Res. 2013, 112, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Tchervenkov, C.I.; Jacobs, J.P.; Weinberg, P.M.; Aiello, V.D.; Béland, M.J.; Colan, S.D.; Elliott, M.J.; Franklin, R.C.G.; Gaynor, J.W.; Krogmann, O.N.; et al. The nomenclature, definition and classification of hypoplastic left heart syndrome. CTY 2006, 16, 339–368. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Raffel, D.M.; Shulkin, B.L.; Corbett, J.R.; Bove, E.L.; Mosca, R.S.; Kulik, T.J. Resting coronary flow and coronary flow reserve in human infants after repair or palliation of congenital heart defects as measured by positron emission tomography. J. Thorac. Cardiovasc. Surg. 1998, 115, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Salih, C.; McCarthy, K.P.; Ho, S.Y. The fibrous matrix of ventricular myocardium in hypoplastic left heart syndrome: A quantitative and qualitative analysis. Ann. Thorac. Surg. 2004, 77, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Yerebakan, C.; Sandica, E.; Prietz, S.; Klopsch, C.; Ugurlucan, M.; Kaminski, A.; Abdija, S.; Lorenzen, B.; Boltze, J.; Nitzsche, B.; et al. Autologous umbilical cord blood mononuclear cell transplantation preserves right ventricular function in a novel model of chronic right ventricular volume overload. Cell Transpl. 2009, 18, 855–868. [Google Scholar] [CrossRef]
- Davies, B.; Elwood, N.J.; Li, S.; Cullinane, F.; Edwards, G.A.; Newgreen, D.F.; Brizard, C.P. Human cord blood stem cells enhance neonatal right ventricular function in an ovine model of right ventricular training. Ann. Thorac. Surg. 2010, 89, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Tarui, S.; Sano, S.; Oh, H. Stem cell therapies in patients with single ventricle physiology. Methodist Debakey Cardiovasc. J. 2014, 10, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R.; Chugh, A.R.; D’Amario, D.; Loughran, J.H.; Stoddard, M.F.; Ikram, S.; Beache, G.M.; Wagner, S.G.; Leri, A.; Hosoda, T.; et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet 2011, 378, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.J.; Berman, D.; Czer, L.S.C.; Marbán, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, H.M.; Qureshi, M.Y.; Peral, S.C.; O’Leary, P.W.; Olson, T.M.; Cetta, F.; Nelson, T.J.; The Wanek Program Clinical Pipeline Group. Regenerative therapy for hypoplastic left heart syndrome: First report of intraoperative intramyocardial injection of autologous umbilical-cord blood-derived cells. J. Thorac. Cardiovasc. Surg. 2014, 149, e35–e37. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, S.; Ohtsuki, S.; Tarui, S.; Ousaka, D.; Eitoku, T.; Kondo, M.; Okuyama, M.; Kobayashi, J.; Baba, K.; Arai, S.; et al. Intracoronary autologous cardiac progenitor cell transfer in patients with hypoplastic left heart syndrome: The TICAP prospective phase 1 controlled trial. Circ. Res. 2015, 116, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-S.; Cheng, K.; Malliaras, K.; Smith, R.R.; Zhang, Y.; Sun, B.; Matsushita, N.; Blusztajn, A.; Terrovitis, J.; Kusuoka, H.; et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J. Am. Coll. Cardiol. 2012, 59, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Rupp, S.; Jux, C.; Bönig, H.; Bauer, J.; Tonn, T.; Seifried, E.; Dimmeler, S.; Zeiher, A.M.; Schranz, D. Intracoronary bone marrow cell application for terminal heart failure in children. CTY 2012, 22, 558–563. [Google Scholar] [CrossRef]
- Rupp, S.; Bauer, J.; Tonn, T.; Schächinger, V.; Dimmeler, S.; Zeiher, A.M.; Schranz, D. Intracoronary administration of autologous bone marrow-derived progenitor cells in a critically ill two-yr-old child with dilated cardiomyopathy. Pediatr. Transpl. 2009, 13, 620–623. [Google Scholar] [CrossRef]
- Rupp, S.; Zeiher, A.M.; Dimmeler, S.; Tonn, T.; Bauer, J.; Jux, C.; Akintuerk, H.; Schranz, D. A regenerative strategy for heart failure in hypoplastic left heart syndrome: Intracoronary administration of autologous bone marrow-derived progenitor cells. J. Heart Lung Transplant. 2010, 29, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Kattman, S.J.; Witty, A.D.; Gagliardi, M.; Dubois, N.C.; Niapour, M.; Hotta, A.; Ellis, J.; Keller, G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 2011, 8, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Witty, A.D.; Mihic, A.; Tam, R.Y.; Fisher, S.A.; Mikryukov, A.; Shoichet, M.S.; Li, R.-K.; Kattman, S.J.; Keller, G. Generation of the epicardial lineage from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernandez, A.; Nelson, T.J.; Reyes, S.; Alekseev, A.E.; Secreto, F.; Perez-Terzic, C.; Beraldi, R.; Sung, H.-K.; Nagy, A.; Terzic, A. iPS cell-derived cardiogenicity is hindered by sustained integration of reprogramming transgenes. Circ. Cardiovasc. Genet 2014, 7, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Addis, R.C.; Epstein, J.A. Induced regeneration—the progress and promise of direct reprogramming for heart repair. Nat. Publ. Group 2013, 19, 829–836. [Google Scholar]
- Lin, B.; Kim, J.; Li, Y.; Pan, H.; Carvajal-Vergara, X.; Salama, G.; Cheng, T.; Li, Y.; Lo, C.W.; Yang, L. High-purity enrichment of functional cardiovascular cells from human iPS cells. Cardiovasc. Res. 2012, 95, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Vergara, X.; Sevilla, A.; D’Souza, S.L.; Ang, Y.-S.; Schaniel, C.; Lee, D.-F.; Yang, L.; Kaplan, A.D.; Adler, E.D.; Rozov, R.; et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature 2010, 465, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.M.; Wolvetang, E.; Mackay-Sim, A. Induced pluripotent stem cells: A new technology to study human diseases. Int. J. Biochem. Cell Biol. 2011, 43, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Marceau, C.; Hamaguchi, R.; Burridge, P.W.; Rajarajan, K.; Churko, J.M.; Wu, H.; Sallam, K.I.; Matsa, E.; Sturzu, A.C.; et al. Human induced pluripotent stem cell-derived cardiomyocytes as an in vitro model for coxsackievirus B3-induced myocarditis and antiviral drug screening platform. Circ. Res. 2014, 115, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Hirt, M.N.; Boeddinghaus, J.; Mitchell, A.; Schaaf, S.; Börnchen, C.; Müller, C.; Schulz, H.; Hubner, N.; Stenzig, J.; Stoehr, A.; et al. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J. Mol. Cell. Cardiol. 2014, 74, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Hirt, M.N.; Hansen, A.; Eschenhagen, T. Cardiac tissue engineering: State of the art. Circ. Res. 2014, 114, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.; Temple, S. Stem cells for retinal repair. Dev. Ophthalmol. 2014, 53, 70–80. [Google Scholar] [PubMed]
- Takasato, M.; Maier, B.; Little, M.H. Recreating kidney progenitors from pluripotent cells. Pediatr. Nephrol. 2014, 29, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Habibollah, S.; Tilgner, K.; Collin, J.; Barta, T.; Al-Aama, J.Y.; Tesarov, L.; Hussain, R.; Trafford, A.W.; Kirkwood, G.; et al. An induced pluripotent stem cell model of hypoplastic left heart syndrome (HLHS) reveals multiple expression and functional differences in HLHS-derived cardiac myocytes. Stem Cells Transl. Med. 2014, 3, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, J.; Yoshida, M.; Tarui, S.; Hirata, M.; Nagai, Y.; Kasahara, S.; Naruse, K.; Ito, H.; Sano, S.; Oh, H. Directed differentiation of patient-specific induced pluripotent stem cells identifies the transcriptional repression and epigenetic modification of NKX2-5, HAND1, and NOTCH1 in hypoplastic left heart syndrome. PLoS ONE 2014, 9, e102796. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Francis, R.; Kim, A.J.; Ramirez, R.; Chen, G.; Subramanian, R.; Anderton, S.; Kim, Y.; Wong, L.; Morgan, J.; et al. Interrogating congenital heart defects with noninvasive fetal echocardiography in a mouse forward genetic screen. Circ. Cardiovasc. Imaging 2014, 7, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, J.A.; Benson, D.W.; Dubin, A.M.; Cohen, M.S.; Maxey, D.M.; Mahle, W.T.; Pahl, E.; Villafañe, J.; Bhatt, A.B.; Peng, L.F.; et al. Hypoplastic left heart syndrome: Current considerations and expectations. J. Am. Coll. Cardiol. 2012, 59, S1–S42. [Google Scholar] [CrossRef] [PubMed]
- Hinton, R.B., Jr.; Martin, L.J.; Tabangin, M.E.; Mazwi, M.L.; Cripe, L.H.; Benson, D.W. Hypoplastic left heart syndrome is heritable. J. Am. Coll. Cardiol. 2007, 50, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.A.; Kirk, E.P.; Yeoh, T.; Chandar, S.; McKenzie, F.; Taylor, P.; Grossfeld, P.; Fatkin, D.; Jones, O.; Hayes, P.; et al. Cardiac homeobox gene NKX2-5 mutations and congenital heart disease: Associations with atrial septal defect and hypoplastic left heart syndrome. JAC 2003, 41, 2072–2076. [Google Scholar]
- Iascone, M.; Ciccone, R.; Galletti, L.; Marchetti, D.; Seddio, F.; Lincesso, A.R.; Pezzoli, L.; Vetro, A.; Barachetti, D.; Boni, L.; et al. Identification of de novo mutations and rare variants in hypoplastic left heart syndrome. Clin. Genet. 2012, 81, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Freylikhman, O.; Tatarinova, T.; Smolina, N.; Zhuk, S.; Klyushina, A.; Kiselev, A.; Moiseeva, O.; Sjoberg, G.; Malashicheva, A.; Kostareva, A. Variants in the NOTCH1 gene in patients with aortic coarctation. Congenit. Heart Dis. 2014, 9, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Muth, A.N.; Ransom, J.F.; Schluterman, M.K.; Barnes, R.; King, I.N.; Grossfeld, P.D.; Srivastava, D. Mutations in NOTCH1 cause aortic valve disease. Nature 2005, 437, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Hinton, R.B.; Martin, L.J.; Rame-Gowda, S.; Tabangin, M.E.; Cripe, L.H.; Benson, D.W. Hypoplastic left heart syndrome links to chromosomes 10q and 6q and is genetically related to bicuspid aortic valve. J. Am. Coll. Cardiol. 2009, 53, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, C.; Chang, W.Y.; Khattak, S.; Hinek, A.; Thompson, T.; de Carvalho Rodrigues, D.; Kennedy, K.; Mahmut, N.; Pasceri, P.; Stanford, W.L.; et al. Modeling and rescue of the vascular phenotype of Williams-Beuren syndrome in patient induced pluripotent stem cells. Stem Cells Transl. Med. 2013, 2, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Carrier, R.L.; Papadaki, M.; Rupnick, M.; Schoen, F.J.; Bursac, N.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Cardiac tissue engineering: Cell seeding, cultivation parameters, and tissue construct characterization. Biotechnol. Bioeng. 1999, 64, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Yamato, M.; Akutsu, T.; Shibata, T.; Isoi, Y.; Kikuchi, A.; Umezu, M.; Okano, T. Electrically communicating three-dimensional cardiac tissue mimic fabricated by layered cultured cardiomyocyte sheets. J. Biomed. Mater. Res. 2002, 60, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, W.-H.; Schneiderbanger, K.; Schubert, P.; Didié, M.; Münzel, F.; Heubach, J.F.; Kostin, S.; Neuhuber, W.L.; Eschenhagen, T. Tissue engineering of a differentiated cardiac muscle construct. Circ. Res. 2002, 90, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.; Shachar, M.; Leor, J.; Cohen, S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol. Bioeng. 2002, 80, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, K.L.K.; Bajpai, V.K.; Andreadis, S.T.; Murry, C.E. Heart regeneration with engineered myocardial tissue. Annu. Rev. Biomed. Eng. 2014, 16, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Dilley, R.J.; Morrison, W.A. Vascularisation to improve translational potential of tissue engineering systems for cardiac repair. Int. J. Biochem. Cell Biol. 2014, 56, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Morritt, A.N.; Bortolotto, S.K.; Dilley, R.J.; Han, X.; Kompa, A.R.; McCombe, D.; Wright, C.E.; Itescu, S.; Angus, J.A.; Morrison, W.A. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation 2007, 115, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Miyagawa, S.; Miki, K.; Saito, A.; Fukushima, S.; Higuchi, T.; Kawamura, T.; Kuratani, T.; Daimon, T.; Shimizu, T.; et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 2012, 126, S29–S37. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.J.H.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.-W.; Weyers, J.J.; Mahoney, W.M.; van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Sivakumaran, P.; Crombie, D.E.; Dusting, G.J.; Pébay, A.; Dilley, R.J. Trichostatin A enhances differentiation of human induced pluripotent stem cells to cardiogenic cells for cardiac tissue engineering. Stem Cells Transl. Med. 2013, 2, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Miyagawa, S.; Fukushima, S.; Saito, A.; Miki, K.; Ito, E.; Sougawa, N.; Kawamura, T.; Daimon, T.; Shimizu, T.; et al. Enhanced survival of transplanted human induced pluripotent stem cell-derived cardiomyocytes by the combination of cell sheets with the pedicled omental flap technique in a porcine heart. Circulation 2013, 128, S87–S94. [Google Scholar] [CrossRef] [PubMed]
- Emmert, M.Y.; Wolint, P.; Wickboldt, N.; Gemayel, G.; Weber, B.; Brokopp, C.E.; Boni, A.; Falk, V.; Bosman, A.; Jaconi, M.E.; et al. Human stem cell-based three-dimensional microtissues for advanced cardiac cell therapies. Biomaterials 2013, 34, 6339–6354. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.-Y.; Lin, B.; Kim, J.; Sullivan, M.; Tobita, K.; Salama, G.; Yang, L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat. Commun. 2013, 4, 2307. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosman, A.; Edel, M.J.; Blue, G.; Dilley, R.J.; Harvey, R.P.; Winlaw, D.S. Bioengineering and Stem Cell Technology in the Treatment of Congenital Heart Disease. J. Clin. Med. 2015, 4, 768-781. https://doi.org/10.3390/jcm4040768
Bosman A, Edel MJ, Blue G, Dilley RJ, Harvey RP, Winlaw DS. Bioengineering and Stem Cell Technology in the Treatment of Congenital Heart Disease. Journal of Clinical Medicine. 2015; 4(4):768-781. https://doi.org/10.3390/jcm4040768
Chicago/Turabian StyleBosman, Alexis, Michael J. Edel, Gillian Blue, Rodney J. Dilley, Richard P. Harvey, and David S. Winlaw. 2015. "Bioengineering and Stem Cell Technology in the Treatment of Congenital Heart Disease" Journal of Clinical Medicine 4, no. 4: 768-781. https://doi.org/10.3390/jcm4040768
APA StyleBosman, A., Edel, M. J., Blue, G., Dilley, R. J., Harvey, R. P., & Winlaw, D. S. (2015). Bioengineering and Stem Cell Technology in the Treatment of Congenital Heart Disease. Journal of Clinical Medicine, 4(4), 768-781. https://doi.org/10.3390/jcm4040768





