Preferences for Prenatal Tests for Cystic Fibrosis: A Discrete Choice Experiment to Compare the Views of Adult Patients, Carriers of Cystic Fibrosis and Health Professionals
Abstract
:1. Introduction
2. Methods
2.1. Ethical Approval
2.2. Recruitment of Participants and Data Collection
2.3. Questionnaire Design
2.4. Analysis
3. Results
3.1. Participants
3.2. Regression Results
| Attributes | Service users (n = 142) | Health professionals (n = 70) | Difference | ||
|---|---|---|---|---|---|
| Coefficient (95% CI) a | p-Value | Coefficient (95% CI) b | p-Value | p-Value | |
| Accuracy | 0.160 (0.126 to 0.194) | <0.0001 | 0.378 (0.318 to 0.438) | <0.0001 | <0.0001 |
| Time of results | −0.108 (−0.149 to −0.067) | <0.0001 | −0.233 (−0.299 to −0.166) | <0.0001 | 0.0016 |
| No miscarriage risk | 1.960 (1.751 to 2.170) | <0.0001 | 0.938 (0.639 to 1.238) | <0.0001 | <0.0001 |
| Attribute | Service user groups | Affected with CF: gender | ||||
|---|---|---|---|---|---|---|
| Affected with CF a (n = 92) | Carrier of CF b (n = 50) | Difference (p-Value) | Females c (n = 42) | Males d (n = 50) | Difference (p-Value) | |
| Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | |||
| Accuracy | 0.154 (0.111 to 0.197) | 0.169 (0.113 to 0.224) | 0.6879 | 0.136 (0.0825 to 0.190) | 0.182 (0.119 to 0.245) | 0.2751 |
| Time of results | −0.113 (−0.167 to −0.060) | −0.102 (−0.166 to 0.037) * | 0.7890 | −0.094 (−0.167 to −0.021) * | −0.137 (−0.218 to −0.056) * | 0.4390 |
| No miscarriage risk | 2.149 (1.873 to 2.425) | 1.698 (1.373 to 2.024) | 0.0355 | 1.849 (1.507 to 2.191) | 2.512 (2.099 to 2.926) | 0.0154 |
3.3. Marginal Rates of Substitution
| Attribute | Number of weeks that respondents are prepared to wait | Reduction in accuracy (%) that respondents are prepared to accept | ||
|---|---|---|---|---|
| Service users | Health professionals | Service users | Health professionals | |
| Test with no risk of miscarriage | 18.15 (1.960/−0.108) | 4.03 (0.938/−0.233) | 12.25 (1.960/0.160) | 2.48 (0.938/0.378) |
| Test with 5% greater accuracy | 7.41 (0.160/−0.108 × 5) | 8.11 (0.378/−0.233 × 5) | - | - |
| Early test | - | - | 0.68 (−0.108/0.160) | 0.62 (−0.233/0.378) |
3.4. Ranking of Attributes
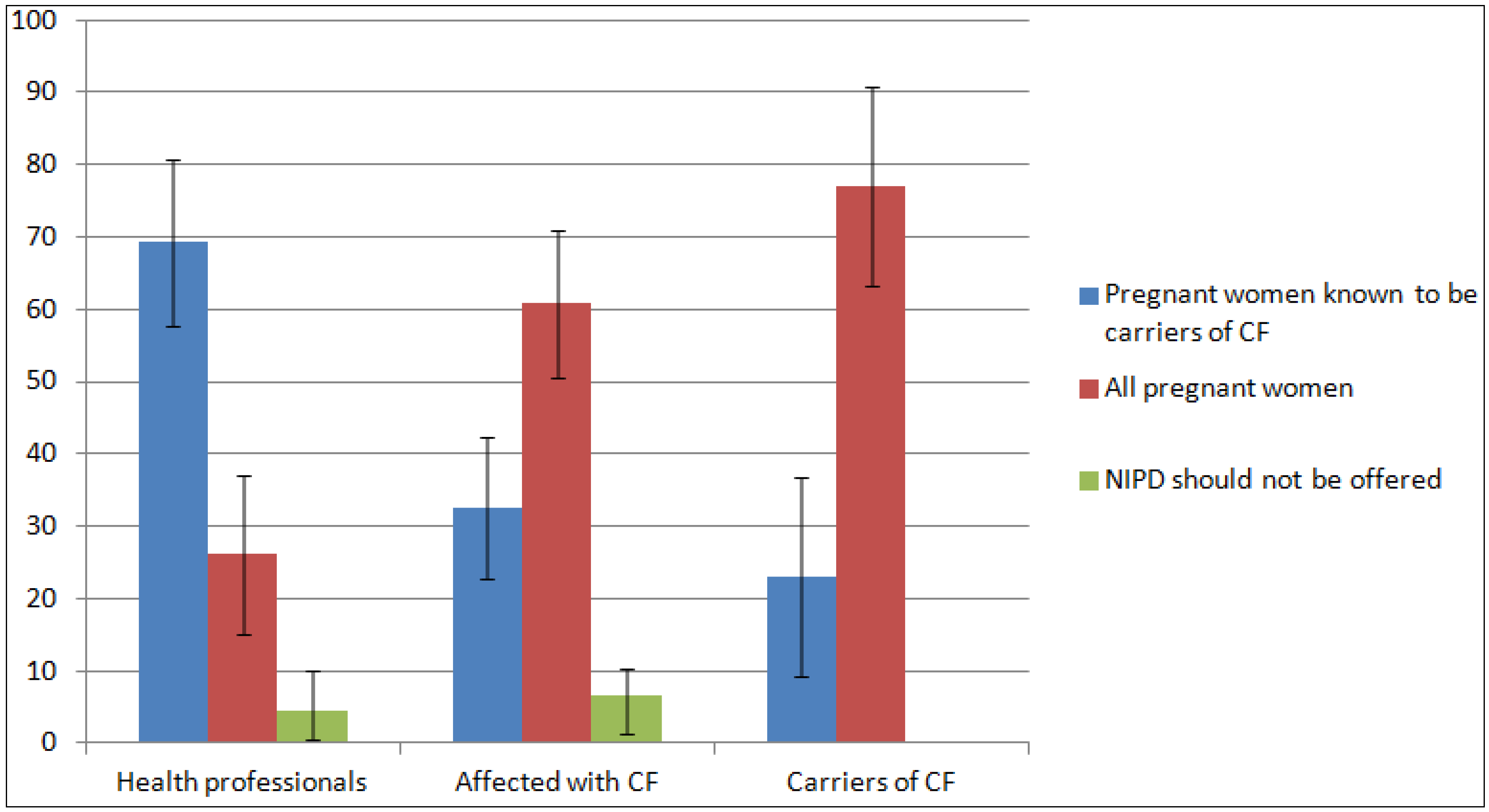
3.5. Views on Prenatal Testing and the Introduction of NIPD
| Questions regarding uptake of prenatal testing | Total (n = 142) | Affected with CF (n = 92) | Carrier of CF (n = 50) | p-Value |
|---|---|---|---|---|
| Have had/likely to have an invasive test for CF | 0.996 | |||
| Strongly Agree/Agree | 57 (43.5%) | 37 (43.5%) | 20 (43.5%) | |
| Strongly Disagree/Disagree | 74 (56.4%) | 48 (56.5%) | 26 (56.5%) | |
| Reason for choosing to have a diagnostic test | 0.288 | |||
| To plan and prepare for the possible birth of a baby with CF | 33 (62.3%) | 22 (66.7%) | 11 (55.0%) | |
| To help make a decision about whether or not to continue the pregnancy | 17 (32.1%) | 9 (27.3%) | 8 (40.0%) | |
| Reason for choosing to have a diagnostic test | 0.288 | |||
| Because my family or my partner would want me to | 2 (3.8%) | 2 (6.0%) | 0 (0%) | |
| Because it is offered as part of the antenatal service | 1 (1.9%) | 0 (0%) | 1 (5.0%) | |
| Other | 0 (0%) | 0 (0%) | 0 (0%) | |
| Would never have an invasive test because would not consider termination of pregnancy | 0.447 | |||
| Strongly Agree/Agree | 63 (47.0%) | 43 (49.4%) | 20 (42.5%) | |
| Strongly Disagree/Disagree | 71 (53.0%) | 44 (50.5%) | 27 (57.4%) | |
| Would never have an invasive test because of the risk of miscarriage | 0.466 | |||
| Strongly Agree/Agree | 71 (56.4%) | 52 (58.4%) | 19 (51.3%) | |
| Strongly Disagree/Disagree | 55 (43.7%) | 37 (41.6%) | 18 (48.6%) | |
| Would have NIPD if available | 0.252 | |||
| Strongly Agree/Agree | 130 (94.9%) | 84 (93.3%) | 46 (97.9%) | |
| Strongly Disagree/Disagree | 7 (5.1%) | 6 (6.7%) | 1 (2.1%) | |
| Willingness to pay for NIPD | 0.126 | |||
| ≤£50 | 56 (41.2%) | 32 (36.0%) | 24 (51.1%) | |
| £100–200 | 53 (39.0%) | 40 (44.9%) | 13 (27.7%) | |
| ≥£200 | 14 (10.3%) | 6 (6.7%) | 8 (17.0%) | |
| Not prepared to pay | 13 (9.6%) | 11 (12.4%) | 2 (4.3%) |
| Questions regarding pressure to have prenatal testing | Total service users (n = 142) | Affected with CF (n = 92) | Carriers of CF (n = 50) | Health professionals (n = 70) | p-Value |
|---|---|---|---|---|---|
| There is pressure on women at risk of having a child with CF to have a diagnostic test in pregnancy | 0.075 | ||||
| Strongly Agree/Agree | 54 (39.4%) | 38 (43.2%) | 16 (32.7%) | 17 (25.8%) | |
| Strongly Disagree/Disagree | 83 (60.6%) | 50 (56.8%) | 33 (67.3%) | 49 (74.2%) | |
| If you agree, where do you think this pressure comes from * | |||||
| Partner | 27 (26.7%) | 21 (27.6%) | 6 (24.0%) | 15 (32.6%) | |
| Family members | 29 (28.7%) | 22 (28.9%) | 7 (28.0%) | 13 (28.3%) | |
| Health professionals | 24 (23.8%) | 20 (26.3%) | 4 (16.0%) | 5 (10.9%) | |
| Society in general | 14 (13.9%) | 8 (10.5%) | 6 (24.0%) | 6 (13.0%) | |
| Your cultural or religious community | 1 (1.0%) | 0 (0%) | 1 (4.0%) | 7 (15.2%) | |
| Other | 6 (5.9%) | 5 (6.6%) | 1 (4.0%) | 0 (0%) | |
| The availability of NIPD will increase the pressure to have prenatal testing | 0.088 | ||||
| Strongly Agree/Agree | 52 (42.6%) | 40 (46.5%) | 12 (33.3%) | 37 (56.1%) | |
| Strongly Disagree/Disagree | 70 (57.4%) | 46 (53.5%) | 24 (66.7%) | 29 (43.9%) |
4. Discussion
Limitations
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Conflicts of Interest
References
- Southern, K.W.; Munck, A.; Pollitt, R.; Travert, G.; Zanolla, L.; Dankert-Roelse, J.; Castellani, C. A survey of newborn screening for cystic fibrosis in Europe. J. Cyst. Fibros. 2007, 6, 57–65. [Google Scholar] [CrossRef]
- O’Sullivan, B.P.; Freedman, S.D. Cystic fibrosis. Lancet 2009, 373, 1891–1904. [Google Scholar] [CrossRef]
- Rowe, S.M.; Miller, S.; Sorscher, E.J. Cystic fibrosis. N. Engl. J. Med. 2005, 352, 1992–2001. [Google Scholar] [CrossRef]
- UK CF Registry. Annual Data Report 2012; UK Cystic Fibrosis Trust: Bromley, UK, 2013. [Google Scholar]
- Henneman, L.; Bramsen, I.; van Os, T.A.; Reuling, I.E.; Heyerman, H.G.; van der Laag, J.; van der Ploeg, H.M.; ten Kate, L.P. Attitudes towards reproductive issues and carrier testing among adult patients and parents of children with cystic fibrosis (CF). Prenat. Diagn. 2001, 21, 1–9. [Google Scholar] [CrossRef]
- Tabor, A.; Alfirevic, Z. Update on procedure-related risks for prenatal diagnosis techniques. Fetal Diagn. Ther. 2010, 27, 1–7. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, M.C.; Garcia-Hoyos, M.; Trujillo, M.J.; Rodriguez de Alba, M.; Lorda-Sanchez, I.; Diaz-Recasens, J.; Gallardo, E.; Ayuso, C.; Ramos, C. Prenatal detection of a cystic fibrosis mutation in fetal DNA from maternal plasma. Prenat. Diagn. 2002, 22, 946–948. [Google Scholar] [CrossRef]
- Bustamante-Aragones, A.; Gallego-Merlo, J.; Trujillo-Tiebas, M.J.; de Alba, M.R.; Gonzalez-Gonzalez, C.; Glover, G.; Diego-Alvarez, D.; Ayuso, C.; Ramos, C. New strategy for the prenatal detection/exclusion of paternal cystic fibrosis mutations in maternal plasma. J. Cyst. Fibros. 2008, 7, 505–510. [Google Scholar] [CrossRef]
- Nasis, O.; Thompson, S.; Hong, T.; Sherwood, M.; Radcliffe, S.; Jackson, L.; Otevrel, T. Improvement in sensitivity of allele-specific PCR facilitates reliable noninvasive prenatal detection of cystic fibrosis. Clin. Chem. 2004, 50, 694–701. [Google Scholar]
- Lench, N.; Barrett, A.; Fielding, S.; McKay, F.; Hill, M.; Jenkins, L.; White, H.; Chitty, L. The clinical implementation of non-invasive prenatal diagnosis for single gene disorders: Challenges and progress made. Prenat. Diagn. 2013, 33, 555–562. [Google Scholar] [CrossRef]
- De Bekker-Grob, E.W.; Ryan, M.; Gerard, K. Discrete choice experiments in health economics: A review of the literature. Health Econ. 2012, 21, 145–172. [Google Scholar] [CrossRef]
- Ryan, M.; Gerard, K.; Amaya-Amaya, M. Using Discrete Choice Experiments to Value Health and Health Care; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Bishop, A.; Marteau, T.; Armstrong, D.; Chitty, L.; Longworth, L.; Buxton, M.; Berlin, C. Women and health professional’s preferences for Down’s syndrome screening tests: A conjoint analysis study. BJOG 2004, 111, 775–779. [Google Scholar] [CrossRef]
- Lewis, S.M.; Cullinane, F.M.; Carlin, J.B.; Halliday, J.L.; Lewis, S.M.; Cullinane, F.M.; Carlin, J.B.; Halliday, J.L. Women’s and health professionals’ preferences for prenatal testing for Down syndrome in Australia. Aust. N. Z. J. Obstet. Gynaecol. 2006, 46, 205–211. [Google Scholar] [CrossRef]
- Lewis, S.M.; Cullinane, F.N.; Bishop, A.J.; Chitty, L.S.; Marteau, T.M.; Halliday, J.L. A comparison of Australian and UK obstetricians’ and midwives’ preferences for screening tests for Down syndrome. Prenat. Diagn. 2006, 26, 60–66. [Google Scholar] [CrossRef]
- Ryan, M.; Diack, J.; Watson, V.; Smith, N. Rapid prenatal diagnostic testing for Down syndrome only or longer wait for full karyotype: The views of pregnant women. Prenat. Diagn. 2005, 25, 1206–1211. [Google Scholar] [CrossRef]
- Chan, Y.M.; Sahota, D.S.; Leung, T.Y.; Choy, K.W.; Chan, O.K.; Lau, T.K. Chinese women’s preferences for prenatal diagnostic procedure and their willingness to trade between procedures. Prenat. Diagn. 2009, 29, 1270–1276. [Google Scholar] [CrossRef]
- Hill, M.; Fisher, J.; Chitty, L.S.; Morris, S. Women’s and health professionals’ preferences for prenatal tests for Down syndrome: A discrete choice experiment to contrast noninvasive prenatal diagnosis with current invasive tests. Genet. Med. 2012, 14, 905–913. [Google Scholar] [CrossRef]
- Hill, M.; Compton, C.; Karunaratna, M.; Lewis, C.; Chitty, L.S. Client views and attitudes to non-invasive prenatal diagnosis for sickle cell disease, thalassaemia and cystic fibrosis. Submitted for publication. 2013. [Google Scholar]
- Hill, M.; Karunaratna, M.; Lewis, C.; Forya, F.; Chitty, L. Views and preferences for the implementation of non-invasive prenatal diagnosis for single gene disorders from health professionals in the United Kingdom. Am. J. Med. Genet. 2013, 161, 1612–1618. [Google Scholar] [CrossRef]
- Lancsar, E.; Louviere, J. Conducting discrete choice experiments to inform healthcare decision making: A user’s guide. Pharmacoeconomics 2008, 26, 661–677. [Google Scholar] [CrossRef]
- Bridges, J.F.; Hauber, A.B.; Marshall, D.; Lloyd, A.; Prosser, L.A.; Regier, D.A.; Johnson, F.R.; Mauskopf, J. Conjoint analysis applications in health—A checklist: A report of the ISPOR good research practices for conjoint analysis task force. Value Health 2011, 14, 403–413. [Google Scholar] [CrossRef]
- Street, D.; Burgess, L.; Viney, R.; Louviere, J. Designing Discete Choice Experiments for Health Care. In Using Discrete Choice Experiments to Value Health and Health Care; Ryan, M., Gerard, K., Amaya-Amaya, M., Eds.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Hahn, G.; Shapiro, S. A Catalogue and Computer Program for the Design and Analysis of Orthoganol Symmetric and Asymmetric Fractional Factorial Experiments; General Electric Research and Development Centre: Schenectady, NY, USA, 1966. [Google Scholar]
- McFadden, D. Conditional Logit Analysis of Qualitative Choice Behavior. In Frontiers in Econometrics; Zarembka, P., Ed.; Academic Press: New York, NY, USA, 1974; pp. 105–142. [Google Scholar]
- Haaijer, R.; Kamakura, W.; Wedel, M. The “no-choice” alternative in conjoint choice experiments. Int. J. Mark. Res. 2001, 43, 93–106. [Google Scholar]
- Stata Statistical Software; release 10; StataCorp LP: College Station, TX, USA, 2007.
- Denayer, L.; Welkenhuysen, M.; Evers-Kiebooms, G.; Cassiman, J.J.; van den Berghe, H. Risk perception after CF carrier testing and impact of the test result on reproductive decision making. Am. J. Med. Genet. 1997, 69, 422–428. [Google Scholar] [CrossRef]
- Sawyer, S.M.; Cerritelli, B.; Carter, L.S.; Cooke, M.; Glazner, J.A.; Massie, J. Changing their minds with time: A comparison of hypothetical and actual reproductive behaviors in parents of children with cystic fibrosis. Pediatrics 2006, 118, 649–656. [Google Scholar] [CrossRef]
- Dudding, T.; Wilcken, B.; Burgess, B.; Hambly, J.; Turner, G. Reproductive decisions after neonatal screening identifies cystic fibrosis. Arch. Dis. Child. Fetal Neonatal Ed. 2000, 82, 124–127. [Google Scholar] [CrossRef]
- Scotet, V.; de Braekeleer, M.; Roussey, M.; Rault, G.; Parent, P.; Dagorne, M.; Journel, H.; Lemoigne, A.; Codet, J.P.; Catheline, M.; et al. Neonatal screening for cystic fibrosis in Brittany, France: Assessment of 10 years’ experience and impact on prenatal diagnosis. Lancet 2000, 356, 789–794. [Google Scholar] [CrossRef]
- Myring, J.; Beckett, W.; Jassi, R.; Roberts, T.; Sayers, R.; Scotcher, D.; McAllister, M. Shock, adjust, decide: Reproductive decision making in cystic fibrosis (CF) carrier couples—A qualitative study. J. Genet. Couns. 2011, 20, 404–417. [Google Scholar] [CrossRef]
- Sanderson, S.C.; O’Neill, S.C.; Bastian, L.A.; Bepler, G.; McBride, C.M. What can interest tell us about uptake of genetic testing? Intention and behavior amongst smokers related to patients with lung cancer. Public Health Genomics 2010, 13, 116–124. [Google Scholar] [CrossRef]
- Lerman, C.; Croyle, R.T.; Tercyak, K.P.; Hamann, H. Genetic testing: Psychological aspects and implications. J. Consult. Clin. Psychol. 2002, 70, 784–797. [Google Scholar] [CrossRef]
- Ropka, M.E.; Wenzel, J.; Phillips, E.K.; Siadaty, M.; Philbrick, J.T. Uptake rates for breast cancer genetic testing: A systematic review. Cancer Epidemiol. Biomark. Prev. 2006, 15, 840–855. [Google Scholar] [CrossRef]
- Hall, A.; Bostanci, A.; Wright, C.F. Non-invasive prenatal diagnosis using cell-free fetal DNA technology: Applications and implications. Public Health Genomics 2010, 13, 246–255. [Google Scholar]
- De Jong, A.; Dondorp, W.J.; de Die-Smulders, C.E.; Frints, S.G.; de Wert, G.M. Non-invasive prenatal testing: Ethical issues explored. Eur. J. Hum. Genet. 2010, 18, 272–277. [Google Scholar] [CrossRef]
- Deans, Z.; Hill, M.; Chitty, L.S.; Lewis, C. Non-invasive prenatal testing for single gene disorders: Exploring the ethics. Eur. J. Hum. Genet. 2013, 21, 713–718. [Google Scholar] [CrossRef]
- Boulton, M.; Cummings, C.; Williamson, R. The views of general practitioners on community carrier screening for cystic fibrosis. Br. J. Gen. Pract. 1996, 46, 299–301. [Google Scholar]
- American College of Obstetricians and Gynecologists Update on Carrier Screening for Cystic Fibrosis. Committee Opinion No. 486. April 2011. Available online: http://www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Genetics/Update_on_Carrier_Screening_for_Cystic_Fibrosis (accessed on 7 December 2013).
- Human Genetics Society of Australasia (HGSA) Genetic Services Committee, Cystic Fibrosis Population Screening Position Paper. Available online: http://www.Hgsa.Org.Au/documents/item/27 (accessed on 7 December 2013).
- UK National Screening Committee. The UK Nsc Policy on Cystic Fibrosis Screening in Pregnancy. Available online: http://www.screening.nhs.uk/cysticfibrosis-pregnancy (accessed on 7 December 2013).
- Conway, S.P.; Allenby, K.; Pond, M.N. Patient and parental attitudes toward genetic screening and its implications at an adult cystic fibrosis centre. Clin. Genet. 1994, 45, 308–312. [Google Scholar] [CrossRef]
- Musci, T.J.; Fairbrother, G.; Batey, A.; Bruursema, J.; Struble, C.; Song, K. Non-invasive prenatal testing with cell-free DNA: U.S. physician attitudes toward implementation in clinical practice. Prenat. Diagn. 2013, 33, 424–428. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hill, M.; Suri, R.; Nash, E.F.; Morris, S.; Chitty, L.S. Preferences for Prenatal Tests for Cystic Fibrosis: A Discrete Choice Experiment to Compare the Views of Adult Patients, Carriers of Cystic Fibrosis and Health Professionals. J. Clin. Med. 2014, 3, 176-190. https://doi.org/10.3390/jcm3010176
Hill M, Suri R, Nash EF, Morris S, Chitty LS. Preferences for Prenatal Tests for Cystic Fibrosis: A Discrete Choice Experiment to Compare the Views of Adult Patients, Carriers of Cystic Fibrosis and Health Professionals. Journal of Clinical Medicine. 2014; 3(1):176-190. https://doi.org/10.3390/jcm3010176
Chicago/Turabian StyleHill, Melissa, Ranjan Suri, Edward F. Nash, Stephen Morris, and Lyn S. Chitty. 2014. "Preferences for Prenatal Tests for Cystic Fibrosis: A Discrete Choice Experiment to Compare the Views of Adult Patients, Carriers of Cystic Fibrosis and Health Professionals" Journal of Clinical Medicine 3, no. 1: 176-190. https://doi.org/10.3390/jcm3010176
APA StyleHill, M., Suri, R., Nash, E. F., Morris, S., & Chitty, L. S. (2014). Preferences for Prenatal Tests for Cystic Fibrosis: A Discrete Choice Experiment to Compare the Views of Adult Patients, Carriers of Cystic Fibrosis and Health Professionals. Journal of Clinical Medicine, 3(1), 176-190. https://doi.org/10.3390/jcm3010176




