Impact of Previous Thoracotomy on Outcomes of Open Thoracoabdominal Aortic Aneurysm Repair: A Retrospective Propensity Score-Matched Analysis
Abstract
1. Introduction
2. Methods
2.1. Patients
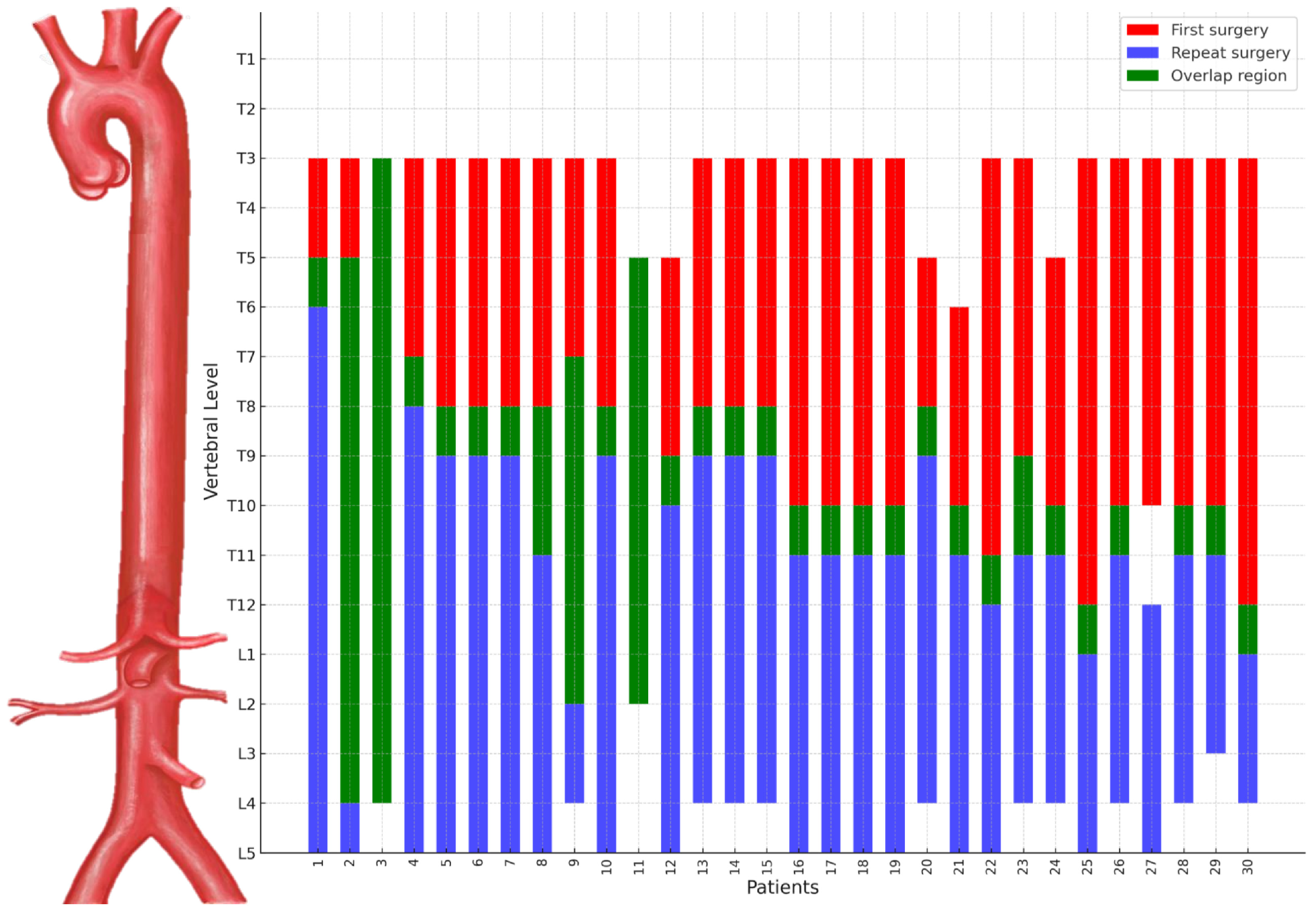
2.2. Definitions
2.3. Surgical Procedure
2.4. Statistical Analysis
3. Results
3.1. Demographics and Baseline Characteristics
3.2. Operative Data and Postoperative Outcomes
4. Discussion
4.1. Challenges and Technical Considerations of Repeat Thoracotomy
4.2. Intraoperative Strategies to Minimize Risks
4.3. Comparative Outcomes
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TAAA | Thoracoabdominal Aortic Aneurysm |
| RT | Repeat Thoracotomy |
| FT | First-Time Thoracotomy |
| MEP | Motor-Evoked Potentials |
| SSEP | Somatosensory-Evoked Potentials |
References
- Murana, G.; Castrovinci, S.; Kloppenburg, G.; Yousif, A.; Kelder, H.; Schepens, M.; de Maat, G.; Sonker, U.; Morshuis, W.; Heijmen, R. Open Thoracoabdominal Aortic Aneurysm Repair in the Modern Era: Results from a 20-Year Single-Centre Experience. Eur. J. Cardio-Thorac. Surg. 2016, 49, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Moulakakis, K.G.; Karaolanis, G.; Antonopoulos, C.N.; Kakisis, J.; Klonaris, C.; Preventza, O.; Coselli, J.S.; Geroulakos, G. Open Repair of Thoracoabdominal Aortic Aneurysms in Experienced Centers. J. Vasc. Surg. 2018, 68, 634–645.e12. [Google Scholar] [CrossRef] [PubMed]
- Cook, I.O.; Green, S.Y.; Rebello, K.R.; Zhang, Q.; Glover, V.A.; Zea-Vera, R.; Moon, M.R.; LeMaire, S.A.; Coselli, J.S. Comparison of Open Thoracoabdominal Repair for Chronic Aortic Dissections and Aneurysms. J. Vasc. Surg. 2024, 80, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Coselli, J.S. Reflection of Pioneers: Redo Thoracoabdominal Aortic Aneurysm Repair Controversies in Thoracic Aortic Aneurysm Surgery. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Coselli, J.S.; Rosu, C.; Amarasekara, H.S.; Green, S.Y.; Zhang, Q.; Price, M.D.; LeMaire, S.A. Reoperative Surgery on the Thoracoabdominal Aorta. J. Thorac. Cardiovasc. Surg. 2018, 155, 474–485.e1. [Google Scholar] [CrossRef] [PubMed]
- Etz, C.D.; Zoli, S.; Kari, F.A.; Mueller, C.S.; Bodian, C.A.; Di Luozzo, G.; Plestis, K.A.; Griepp, R.B. Redo Lateral Thoracotomy for Reoperative Descending and Thoracoabdominal Aortic Repair: A Consecutive Series of 60 Patients. Ann. Thorac. Surg. 2009, 88, 758–767. [Google Scholar] [CrossRef]
- Afifi, R.O.; Sandhu, H.K.; Trott, A.E.; Nguyen, T.C.; Miller, C.C.; Estrera, A.L.; Safi, H.J. Redo Thoracoabdominal Aortic Aneurysm Repair: A Single-Center Experience over 25 Years. Ann. Thorac. Surg. 2017, 103, 1421–1428. [Google Scholar] [CrossRef]
- Lau, C.; Gaudino, M.; Gambardella, I.; Mills, E.; Munjal, M.; Elsayed, M.; Girardi, L. Reoperative Repair of Descending Thoracic and Thoracoabdominal Aneurysms†. Eur. J. Cardio-Thorac. Surg. 2017, 52, 501–507. [Google Scholar] [CrossRef]
- Kawaharada, N.; Morishita, K.; Fukada, J.; Hachiro, Y.; Takahashi, K.; Abe, T. Thoracoabdominal Aortic Aneurysm Repair through Redo Left-Sided Thoracotomy. Ann. Thorac. Surg. 2004, 77, 1304–1308. [Google Scholar] [CrossRef]
- Coselli, J.S.; de Figueiredo, L.F.; LeMaire, S.A. Impact of Previous Thoracic Aneurysm Repair on Thoracoabdominal Aortic Aneurysm Management. Ann. Thorac. Surg. 1997, 64, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Sef, D.; Thet, M.S.; Miskolczi, S.; Velissaris, T.; De Silva, R.; Luthra, S.; Turina, M.I. Perioperative Neuromonitoring During Thoracoabdominal Aortic Aneurysm Open Repair: A Systematic Review. Eur. J. Cardio-Thorac. Surg. 2023, 63, ezad221. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.R.; Smalley, Z.; Nesvick, C.L.; Lee, S.L.; Michael, L.M. The Use of Lumbar Drains in Preventing Spinal Cord Injury Following Thoracoabdominal Aortic Aneurysm Repair: An Updated Systematic Review and Meta-Analysis. J. Neurosurg. Spine 2016, 25, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M.; Preventza, O.; Black, J.H., 3rd; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 Acc/Aha Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef] [PubMed]
- Afifi, R.O.; Sandhu, H.K.; Zaidi, S.T.; Trinh, E.; Tanaka, A.; Miller, C.C., 3rd; Safi, H.J.; Estrera, A.L. Intercostal Artery Management in Thoracoabdominal Aortic Surgery: To Reattach or Not to Reattach? J. Thorac. Cardiovasc. Surg 2018, 155, 1372–1378 e1. [Google Scholar] [CrossRef] [PubMed]
- Etz, C.D.; Zoli, S.; Mueller, C.S.; Bodian, C.A.; Di Luozzo, G.; Lazala, R.; Plestis, K.A.; Griepp, R.B. Staged Repair Significantly Reduces Paraplegia Rate after Extensive Thoracoabdominal Aortic Aneurysm Repair. J. Thorac. Cardiovasc. Surg. 2010, 139, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Zoli, S.; Etz, C.D.; Roder, F.; Brenner, R.M.; Bodian, C.A.; Kleinman, G.; Di Luozzo, G.; Griepp, R.B. Experimental Two-Stage Simulated Repair of Extensive Thoracoabdominal Aneurysms Reduces Paraplegia Risk. Ann. Thorac. Surg. 2010, 90, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, J.V.; Carpenter, J.P.; Pochettino, A.; Sonnad, S.S.; Bavaria, J.E. Thoracoabdominal Aortic Aneurysm Repair after Prior Aortic Surgery. J. Vasc. Surg. 2003, 38, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
| Variable | Overall Cohort | Propensity-Matched Cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n = 214) | FT Group (n = 184) | RT Group (n = 30) | p Value | Overall (n = 67) | FT Group (n = 45) | RT Group (n = 22) | p Value | |
| Age (years) | 57.3 (42.8–69.2) | 60.2 (43.8–69.9) | 49.1 (40.1–56.3) | 0.019 | 53.1 (42.8–68.2) | 53.3 (42.5–68.0) | 53.0 (47.2–68.3) | 0.793 |
| Male | 152 (71.0) | 127 (69.0) | 25 (83.3) | 0.166 | 46 (68.7) | 29 (64.4) | 17 (77.3) | 0.365 |
| Connective tissue disease | 70 (32.7) | 55 (29.9) | 15 (50.0) | 0.049 | 29 (43.3) | 21 (46.7) | 8 (36.4) | 0.182 |
| Marfan’s syndrome | 61 (28.5) | 49 (26.6) | 12 (40.0) | 0.198 | 25 (37.3) | 18 (40.0) | 7 (31.8) | 0.189 |
| Loeys Dietz syndrome | 9 (4.2) | 6 (3.3) | 3 (10.0) | 0.116 | 4 (6.0) | 3 (6.7) | 1 (4.5) | >0.999 |
| Chronic Aortic dissection | 139 (65.0) | 114 (62.0) | 25 (83.3) | 0.039 | 50 (74.6) | 33 (73.3) | 17 (77.3) | 0.652 |
| Crawford type | <0.001 | 0.786 | ||||||
| I | 15 (7.0) | 14 (7.6) | 1 (3.3) | 3 (4.5) | 2 (4.4) | 1 (4.5) | ||
| II | 90 (42.1) | 87 (47.3) | 3 (10.0) | 11 (16.4) | 9 (20.0) | 2 (9.1) | ||
| III | 66 (30.8) | 58 (47.3) | 23 (76.7) | 43 (64.2) | 27 (60.0) | 16 (72.7) | ||
| IV | 28 (13.1) | 25 (13.6) | 3 (10.0) | 10 (14.9) | 7 (15.6) | 3 (13.6) | ||
| Hypertension | 135 (63.1) | 113 (61.4) | 22 (73.3) | 0.293 | 45 (67.2) | 27 (60.0) | 18 (81.8) | 0.093 |
| Diabetes | 15 (7.0) | 13 (7.1) | 2 (6.7) | >0.999 | 4 (6.0) | 2 (4.4) | 2 (9.1) | 0.263 |
| COPD | 5 (2.3) | 4 (2.2) | 1 (3.3) | 0.534 | 2 (3.0) | 1 (2.2) | 1 (4.5) | >0.999 |
| Smoking history | 91 (42.9) | 76 (41.8) | 15(50.0) | 0.518 | 35 (52.2) | 21 (46.7) | 14 (63.6) | 0.110 |
| Chronic kidney disease * | 13 (6.1) | 11 (6.0) | 2 (6.7) | >0.999 | 2 (3.0) | 0 | 2 (9.1) | - |
| Peripheral artery disease | 16 (7.5) | 13 (7.1) | 3 (10.0) | 0.476 | 7 (10.4) | 4 (8.9) | 3 (13.6) | 0.433 |
| History of CVA | 16 (7.5) | 15 (8.2) | 1 (3.3) | 0.706 | 2 (3.0) | 1 (2.2) | 1 (4.5) | 0.433 |
| Previous AAA open repair | 20 (9.3) | 18 (9.8) | 2 (6.7) | 0.746 | 9 (13.4) | 8 (17.8) | 1 (4.5) | 0.215 |
| Previous TEVAR | 10 (4.7) | 8 (4.3) | 2 (6.7) | 0.635 | 4 (6.0) | 3 (6.7) | 1 (4.5) | 0.893 |
| Previous EVAR | 2 (0.9) | 2 (1.1) | 0 | >0.999 | 0 | 0 | 0 | - |
| Previous cardiac surgery | 94 (43.9) | 75 (40.8) | 19 (63.3) | 0.035 | 40 (59.7) | 29 (64.4) | 11 (50.0) | 0.126 |
| Variable | Number (n = 30) |
|---|---|
| Type of previous aortic surgery | |
| Descending thoracic aorta replacement | 26 (86.7) |
| Thoracoabdominal aorta replacement | 3 (10.0) |
| Descending thoracic aorta wrapping | 1 (3.3) |
| Time interval to Repeat surgery (year) | 3.4 (1.0–4.9) |
| Indication of Repeat aortic surgery | |
| Aneurysm | 26 (86.6) |
| Aneurysm impending rupture | 1 (3.3) |
| Anastomosis site pseudoaneurysm | 2 (6.7) |
| Graft infection | 1 (3.3) |
| Previous surgical approach | |
| 4th ICS | 4 (16.0) |
| 5th ICS | 13 (52.0) |
| 6th ICS | 5 (20.0) |
| 7th ICS | 3 (12.0) |
| Repeat surgical approach | |
| 6th ICS | 4 (13.3) |
| 7th ICS | 22 (73.3) |
| 8th ICS | 4 (13.3) |
| Variable | Overall Cohort | Propensity-Matched Cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n = 214) | FT Group (n = 184) | RT Group (n = 30) | p Value | Overall (n = 67) | FT Group (n = 45) | RT Group (n = 22) | p Value | |
| Emergency operation | 16 (7.5) | 13 (7.1) | 3 (10.0) | 0.476 | 2 (3.0) | 0 | 2 (9.1) | - |
| Operation time(min) | 497.0 (426.0–554.0) | 492.5 (421.0–554.0) | 500.5 (476.0–559.0) | 0.369 | 484.0 (434.5–530.0) | 459.0 (426.0–514.0) | 500.0 (476.0–552.0) | 0.037 |
| CPB time (min) | 169.5 (128.0–207.0) | 171.0 (125.5–213.0) | 168.0 (128.0–192.0) | 0.618 | 168.3 ± 47.1 | 168.3 ± 46.8 | 165.2 ± 48.8 | 0.668 |
| ACC time(min) | 157.0 (118.0–180.0) | 157.0 (110.0–184.0) | 159.0 (128.0–174.0) | 0.945 | 159.0 (135.0–174.0) | 159.0 (138.5–180.0) | 155.0 (131.0–168.0) | 0.170 |
| Rectal temperature (°C) | 30.3 (29.6–31.4) | 30.4 (29.6–31.5) | 30.1 (29.4–30.7) | 0.051 | 30.1 (29.5–30.8) | 30.1 (29.5–30.9) | 30.1 (29.4–30.7) | 0.123 |
| Lumbar drainage | 191 (89.3) | 162 (88) | 29 (96.7) | 0.213 | 61 (91.0) | 40 (88.9) | 19 (86.4) | 0.150 |
| MEP and SSEP monitoring | 159 (74.3) | 132 (71.2) | 27 (90.0) | 0.058 | 54 (80.6) | 35 (77.8) | 19 (86.4) | 0.171 |
| Variable | Overall Cohort a | Propensity-Matched Cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 214) | FT Group (n = 184) | RT Group (n = 30) | OR/Coef | 95% CI | p Value | Overall (n = 67) | FT Group (n = 45) | RT Group (n = 22) | OR/Coef | 95% CI | p Value | |
| 30-day mortality b | 11 (5.1) | 10 (5.4) | 1 (3.3) | 0.626 | 0.033–3.653 | 0.666 | 2 (3.0) | 1 (2.2) | 1 (4.5) | 3.095 | 0.184–52.158 | 0.433 |
| Paraplegia b | 8 (3.7) | 8 (4.3) | 0 (0.0) | - | 0 | 0 | 0 | - | ||||
| Dialysis b | 23 (10.7) | 20 (10.9) | 3 (10.0) | 3.421 | 0.642–15.362 | 0.117 | 8 (11.9) | 5 (11.1) | 3 (13.6) | 1.232 | 0.247–6.135 | 0.799 |
| major stroke b | 13 (6.1) | 10 (5.4) | 3 (10.0) | 2.872 | 0.579–11.480 | 0.152 | 2 (3.0) | 0 | 2 (9.1) | - | ||
| Respiratory complication b | 39 (18.2) | 36 (19.6) | 3 (10.0) | 1.315 | 0.272–4.891 | 0.701 | 9 (13.4) | 6 (13.3) | 3 (13.6) | 1.068 | 0.222–5.149 | 0.935 |
| Postoperative bleeding b | 19 (8.9) | 16 (8.7) | 3 (10.0) | 4.765 | 0.859–23.927 | 0.057 | 4 (6.0) | 1 (2.2) | 3 (13.6) | 10.263 | 0.997–105.621 | 0.050 |
| Gastrointestinal complication b | 5 (2.3) | 4 (2.2) | 1 (3.3) | 5.12 | 0.123–81.722 | 0.247 | 1 (1.5) | 0 | 1 (4.5) | - | ||
| Graft infection b | 1 (0.5) | 0 (0.0) | 1 (3.3) | - | 1 (1.5) | 0 | 1 (4.5) | - | ||||
| ECLS b | 3 (1.4) | 3 (1.6) | 0 (0.0) | - | 1 (1.5) | 1 (2.2) | 0 | - | ||||
| length of stay c | 14.5 (10.0–21.0) | 15.0 (10.0–21.5) | 11.0 (9.0–20.0) | 4.209 | −10.041–18.459 | 0.561 | 14.0 (10.0–20.5) | 14.0 (10.0–20.0) | 12.5 (10.0–23.0) | −0.273 | 0.406–1.426 | 0.394 |
| ICU duration c | 3.0 (2.0–5.0) | 3.0 (2.0–5.0) | 3.0 (2.0–4.0) | 3.210 | −8.138–14.558 | 0.578 | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | −0.214 | 0.477–1.368 | 0.314 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Heo, M.; Oh, S.; Chung, S.; Jeong, D.S.; Kim, W.S.; Cho, Y.H.; Sung, K. Impact of Previous Thoracotomy on Outcomes of Open Thoracoabdominal Aortic Aneurysm Repair: A Retrospective Propensity Score-Matched Analysis. J. Clin. Med. 2026, 15, 963. https://doi.org/10.3390/jcm15030963
Heo M, Oh S, Chung S, Jeong DS, Kim WS, Cho YH, Sung K. Impact of Previous Thoracotomy on Outcomes of Open Thoracoabdominal Aortic Aneurysm Repair: A Retrospective Propensity Score-Matched Analysis. Journal of Clinical Medicine. 2026; 15(3):963. https://doi.org/10.3390/jcm15030963
Chicago/Turabian StyleHeo, Muhyung, Siwon Oh, Suryeun Chung, Dong Seop Jeong, Wook Sung Kim, Yang Hyun Cho, and Kiick Sung. 2026. "Impact of Previous Thoracotomy on Outcomes of Open Thoracoabdominal Aortic Aneurysm Repair: A Retrospective Propensity Score-Matched Analysis" Journal of Clinical Medicine 15, no. 3: 963. https://doi.org/10.3390/jcm15030963
APA StyleHeo, M., Oh, S., Chung, S., Jeong, D. S., Kim, W. S., Cho, Y. H., & Sung, K. (2026). Impact of Previous Thoracotomy on Outcomes of Open Thoracoabdominal Aortic Aneurysm Repair: A Retrospective Propensity Score-Matched Analysis. Journal of Clinical Medicine, 15(3), 963. https://doi.org/10.3390/jcm15030963






