Factors Predicting Guselkumab Treatment Response in Patients with Moderate-to-Severe Plaque Psoriasis: A Post Hoc Analysis of Korean Real-World Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Sources and Data Collection
2.3. Post Hoc Study Population and Definitions
2.4. Statistical Analyses
2.5. Ethical Considerations
3. Results
3.1. Patients
3.2. Baseline Demographics, Medical History, and Disease Characteristics
3.3. Predictors of PASI 90 Response to Guselkumab at Week 28
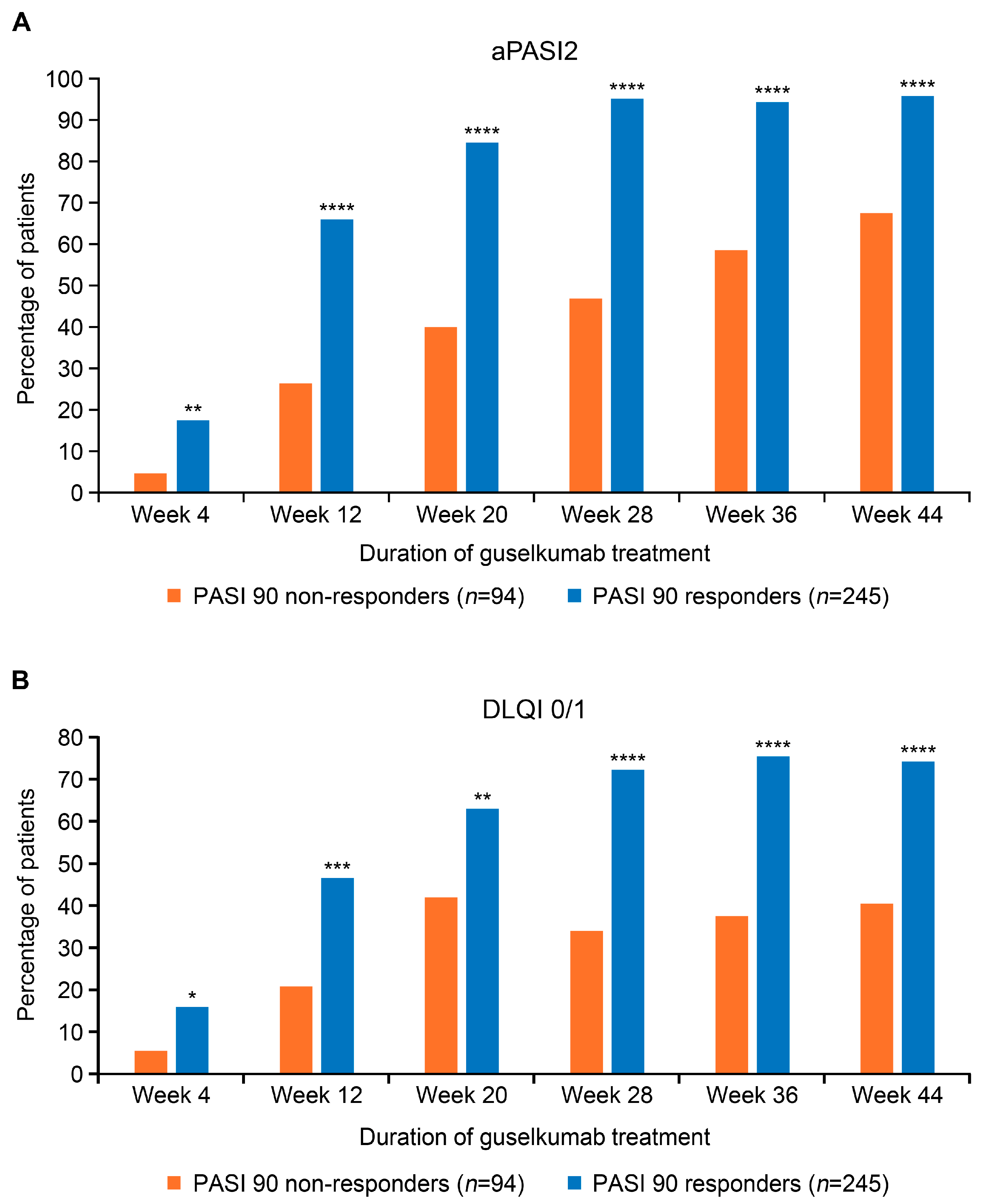
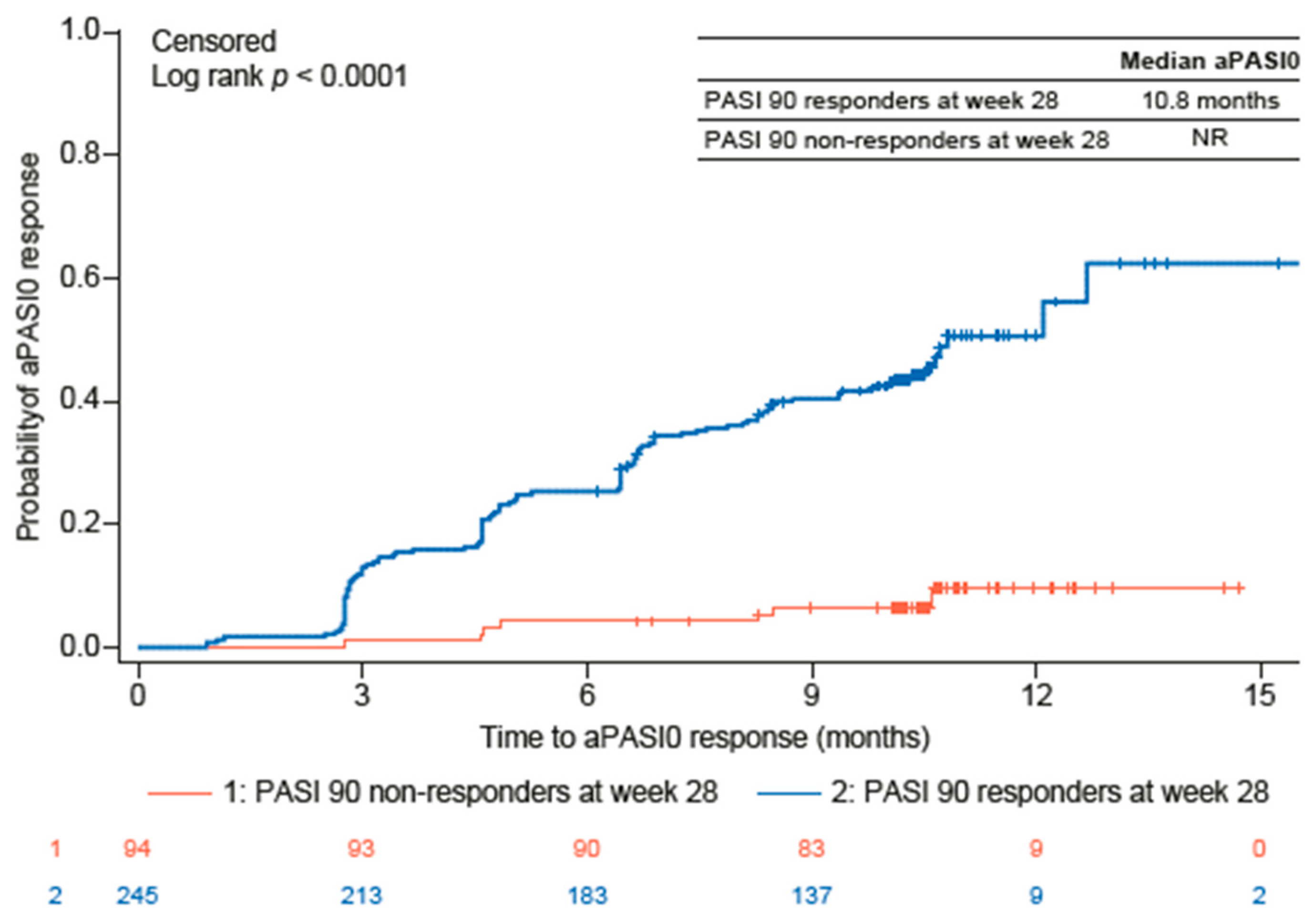
3.4. Guselkumab Treatment Effectiveness Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aPASI | Absolute Psoriasis Area and Sensitivity Index |
| aPASI0 | Absolute Psoriasis Activity Severity Index score of 0 |
| aPASI1 | Absolute Psoriasis Activity Severity Index score of ≤1 |
| aPASI2 | Absolute Psoriasis Activity Severity Index score of ≤2 |
| BMI | Body mass index |
| BSA | Body surface area |
| CI | Confidence interval |
| DLQI | Dermatology Life Quality Index |
| DLQI 0/1 | Dermatology Life Quality Index score of 0 or 1 |
| IGA | Investigator’s Global Assessment |
| IL | Interleukin |
| OR | Odds ratio |
| PASI | Psoriasis Area and Sensitivity Index |
| QoL | Quality of life |
| SD | Standard deviation |
References
- Bolick, N.L.; Ghamrawi, R.I.; Feldman, S.R. Management of plaque psoriasis: A review and comparison of IL-23 inhibitors. EMJ Dermatol. 2020, 8, 84–95. [Google Scholar]
- Reich, K.; Gordon, K.B.; Strober, B.; Langley, R.G.; Miller, M.; Yang, Y.W.; Shen, Y.K.; You, Y.; Zhu, Y.; Foley, P.; et al. Super-response to guselkumab treatment in patients with moderate-to-severe psoriasis: Age, body weight, baseline Psoriasis Area and Severity Index, and baseline Investigator’s Global Assessment scores predict complete skin clearance. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 2393–2400. [Google Scholar] [CrossRef]
- Korman, N.J.; Zhao, Y.; Pike, J.; Roberts, J.; Sullivan, E. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol. Online J. 2015, 21, 13030. [Google Scholar] [CrossRef]
- Armstrong, A.; Bohannan, B.; Mburu, S.; Alarcon, I.; Kasparek, T.; Toumi, J.; Frade, S.; Barrio, S.F.; Augustin, M. Impact of psoriatic disease on quality of life: Interim results of a global survey. Dermatol. Ther. 2022, 12, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.M.; Jo, S.J.; Naldi, L.; Romiti, R.; Guevara-Sangines, E.; Howe, T.; Pietri, G.; Gilloteau, I.; Richardson, C.; Tian, H.; et al. A multidimensional assessment of the burden of psoriasis: Results from a multinational dermatologist and patient survey. Br. J. Dermatol. 2018, 179, 173–181. [Google Scholar] [CrossRef]
- Blauvelt, A.; Wu, J.J.; Armstrong, A.; Menter, A.; Liu, C.; Jacobson, A. Importance of complete skin clearance in psoriasis as a treatment goal: Implications for patient-reported outcomes. J. Drugs Dermatol. 2020, 19, 487–492. [Google Scholar] [CrossRef]
- Blauvelt, A.; Papp, K.A.; Griffiths, C.E.; Randazzo, B.; Wasfi, Y.; Shen, Y.K.; Li, S.; Kimball, A.B. Efficacy and safety of gusel-kumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J. Am. Acad. Dermatol. 2017, 76, 405–417. [Google Scholar] [CrossRef]
- Reich, K.; Armstrong, A.W.; Foley, P.; Song, M.; Wasfi, Y.; Randazzo, B.; Li, S.; Shen, Y.K.; Gordon, K.B. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J. Am. Acad. Dermatol. 2017, 76, 418–431. [Google Scholar] [CrossRef]
- Leonardi, C.; Langley, R.G.; Papp, K.; Tyring, S.K.; Wasel, N.; Vender, R.; Unnebrink, K.; Gupta, S.R.; Valdecantos, W.C.; Bagel, J. Adalimumab for treatment of moderate to severe chronic plaque psoriasis of the hands and feet: Efficacy and safety results from REACH, a randomized, placebo-controlled, double-blind trial. Arch. Dermatol. 2011, 147, 429–436. [Google Scholar] [CrossRef]
- Papp, K.A.; Langley, R.G.; Lebwohl, M.; Krueger, G.G.; Szapary, P.; Yeilding, N.; Guzzo, C.; Hsu, M.-C.; Wang, Y.; Li, S.; et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008, 371, 1675–1684. [Google Scholar] [CrossRef]
- Langley, R.G.; Elewski, B.E.; Lebwohl, M.; Reich, K.; Griffiths, C.E.M.; Papp, K.; Puig, L.; Nakagawa, H.; Spelman, L.; Sigurgeirsson, B.; et al. Secukinumab in plaque psoriasis—Results of two phase 3 trials. N. Engl. J. Med. 2014, 371, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, Y.; Cui, L.; Shi, Y.; Guo, C. Advances in the pathogenesis of psoriasis: From keratinocyte perspective. Cell Death Dis. 2022, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Abreu, M.T.; Krueger, J.G.; Eyerich, K.; Sachen, K.; Greving, C.; Hammaker, D.; Bao, P.; Lacy, E.; Sarabia, I.; et al. P504 guselkumab, an IL-23p19 subunit–specific monoclonal antibody, binds CD64+ myeloid cells and potently neutralises IL-23 produced from the same cells. J. Crohns Colitis 2023, 17, i634–i635. [Google Scholar] [CrossRef]
- McGonagle, D.; Atreya, R.; Abreu, M.; Krueger, J.; Eyerich, K.; Sachen, K.; Greving, C.; Hammaker, D.; Bao, P.; Lacy, E.; et al. POS1531 guselkumab, an IL-23P19 subunit–specific monoclonal antibody, binds CD64+ myeloid cells and potently neutralises IL-23 produced from the same cells. Ann. Rheum. Dis. 2023, 82, 1128–1129. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Tremfya Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/tremfya-epar-product-information_en.pdf (accessed on 6 February 2025).
- Ministry of Food and Drug Safety. Trempier Prefilled Syringe (Guselkumab, Genetically Modified). Available online: https://nedrug.mfds.go.kr/pbp/CCBBB01/getItemDetailCache?cacheSeq=201801604aupdateTs2023-12-26%2014:24:05.0b (accessed on 6 February 2025).
- Tada, Y.; Sugiura, Y.; Kamishima, M.; Tanaka, Y.; Tsuchiya, H.; Masuda, J.; Yamanaka, K. Safety and effectiveness of guselkumab in Japanese patients with psoriasis: 20-week interim analysis of a postmarketing surveillance study. J. Dermatol. 2024, 51, 779–790. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. TREMFYA (Guselkumab) Injection, for Subcutaneous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761061s000lbl.pdf (accessed on 6 February 2025).
- Reich, K.; Gordon, K.B.; Strober, B.E.; Armstrong, A.W.; Miller, M.; Shen, Y.K.; You, Y.; Han, C.; Yang, Y.W.; Foley, P.; et al. Five-year maintenance of clinical response and health-related quality of life improvements in patients with moderate-to-severe psoriasis treated with guselkumab: Results from VOYAGE 1 and VOYAGE 2. Br. J. Dermatol. 2021, 185, 1146–1159. [Google Scholar] [CrossRef]
- Ruiz-Villaverde, R.; Rodriguez-Fernandez-Freire, L.; Armario-Hita, J.C.; Pérez-Gil, A.; Galán-Gutiérrez, M. Super responders to guselkumab treatment in moderate-to-severe psoriasis: A real clinical practice pilot series. Int. J. Dermatol. 2022, 61, 1029–1033. [Google Scholar] [CrossRef]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef]
- Kim, J.; Oh, C.H.; Jeon, J.; Baek, Y.; Ahn, J.; Kim, D.J.; Lee, H.S.; Correa da Rosa, J.; Suárez-Fariñas, M.; Lowes, M.A.; et al. Molecular phenotyping small (Asian) versus large (Western) plaque psoriasis shows common activation of IL-17 pathway genes but different regulatory gene sets. J. Investig. Dermatol. 2016, 136, 161–172. [Google Scholar] [CrossRef]
- Shin, B.S.; Kim, M.; Suh, M.K.; Lee, Y.B.; Youn, S.W.; Lee, J.Y.; Kim, C.W.; Lee, G.-Y.; Son, S.W.; Kim, K.H.; et al. Effectiveness and safety of guselkumab in patients with moderate-to-severe plaque psoriasis in real-world practice in Korea: A prospective, multicenter, observational, postmarketing surveillance study. J. Dermatol. 2025, 52, 1125–1137. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, K.; Jian, L.; Duan, Y.; Zhang, M.; Kuang, Y. Comparison between super-responders and non-super-responders in psoriasis under adalimumab treatment: A real-life cohort study on the effectiveness and drug survival over one-year. J. Dermatolog. Treat. 2024, 35, 2331782. [Google Scholar] [CrossRef]
- Schwarz, C.W.; Loft, N.; Rasmussen, M.K.; Nissen, C.V.; Dam, T.N.; Ajgeiy, K.K.; Egeberg, A.; Skov, L. Predictors of response to biologics in patients with moderate-to-severe psoriasis: A Danish nationwide cohort study. Acta Derm. Venereol. 2021, 101, adv00579. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.J.; Langley, R.G.; Gordon, K.B.; Pinter, A.; Ferris, L.K.; Rubant, S.; Photowala, H.; Xue, Z.; Wu, T.; Zhan, T.; et al. Efficacy of risankizumab versus secukinumab in patients with moderate-to-severe psoriasis: Subgroup analysis from the IMMerge study. Dermatol. Ther. 2022, 12, 561–575. [Google Scholar] [CrossRef]
- Seneschal, J.; Lacour, J.P.; Bewley, A.; Faurby, M.; Paul, C.; Pellacani, G.; De Simone, C.; Horne, L.; Sohrt, A.; Augustin, M.; et al. A multinational, prospective, observational study to estimate complete skin clearance in patients with moderate-to-severe plaque PSOriasis treated with BIOlogics in a REAL world setting (PSO-BIO-REAL). J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2566–2573. [Google Scholar] [CrossRef]
- Caldarola, G.; Galluzzo, M.; Bernardini, N.; Calabrese, L.; Grimaldi, M.; Moretta, G.; Pagnanelli, G.; Shumak, R.G.; Talamonti, M.; Tofani, L.; et al. Tildrakizumab in moderate-to-severe plaque psoriasis: A multicenter, retrospective, real-life study. Dermatol. Ther. 2022, 35, e15488. [Google Scholar] [CrossRef]
- Caldarola, G.; Zangrilli, A.; Bernardini, N.; Bavetta, M.; De Simone, C.; Graceffa, D.; Bonifati, C.; Faleri, S.; Giordano, D.; Mariani, M.; et al. Risankizumab for the treatment of moderate-to-severe psoriasis: A multicenter, retrospective, 1 year real-life study. Dermatol. Ther. 2022, 35, e15489. [Google Scholar] [CrossRef]
- Mastorino, L.; Siliquini, N.; Avallone, G.; Zenone, M.; Ortoncelli, M.; Quaglino, P.; Dapavo, P.; Ribero, S. Guselkumab shows high efficacy and maintenance in the improvement of response until week 48, a real-life study. Dermatol. Ther. 2022, 35, e15670. [Google Scholar] [CrossRef]
- Rompoti, N.; Politou, M.; Stefanaki, I.; Vavouli, C.; Papoutsaki, M.; Neofotistou, A.; Rigopoulos, D.; Stratigos, A.; Nicolaidou, E. Brodalumab in plaque psoriasis: Real-world data on effectiveness, safety and clinical predictive factors of initial response and drug survival over a period of 104 weeks. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 689–697. [Google Scholar] [CrossRef]
- Kyrmanidou, E.; Kemanetzi, C.; Stavros, C.; Trakatelli, M.G.; Patsatsi, A.; Madia, X.; Ignatiadi, D.; Kalloniati, E.; Apalla, Z.; Lazaridou, E. A real-life 208 week single-centred, register-based retrospective study assessing secukinumab survival and long-term efficacy and safety among Greek patients with moderate to severe plaque psoriasis, including difficult-to-treat manifestations such as genitals and scalp. Dermatol. Pract. Concept. 2024, 14, e2024119. [Google Scholar] [CrossRef]
- Mastorino, L.; Susca, S.; Cariti, C.; Verrone, A.; Stroppiana, E.; Ortoncelli, M.; Dapavo, P.; Ribero, S.; Quaglino, P. “Super-responders” at biologic treatment for psoriasis: A comparative study among IL17 and IL23 inhibitors. Exp. Dermatol. 2023, 32, 2187–2188. [Google Scholar] [CrossRef]
- Conrad, C.; Ortmann, C.E.; Vandemeulebroecke, M.; Kasparek, T.; Reich, K. Nail involvement as a predictor of differential treatment effects of secukinumab versus ustekinumab in patients with moderate to severe psoriasis. Dermatol. Ther. 2022, 12, 233–241. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Riedl, E.; Brunner, P.M.; Piaserico, S.; Visser, W.I.; Haustrup, N.; Konicek, B.W.; Kadziola, Z.; Nunez, M.; Brnabic, A.; et al. Identifying predictors of PASI100 responses up to month 12 in patients with moderate-to-severe psoriasis receiving biologics in the Psoriasis Study of Health Outcomes (PSoHO). Acta Derm. Venereol. 2024, 104, adv40556. [Google Scholar] [CrossRef]
- Schäkel, K.; Reich, K.; Asadullah, K.; Pinter, A.; Jullien, D.; Weisenseel, P.; Paul, C.; Gomez, M.; Wegner, S.; Personke, Y.; et al. Early disease intervention with guselkumab in psoriasis leads to a higher rate of stable complete skin clearance (‘clinical super response’): Week 28 results from the ongoing phase IIIb randomized, double-blind, parallel-group, GUIDE study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2016–2027. [Google Scholar] [CrossRef]
- Shin, J.-O.; Shin, B.S.; Bae, K.-N.; Shin, K.; Kim, H.-S.; Ko, H.-C.; Kim, M.-B.; Kim, B. Review of the reasons for and effectiveness of switching biologics for psoriasis treatment in Korea. Indian. J. Dermatol. Venereol. Leprol. 2023, 89, 928. [Google Scholar] [CrossRef]
- Patel, V.; Park, S.H.; Zhong, Y.; Sima, A.P.; Zhuo, J.; Roberts-Toler, C.; Becker, B.; Hovland, S.; Strober, B. The association between patient-reported disease burden and treatment switching in patients with plaque psoriasis treated with nonbiologic systemic therapy. Psoriasis 2024, 14, 167–174. [Google Scholar] [CrossRef]
- Strober, B.; Papp, K.A.; Lebwohl, M.; Reich, K.; Paul, C.; Blauvelt, A.; Gordon, K.B.; Milmont, C.E.; Viswanathan, H.N.; Li, J.; et al. Clinical meaningfulness of complete skin clearance in psoriasis. J. Am. Acad. Dermatol. 2016, 75, 77–82.e77. [Google Scholar] [CrossRef]
- Youn, S.W.; Lee, J.H.; Yu, D.Y.; Kim, Y.; Kim, B.S.; Seo, S.J.; Choe, Y.B.; Yun, S.K.; Park, J.; Kim, N.I.; et al. The relationship between clinical characteristics including presence of exposed lesions and health-related quality of life (HRQoL) in patients with psoriasis: Analysis from the nationwide epidemiologic study for psoriasis in Korea (EPI-PSODE study). J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1499–1506. [Google Scholar] [CrossRef]
| PASI 90 Responders (n = 245) | PASI 90 Non-Responders (n = 94) | p-Value | |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 166 (67.8) | 65 (69.1) | 0.8052 |
| Female | 79 (32.2) | 29 (30.9) | |
| Age, years | 42.0 | 44.5 | 0.0712 |
| BMI | |||
| n | 123 | 47 | |
| BMI (kg/m2) | 25.3 | 26.2 | 0.1909 |
| Smoking status, n (%) | |||
| n | 220 | 75 | |
| Non-smoker | 132 (60.0) | 41 (54.7) | 0.0402 |
| Past smoker | 18 (8.2) | 14 (18.7) | |
| Current smoker | 70 (31.8) | 20 (26.7) | |
| Alcohol use, n (%) | |||
| n | 220 | 74 | |
| Never used alcohol | 72 (32.7) | 29 (39.2) | 0.1097 |
| Formerly used alcohol | 24 (10.9) | 13 (17.6) | |
| Currently using alcohol | 124 (56.4) | 32 (43.2) | |
| Comorbidities, n (%) | |||
| Autoimmune rheumatic disease | 23 (9.4) | 12 (12.8) | 0.3601 |
| Hyperlipidemia | 22 (9.0) | 8 (8.5) | 0.8917 |
| Hypertension | 21 (8.6) | 16 (17.0) | 0.0255 |
| Diabetes mellitus | 10 (4.1) | 10 (10.6) | 0.0218 |
| Cardiovascular disease | 2 (0.8) | 2 (2.1) | 0.3081 |
| Impaired glucose regulation | 2 (0.8) | 0 | 1.0000 |
| Autoimmune thyroiditis/hypo-/ hyperthyroidism | 2 (0.8) | 0 | 1.0000 |
| Psychiatric disorders | 1 (0.4) | 3 (3.2) | 0.0664 |
| Uveitis | 1 (0.4) | 0 | 1.0000 |
| Malignancy | 1 (0.4) | 0 | 1.0000 |
| Non-alcoholic fatty liver disease | 0 | 1 (1.1) | 0.2773 |
| Palmoplantar pustulosis | 0 | 1 (1.1) | 0.2773 |
| PASI 90 Responders (n = 245) | PASI 90 Non-Responders (n = 94) | p-Value | |
|---|---|---|---|
| Family history of psoriasis, n (%) | |||
| n | 240 | 86 | |
| Yes | 16 (6.7) | 13 (15.1) | 0.0182 |
| Disease duration, months | 85.3 | 125.4 | 0.0162 |
| Psoriasis morphology, n (%) | |||
| Scalp | 131 (53.5) | 43 (45.7) | 0.2027 |
| Face | 102 (41.6) | 42 (44.7) | 0.6113 |
| Neck | 95 (38.8) | 40 (42.6) | 0.5247 |
| Chest | 159 (64.9) | 64 (68.1) | 0.5798 |
| Abdomen | 193 (78.8) | 70 (74.5) | 0.3946 |
| Back | 190 (77.6) | 78 (83.0) | 0.2716 |
| Upper arm | 197 (80.4) | 73 (77.7) | 0.5737 |
| Elbow | 173 (70.6) | 63 (67.0) | 0.5199 |
| Lower arm | 192 (78.4) | 75 (79.8) | 0.7748 |
| Palm | 23 (9.4) | 7 (7.4) | 0.5732 |
| Back of hand | 56 (22.9) | 26 (27.7) | 0.3553 |
| Nail | 46 (18.8) | 23 (24.5) | 0.2439 |
| Upper leg | 193 (78.8) | 77 (81.9) | 0.5204 |
| Knee | 165 (67.3) | 62 (66.0) | 0.8076 |
| Lower leg | 218 (89.0) | 89 (94.7) | 0.1080 |
| Foot sole | 30 (12.2) | 13 (13.8) | 0.6947 |
| Back of foot | 44 (18.0) | 25 (26.6) | 0.0771 |
| Buttock | 119 (48.6) | 49 (52.1) | 0.5577 |
| Genitalia | 16 (6.5) | 9 (9.6) | 0.3371 |
| Concurrent psoriatic arthropathy, n (%) | 20 (8.2) | 10 (10.6) | 0.4726 |
| PASI score (0–72), mean (SD) | 16.7 (6.5) | 13.8 (4.9) | <0.0001 |
| BSA, mean (SD) | 22.4 (15.1) | 19.3 (14.6) | 0.0108 |
| IGA score, n (%) | |||
| n | 148 | 51 | |
| Minimal | 3 (2.0) | 1 (2.0) | 0.0034 |
| Mild | 47 (31.8) | 27 (52.9) | |
| Moderate | 88 (59.5) | 16 (31.4) | |
| Severe | 10 (6.8) | 7 (13.7) | |
| DLQI, n (%) | |||
| n | 152 | 59 | |
| 0–1 | 6 (3.9) | 2 (3.4) | 0.3908 |
| 2–5 | 14 (9.2) | 3 (5.1) | |
| 6–10 | 22 (14.5) | 15 (25.4) | |
| 11–20 | 60 (39.5) | 22 (37.3) | |
| 21–30 | 50 (32.9) | 17 (28.8) | |
| Number of prior biologics, n (%) | |||
| 0 | 204 (83.3) | 75 (79.8) | 0.6593 |
| 1 | 35 (14.3) | 17 (18.1) | |
| 2 | 5 (2.0) | 1 (1.1) | |
| ≥3 | 1 (0.4) | 1 (1.1) | |
| Type of prior treatment, n (%) | |||
| Topical | 165 (67.3) | 47 (50.0) | 0.0031 |
| Phototherapy | 179 (73.1) | 60 (63.8) | 0.0952 |
| Systemic oral agent | 226 (92.2) | 84 (89.4) | 0.3955 |
| Steroid | 6 (2.4) | 5 (5.3) | 0.1859 |
| Methotrexate | 123 (50.2) | 34 (36.2) | 0.0204 |
| Cyclosporine | 137 (55.9) | 59 (62.8) | 0.2531 |
| Acitretin | 10 (4.1) | 5 (5.3) | 0.5697 |
| Others | 2 (0.8) | 5 (5.3) | 0.0193 |
| Biologics | 41 (16.7) | 19 (20.2) | 0.4526 |
| Concomitant treatment, n (%) | 96 (39.2) | 63 (67.0) | <0.0001 |
| Type of concomitant treatment, n (%) | |||
| Systemic oral agent | 17 (6.9) | 6 (6.4) | 0.8555 |
| Phototherapy | 2 (0.8) | 1 (1.1) | 1.0000 |
| Topical | 89 (36.3) | 62 (66.0) | <0.0001 |
| Univariate Analysis | Multivariate Logistic Regression | |||||
|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| Female sex | 1.067 | 0.639–1.782 | 0.8053 | 0.943 | 0.446–1.991 | 0.8774 |
| Age, years | 0.981 | 0.964–0.998 | 0.0302 | 0.995 | 0.971–1.019 | 0.6763 |
| Positive family history of psoriasis | 0.401 | 0.184–0.873 | 0.0214 | 0.345 | 0.135–0.884 | 0.0266 |
| Smoker status | ||||||
| Current | 1.087 | 0.592–1.997 | 0.7877 | 0.974 | 0.441–2.155 | 0.9489 |
| Past | 0.399 | 0.183–0.872 | 0.0213 | 0.428 | 0.165–1.109 | 0.0807 |
| Disease duration, months | 0.997 | 0.995–1.000 | 0.0173 | 0.999 | 0.996–1.002 | 0.5695 |
| Psoriasis morphology | ||||||
| Back of foot | 0.604 | 0.344–1.060 | 0.0787 | 0.522 | 0.242–1.127 | 0.0978 |
| Comorbidities | ||||||
| Diabetes mellitus | 0.358 | 0.144–0.890 | 0.0270 | 0.713 | 0.195–2.607 | 0.6092 |
| Hypertension | 0.457 | 0.227–0.920 | 0.0282 | 0.754 | 0.286–1.984 | 0.5670 |
| Psychiatric disorders | 0.124 | 0.013–1.211 | 0.0726 | 0.192 | 0.015–2.451 | 0.2039 |
| Baseline PASI score | 1.111 | 1.051–1.175 | 0.0002 | 1.220 | 1.089–1.367 | 0.0006 |
| Baseline BSA score | 1.016 | 0.997–1.034 | 0.0947 | 0.948 | 0.908–0.989 | 0.0127 |
| Prior treatment use | ||||||
| Topical agents | 2.063 | 1.271–3.348 | 0.0034 | 1.464 | 0.789–2.717 | 0.2270 |
| Phototherapy | 1.537 | 0.926–2.551 | 0.0964 | 2.444 | 1.229–4.858 | 0.0108 |
| Concomitant treatment use | ||||||
| Topical agents | 0.294 | 0.179–0.485 | <0.0001 | 0.409 | 0.222–0.756 | 0.0044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Lee, Y.B.; Shin, B.S.; Kim, M.; Suh, M.K.; Youn, S.W.; Lee, J.Y.; Kim, C.W.; Lee, G.-Y.; Kim, K.H.; An, J.; et al. Factors Predicting Guselkumab Treatment Response in Patients with Moderate-to-Severe Plaque Psoriasis: A Post Hoc Analysis of Korean Real-World Data. J. Clin. Med. 2026, 15, 704. https://doi.org/10.3390/jcm15020704
Lee YB, Shin BS, Kim M, Suh MK, Youn SW, Lee JY, Kim CW, Lee G-Y, Kim KH, An J, et al. Factors Predicting Guselkumab Treatment Response in Patients with Moderate-to-Severe Plaque Psoriasis: A Post Hoc Analysis of Korean Real-World Data. Journal of Clinical Medicine. 2026; 15(2):704. https://doi.org/10.3390/jcm15020704
Chicago/Turabian StyleLee, Young Bok, Bong Seok Shin, Miri Kim, Moo Kyu Suh, Sang Woong Youn, Ji Yeoun Lee, Chul Woo Kim, Ga-Young Lee, Kwang Ho Kim, Jihye An, and et al. 2026. "Factors Predicting Guselkumab Treatment Response in Patients with Moderate-to-Severe Plaque Psoriasis: A Post Hoc Analysis of Korean Real-World Data" Journal of Clinical Medicine 15, no. 2: 704. https://doi.org/10.3390/jcm15020704
APA StyleLee, Y. B., Shin, B. S., Kim, M., Suh, M. K., Youn, S. W., Lee, J. Y., Kim, C. W., Lee, G.-Y., Kim, K. H., An, J., Kim, Y., Kim, K. J., Kim, D. H., & Son, S. W. (2026). Factors Predicting Guselkumab Treatment Response in Patients with Moderate-to-Severe Plaque Psoriasis: A Post Hoc Analysis of Korean Real-World Data. Journal of Clinical Medicine, 15(2), 704. https://doi.org/10.3390/jcm15020704







