Italian Evidence-Based Clinical Recommendations on the Appropriateness of Prescriptions and Diagnostic Tests in Pediatric Allergology: Focus on Anaphylaxis, Drug Allergy and Hymenoptera Venom Allergy
Abstract
1. Introduction
2. Methods
2.1. Working Group and Panel of Experts
2.2. Formulation of Clinical Questions
2.3. Systematic Review
- Design of the source studies: only randomized controlled trials (RCTs), observational cohort studies with a comparator, case–control studies were included; regarding secondary sources, systematic reviews with and without meta-analyses and guidelines were initially retrieved and then assessed for obtaining additional entries through a snowball approach. Conversely, case reports, letters to the editor, brief reports, and conference abstracts were excluded;
- Age groups, only studies reporting on subjects aged 0–18 years were considered;
- Language and timeframe: the search was arbitrarily limited to results in English, published between January 2015 and January 2025.
2.4. Data Extraction
2.5. Meta-Analysis
2.6. GRADE and GRADE Framework
2.7. Consensus Panel for the Strength of Recommendations
3. Results
3.1. Anaphylaxis
3.1.1. Summary of Literature Search
3.1.2. Clinical Questions for Anaphylaxis
PICO 1
- The diagnostic accuracy of allergy tests in identifying the causal agent and initiating a specific management pathway.
- The risk of recurrent anaphylactic episodes.
PICO 2
PICO 3
PICO 4
3.2. Drug Allergy
3.2.1. Summary of Literature Research
- The diagnostic accuracy of allergy tests to identify the causal agent;
- The inappropriate use of antibiotics.
3.2.2. Clinical Questions on Drug Allergy
PICO 1
PICO 2
3.3. Hymenoptera Venom Allergy
3.3.1. Summary of Literature Research
- Risk of anaphylaxis;
- Diagnostic accuracy to initiate appropriate treatment.
3.3.2. Clinical Questions for Hymenoptera Venom Allergy
PICO 1
PICO 2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Topic | PICO | PubMed | Embase |
|---|---|---|---|
| Anaphylaxis | 1 | (“Ambulatory Care”[Mesh] OR “Day Care, Medical”[Mesh] OR “home visit*” OR “outpatient*”) AND (“Anaphylaxis”[Mesh] OR “Hypersensitivity, Immediate”[Mesh]) AND (“Emergency Medical Services”[Mesh] OR “Self Administration”[Mesh] OR “Epinephrine”[Mesh]) AND (“Tryptases”[MeSH] OR tryptase[tiab] OR “serum tryptase”[tiab] OR “mast cell tryptase”[tiab] OR “tryptase level”[tiab] OR “baseline tryptase”[tiab] OR “acute tryptase”[tiab]) AND (“Child”[MeSH] OR “Adolescent”[MeSH] OR child*[tiab] OR pediatric*[tiab] OR pediatric*[tiab] OR adolescen*[tiab]) | (‘ambulatory care’/exp OR ‘ambulatory care’ OR ‘day care’ OR ‘home visit’ OR ‘outpatient’) AND (‘anaphylaxis’ OR ‘hypersensitivity’) AND (‘emergency health service’ OR ‘drug self administration’ OR ‘epinephrine’) AND (‘tryptase’) AND ([adolescent]/lim OR [child]/lim OR [newborn]/lim OR [preschool]/lim) |
| 2 | (“Anaphylaxis”[MeSH] OR anaphylaxis[tiab] OR anaphylactic[tiab]) AND (“Tryptases”[MeSH] OR tryptase[tiab] OR “serum tryptase”[tiab] OR “mast cell tryptase”[tiab] OR “tryptase level”[tiab] OR “baseline tryptase”[tiab] OR “acute tryptase”[tiab]) AND (“Child”[MeSH] OR “Adolescent”[MeSH] OR child*[tiab] OR pediatric*[tiab] OR pediatric*[tiab] OR adolescen*[tiab]) | (‘anaphylaxis’ OR ‘hypersensitivity’) AND (‘tryptase’ OR ‘tryptase test kit’ OR ‘tryptase blood level’) AND ([adolescent]/lim OR [child]/lim OR [newborn]/lim OR [preschool]/lim) | |
| 3 | ((“Food Hypersensitivity”[MeSH] OR “Food Hypersensitivity”[tiab] OR “food allergy”[tiab] OR “food-induced allergy”[tiab] OR “food-induced anaphylaxis”[tiab] OR “food allergy–related anaphylaxis”[tiab]) AND (“Anaphylaxis”[MeSH] OR anaphylaxis[tiab]) AND (“Skin Tests”[MeSH] OR “skin prick test”[tiab] OR “skin-prick test”[tiab] OR “SPT”[tiab] OR “prick test”[tiab]) AND (“Child”[MeSH] OR “Adolescent”[MeSH] OR child*[tiab] OR pediatric*[tiab] OR pediatric*[tiab])) | (‘food allergy’ AND (‘anaphylaxis’ OR ‘hypersensitivity’)) AND ([adolescent]/lim OR [child]/lim OR [newborn]/lim OR [preschool]/lim) AND (‘skin test’ OR ‘prick test’) | |
| 4 | (“Anaphylaxis”[MeSH] OR anaphylaxis[tiab] OR anaphylactic[tiab]) AND (“Immunoglobulin E”[MeSH] OR “specific IgE”[tiab] OR “specific IgE”[MeSH] OR “component resolved”[tiab] OR “component-resolved”[tiab] OR “component resolved diagnostics”[tiab] OR “component-resolved diagnostics”[tiab] OR CRD[tiab] OR “molecular allergology”[tiab]) AND (“Child”[MeSH] OR “Adolescent”[MeSH] OR child*[tiab] OR pediatric*[tiab] OR pediatric*[tiab] OR adolescen*[tiab]) | (‘anaphylaxis’ OR ‘hypersensitivity’) AND ([adolescent]/lim OR [child]/lim OR [newborn]/lim OR [preschool]/lim) AND (‘immunoglobulin’ OR ‘immunoglobulin e’ OR ‘component resolved diagnosis’ OR ‘component resolved diagnostics’ OR (molecular AND allergology) | |
| Drug Allergy | 1 | (“Hypersensitivity/drug therapy”[MeSH] OR “Drug Hypersensitivity”[MeSH] OR “beta-lactams” [tiab] OR “beta lactam”[tiab] OR “penicillin allergy”[tiab] OR “penicillin hypersensitivity”[tiab] OR “cephalosporin allergy”[tiab] OR “drug allergy”[tiab]) AND (“Skin Tests”[MeSH] OR “skin prick”[tiab] OR “prick test”[tiab] OR intradermal[tiab] OR “intradermal test”[tiab]) AND (“Drug Provocation Test”[tiab] OR “oral provocation test”[tiab] OR “oral challenge”[tiab] OR “drug challenge”[tiab]) AND (“Immunoglobulin E”[MeSH] OR “specific IgE”[tiab] OR sIgE[tiab] OR “drug-specific IgE”[tiab]) AND (diagnos*[tiab] OR “diagnostic accuracy”[tiab] OR “misdiagnosis”[tiab] OR “incorrect diagnosis”[tiab] OR “false allergy”[tiab] OR sensitivity[tiab] OR specificity[tiab] OR “alternative treatment”[tiab] OR “treatment alternatives”[tiab] OR “antibiotic stewardship”[tiab]) (“Anaphylaxis”[MeSH] OR anaphylaxis[tiab] OR anaphylactic[tiab]) AND (“Tryptases”[MeSH] OR tryptase[tiab] OR “serum tryptase”[tiab] OR “mast cell tryptase”[tiab] OR “tryptase level”[tiab] OR “baseline tryptase”[tiab] OR “acute tryptase”[tiab]) AND (“Child”[MeSH] OR “Adolescent”[MeSH] OR child*[tiab] OR pediatric*[tiab] OR pediatric*[tiab] OR adolescen*[tiab]) | “(‘drug hypersensitivity’ OR ‘penicillin allergy’ OR ‘beta lactam allergy’) AND ((‘prick test’ OR ‘immunoglobulin e’ OR ‘immunological procedures’ OR ‘anamnesis’) AND (‘follow up’ OR ‘accuracy’ OR ‘diagnostic accuracy’ OR ‘therapy’))” |
| 2 | ((“beta-Lactams”[Mesh] OR “beta Lactam Antibiotics”[Mesh] OR “beta Lactam Antibiotics” [Pharmacological Action]) OR “Penicillins”[Mesh]) AND (“Drug Hypersensitivity Syndrome”[Mesh] OR “Drug Hypersensitivity”[Mesh]) AND (“Skin Tests”[Mesh] OR “Immunoglobulin E”[Mesh] OR “Follow-Up Studies”[Mesh] OR “Medical History Taking”[Mesh]) AND (“Case Management”[Mesh] OR “Patient Care Management”[Mesh] OR “Missed Diagnosis”[Mesh] OR “Quality of Life”[Mesh]) (“Anaphylaxis”[MeSH] OR anaphylaxis[tiab] OR anaphylactic[tiab]) AND (“Tryptases”[MeSH] OR tryptase[tiab] OR “serum tryptase”[tiab] OR “mast cell tryptase”[tiab] OR “tryptase level”[tiab] OR “baseline tryptase”[tiab] OR “acute tryptase”[tiab]) AND (“Child”[MeSH] OR “Adolescent”[MeSH] OR child*[tiab] OR pediatric*[tiab] OR pediatric*[tiab] OR adolescen*[tiab]) | (‘drug hypersensitivity’ OR ‘penicillin allergy’ OR ‘beta lactam allergy’) AND ((‘prick test’ OR ‘immunoglobulin e’ OR ‘immunological procedures’ OR ‘anamnesis’) AND (‘follow up’ OR ‘accuracy’ OR ‘diagnostic accuracy’ OR ‘therapy’))” | |
| Hymenoptera Venom Allergy | 1 | (“Arthropod Venoms”[Mesh] OR “Bee Venoms”[Mesh] OR “Venom Hypersensitivity”[Mesh]) AND ((“Allergy and Immunology”[Mesh] AND (“Ambulatory Care”[Mesh] OR “Office Visits”[Mesh])) OR (“Pediatrics”[Mesh] AND (“Ambulatory Care”[Mesh] OR “Office Visits”[Mesh])) AND (“Anaphylaxis”[Mesh]) OR “Recurrence”[Mesh] OR “Immunomodulation”[Mesh] OR “Immunotherapy, Active”[Mesh]) (“Anaphylaxis”[MeSH] OR anaphylaxis[tiab] OR anaphylactic[tiab]) AND (“Tryptases”[MeSH] OR tryptase[tiab] OR “serum tryptase”[tiab] OR “mast cell tryptase”[tiab] OR “tryptase level”[tiab] OR “baseline tryptase”[tiab] OR “acute tryptase”[tiab]) AND (“Child”[MeSH] OR “Adolescent”[MeSH] OR child*[tiab] OR pediatric*[tiab] OR pediatric*[tiab] OR adolescen*[tiab]) | ((‘hymenoptera’/exp OR ‘hymenoptera’) OR ‘insect venom’ OR ‘hymenoptera venom’ OR ‘hymenoptera venom allergy’) AND (‘immunology’ AND (‘outpatient’ OR ‘ambulatory care’ OR ‘hospital visit’) OR (‘pediatrics’ AND (‘outpatient’ OR ‘ambulatory care’ OR ‘hospital visit’))) AND (‘immunomodulation’ OR ‘immunotherapy’ OR ‘anaphylaxis’ OR ‘relapse’ OR ‘recurrent disease’ OR ‘recurrence risk’) |
| 2 | (“Hymenoptera Venoms”[MeSH] OR “Insect Stings”[MeSH] OR hymenoptera[tiab] OR “insect sting”[tiab]) AND (“Skin Tests”[MeSH] OR prick[tiab] OR intradermal[tiab]) AND (“Immunoglobulin E”[MeSH] OR “specific IgE”[tiab] OR sIgE[tiab]) AND (“Basophil Activation Test”[tiab] OR BAT[tiab]) AND (diagnos*[tiab] OR “diagnostic accuracy”[tiab] OR sensitivity[tiab] OR specificity[tiab]) (“Anaphylaxis”[MeSH] OR anaphylaxis[tiab] OR anaphylactic[tiab]) AND (“Tryptases”[MeSH] OR tryptase[tiab] OR “serum tryptase”[tiab] OR “mast cell tryptase”[tiab] OR “tryptase level”[tiab] OR “baseline tryptase”[tiab] OR “acute tryptase”[tiab]) AND (“Child”[MeSH] OR “Adolescent”[MeSH] OR child*[tiab] OR pediatric*[tiab] OR pediatric*[tiab] OR adolescen*[tiab]) | (‘hymenoptera venom allergy’ OR ‘bee venom extract’ OR ‘insect allergy’) AND (‘prick test’ OR ‘immunoglobulin e’ OR ‘immunological procedures’ OR ‘anamnesis’) AND (‘follow up’ OR ‘accuracy’ OR ‘diagnostic accuracy’ OR ‘therapy’) |
| Author | Year | D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | D9 | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anaphylaxis | |||||||||||
| De Schryver S et al. [33] | 2016 | + | + | + | + | + | + | + | 7 | ||
| Cavkaytar et al. [34] | 2016 | + | + | + | + | + | + | 6 | |||
| Le M. et al. [78] | 2018 | + | + | + | + | + | 5 | ||||
| Vetander M. et al. [38] | 2016 | + | + | + | + | + | 5 | ||||
| Clark E. et al. [32] | 2023 | + | + | + | + | + | 5 | ||||
| Burrell S. et al. [27] | 2021 | + | + | + | + | + | + | 6 | |||
| Drug allergy | |||||||||||
| Moral et al. [53] | 2022 | + | + | + | + | + | + | 6 | 1 | ||
| Goh et al. [49] | 2021 | + | + | + | + | + | + | 6 | 1 | ||
| Labrosse et al. [52] | 2020 | + | + | + | + | + | + | 6 | 1 | ||
| Mill et al. [57] | 2016 | + | + | + | + | + | + | 6 | 1 | ||
| Hymenoptera Venom Allergy | |||||||||||
| Worm et al. [60] | 2014 | + | + | + | 3 | ||||||
| Quercia et al. [58] | 2014 | + | + | + | + | 4 | |||||
| Graif et al. [79] | 2009 | + | + | + | + | + | 5 | ||||
| Tischler et al. [76] | 2025 | + | + | + | + | + | + | 6 | |||
| Baker et al. [71] | 2016 | + | + | + | + | 4 | |||||
| Kokcu Karadag et al. [75] | 2024 | + | + | 2 | |||||||
| Vos et al. [74] | 2013 | + | + | + | + | + | + | 6 |

 : definitively high;
: definitively high;  : probably high; ☺: probably low; ☺☺: definitively low.
: probably high; ☺: probably low; ☺☺: definitively low.

 : definitively high;
: definitively high;  : probably high; ☺: probably low; ☺☺: definitively low.
: probably high; ☺: probably low; ☺☺: definitively low.| Study | D1 | D2 | D3 | D4 | D5 | D6 |
|---|---|---|---|---|---|---|
| Umasunthar T. et al. 2015 [25] | ☺☺ | ☺☺ | ☺ |  | ☺ |  |
| Kukkonen et al. 2016 [44] | ☺☺ | ☺☺ | ☺ |  | ☺ |  |
| Van Veen et al. 2016 [45] | ☺☺ | ☺☺ | ☺ | ☺ | ☺ |  |
| Authors | Year | Type of Study | Objectives | Population (General Characteristics) | Sample Size | Outcome | Results (Quantitative Elements) | Results (Description) | Limitations | Pico |
|---|---|---|---|---|---|---|---|---|---|---|
| Clark E. et al. [32] | 2023 | CS, R, M | To assess the appropriateness of using epinephrine in children experiencing anaphylaxis. | Children (aged 0–18) who presented with suspected anaphylaxis at the Pediatric Emergency Department of Montpellier University Hospital between 2016 and 2020. | 1.056 Children | To assess whether patients with anaphylaxis received adrenaline as first-line treatment, verify the appropriateness of prescribing adrenaline auto-injectors upon discharge. | Of the 224 children diagnosed with anaphylaxis, only 17.0% received an adrenaline/epinephrine injection, while 57.1% consulted an allergist after the acute episode. Upon discharge from the emergency department, 63 patients (28.1%) were prescribed an auto-injector. | There was inadequate clinical management (less than half of the children received adrenaline) and insufficient preparation of patients and families for preventing future anaphylactic reactions (adrenaline auto-injectors were not prescribed as widely upon discharge). | The study is based on previously recorded clinical data; single-centre study. | 1 |
| Burrell S. et al. [27] | 2021 | CS, R, M | To assess the impact of self-administering adrenaline during oral challenge tests on the health-related quality of life (HRQoL) and self-efficacy of children with a peanut allergy. | Children (aged 8–16) who experienced reactions during oral provocation tests at a hospital’s specialist allergy centre. | 56 Children | Health-related quality of life (HRQoL); self-efficacy in managing anaphylaxis; emotional responses to experiencing anaphylaxis in a controlled hospital setting; the impact of self-administering adrenaline on reducing fear and anxiety; the rate of successful self-injections in real-life clinical circumstances. | There was an average improvement of 2.6 points in HRQoL scores and an average increase of 4.1 points in self-efficacy scores after the food challenge. There was also an average improvement of 10.3 points in the child’s HRQoL based on parental opinion. The occurrence of anaphylaxis during the food challenge did not negatively impact HRQoL or self-efficacy. | Self-administration of adrenaline improves HRQoL and self-efficacy. Parents perceive an even greater impact. Encouragement for autonomous use is provided during hospital challenges. | Small sample size; secondary analysis; selection bias; short follow-up period; subjective outcome measures; and limited data on adrenaline usage experience. | 1 |
| Le M. et al. [77] | 2019 | CS, P, M | To evaluate and compare the demographic and clinical characteristics, as well as the management, of cases of anaphylaxis without identifiable triggers in adults and children in Canada. | Adults and children with anaphylaxis (mean age 9.0 years, interquartile range 5.0–14.3, for children and 38.1 years, interquartile range 28.1–51.3 for adults) without identifiable triggers recorded between 2011 and 2018 in the emergency departments of eight centres in Canada as part of the Cross Canada Anaphylaxis Registry (C CARE). | 222 children (aged 0–18) and 73 adults | The clinical management and long-term follow-up of patients who have experienced anaphylaxis for which no trigger was identified. | A total of 136 patients received adrenaline in the pre-hospital setting, with a higher percentage of children than adults being treated with adrenaline (74 and 10 cases, respectively). In the emergency department, antihistamines and corticosteroids were used more often in adults than in children. Tryptase levels were measured in only 26 cases (9.8%), and only two patients underwent periodic tryptase monitoring, one of whom had an elevated tryptase level at the time of the reaction. Adrenaline auto-injectors were prescribed in 185 cases, and 40 patients had already received a prescription. Fifty-three patients (43 children and 10 adults) were seen by an allergist or an internist/otorhinolaryngologist specializing in allergology. | There were low referral rates to allergists; children were referred more often than adults. Treatment was inconsistent and frequently suboptimal (there was excessive use of antihistamines and reduced use of adrenaline). Treatment was better for children than adults. Tryptase levels were not measured in most patients at the time of the reaction. | The results are limited to eight centres. Participant recruitment was conducted primarily in academic institutions, where participants are more likely to consult allergists or specialists practising allergy medicine. Recruitment was conducted in the province of Quebec, as three sites in Quebec participated in this study. | 1 |
| De Schryver, S et al. [33] | 2016 | CS, P, S | To assess tryptase levels in children presenting with anaphylaxis, to identify factors associated with elevated tryptase levels, and to evaluate the difference between tryptase levels during the acute reaction and levels post-reaction baseline. | Children (aged 0–18) who presented to the emergency department of the Montreal Children’s Hospital with a diagnosis of anaphylaxis between April 2011 and April 2015 | 203 Children | The usefulness of tryptase levels as a diagnostic biomarker in pediatric anaphylaxis. | During the reaction, 19.2% of children had tryptase levels of at least 11.4 μg/L. Levels above the threshold (2 ng/mL + 1.2 x baseline or post-reaction tryptase level) were found in 85.7% of severe reactions, 54.2% of moderate reactions and 69.2% of mild reactions. Of the 68 children for whom post-reaction tryptase levels were measured, the mean level at the time of the reaction was 9.9 μg/L, compared to 3.6 μg/L post-reaction, representing a difference of 6.3 μg/L. | Severe reactions were more frequently associated with elevated tryptase levels. However, many children with clinically evident anaphylaxis had normal tryptase levels, indicating that this biomarker has limited sensitivity. Tryptase levels are generally higher during the reaction than the post-reaction baseline, confirming that increased tryptase is a marker of mast cell activation during anaphylaxis. | Only 68 patients had measurements taken during and after the reaction: single-centre study | 2 |
| Cavkaytar et al. [34] | 2016 | CS, R, M | To determine whether baseline serum tryptase levels pose a risk of immediate-type drug hypersensitivity reactions in children. | Children (aged 0–18 years) admitted to the Pediatric Allergy Department at Hacettepe University between January 2012 and August 2015, who had a history of suspected immediate drug hypersensitivity with an onset within six hours of taking the responsible drug. | 345 Children | The usefulness of baseline serum tryptase levels as a marker of risk for immediate drug hypersensitivity in children. | A total of 106 children (30.7%) had a history of drug hypersensitivity reaction (DHR), as confirmed by skin and/or provocation tests. The median baseline tryptase level (interquartile range) of patients with DHR was 2.6 μg/L, with a maximum level of 8.2 μg/L. There was no significant difference in baseline tryptase levels between patients who were hypersensitive to the drug, with or without anaphylaxis, [2.6 (2.0–3.6) μg/L vs. 2.8 (1.6–4.3) μg/L, p > 0.05]. | Basal tryptase levels are not an effective predictor of immediate allergic reactions to drugs in children. Immediate drug hypersensitivity can occur even when these levels are normal. | The absence of serial measurements of serum tryptase levels in patients who are hypersensitive to the drug during actual hypersensitivity reactions to the drug; single-centre study. | 2 |
| Vetander M. et al. [38] | 2016 | CS, P, M | To assess the incidence of food anaphylaxis among adolescents and identify the associated risk factors. | Children aged 0–16 were recruited at birth (1994–1996) in four districts of Stockholm and were then followed up until they reached the age of 16. | 3153 Children | Incidence of food anaphylaxis among adolescents, and identification of associated risk factors. | The overall incidence of anaphylaxis (self-reported) was 0.8% among adolescents. One third of adolescents had access to healthcare during an anaphylactic reaction. Among those who experienced an anaphylactic reaction, 67% had a prescription for an epinephrine auto-injector (e.g., an EpiPen) and 15% received adrenaline treatment during the reaction. Factors associated with an anaphylactic reaction at the age of 16 were: reactions to food at the age of 1–2 (OR: 17.7; 95% CI: 6.91–45.2); sensitization at the age of 4 (OR: 20.9; 95% CI: 6.8–64); polysensitization; having eczema or asthma | Low demand for medical assistance; limited use of adrenaline; lack of awareness of food risks. | Diagnoses of anaphylaxis were based in part on questionnaires completed by parents or participants, without clinical confirmation; the study was conducted in an urban Swedish cohort, so the results may not be generalisable to populations in other countries | 3 |
| Authors | Year | Objectives | Population | Outcome | Results (Quantitative Elements) | Results (Description) | Pico |
|---|---|---|---|---|---|---|---|
| Umasunthar T. et al. [25] | 2015 | To evaluate the ability of patients and parents of children with food allergies to recognize anaphylaxis and administer adrenaline auto-injectors (AAIs) appropriately during a simulated emergency. | Children with food allergies were recruited from allergy clinics in the UK (St Mary’s Hospital and Imperial College, both in London). | Administering adrenaline properly via an auto-injector during a simulated anaphylaxis scenario. | After six weeks, 42% of Anapen users (30 out of 71) and 43% of EpiPen users (31 out of 73) successfully administered adrenaline in a simulated anaphylaxis scenario. Success rates at one year were similar to those at six weeks. A study on device switching after one year of follow-up without retraining found that success rates were lower for those who switched from one device to another with a different operating mechanism than for those who switched from one device to another with a similar operating mechanism. | The effectiveness of self-administering adrenaline depends heavily on the type of device used. Auto-injectors equipped with voice instructions can significantly improve the likelihood of correct administration. Adequate and ongoing training is necessary to minimize errors. | 1 |
| Kukkonen et al. [44] | 2015 | To assess the association between sensitization to specific peanut components and the severity of allergic reactions in a pediatric population | Children aged 6–18 years suspected of having a peanut allergy and undergoing an oral challenge. | Measuring sIgE against peanut components as a diagnostic tool for predicting severe allergic reactions. | Of the 69 patients who tested positive (68%), 36% experienced severe reactions, 52% experienced moderate reactions and 12% experienced mild reactions. Thirty-eight people (37%) received adrenaline during the challenge. Ara h6 was the best indicator for discriminating between moderate and severe allergy, with an area under the curve (AUC) of 0.98 (95% CI: 0.96–1.00), while Ara h8 showed no diagnostic value with an AUC of 0.42 (95% CI: 0.30–0.52). Combining Ara h2 and Ara h6 enabled all severe reactions to be identified, even at low allergen doses. | Using Component-Resolved Diagnostics (CRD) that focus on Ara h6, or combining them with Ara h2, can significantly reduce the need for oral challenge tests (OFTs), thereby improving the accuracy of diagnoses of severe peanut allergies. | 4 |
| Van Veen et al. [45] | 2016 | To evaluate the diagnostic effectiveness of component-resolved (CRD) tests and establish whether they could replace oral food challenge tests. | Children with suspected peanut allergy, recruited between 2012 and 2013 at Reinier de Graaf Hospital in Delft, the Netherlands. | Diagnostic utility of CRD (component-resolved) tests. | The double-blind, placebo-controlled food challenge with peanuts produced a positive result in 33 children (53%). No correlation was found between the trigger dose and the severity of the reaction. However, a statistically significant correlation was found between the skin prick test, peanut-specific IgE, Ara h 1, Ara h 2 and Ara h 6, and the outcome of the food challenge test in terms of positivity or negativity (p < 0.001). No correlation was found between specific IgE for Ara h 3, Ara h 8 and Ara h 9, and the clinical outcome of the food challenge test. | Molecular diagnostics (also known as component-resolved diagnostics) are not superior to traditional tests such as extract and SPT, and they cannot replace the gold standard of DBPCFC, particularly when it comes to measuring reaction dose or clinical severity of allergy. | 4 |
| Authors | Year | Systematic Review with/Without Meta-Analysis | Objectives | Population | Outcome | Results (Quantitative Elements) | Results (Description) | Pico |
|---|---|---|---|---|---|---|---|---|
| Sim M. et al. [28] | 2025 | SR without MA | To provide a critical evaluation of the effectiveness of adrenaline auto-injectors in preventing fatal anaphylactic outcomes. | Adults and children | The effectiveness of adrenaline auto-injectors (AAIs) in preventing fatal anaphylaxis. The pharmacokinetics and pharmacodynamics of intramuscular adrenaline. Limitations and delays in the use of AAIs. A comparison with intravenous adrenaline. The need for alternative methods of administration. | Studies of animal physiology have shown that intravenous infusions of adrenaline at doses of 0.05–0.5 µg/kg/min for one to two hours (approximately 10 µg/kg in total) are effective in treating severe anaphylaxis, ensuring stable and prolonged plasma levels. In contrast, intramuscular injections, including auto-injectors (AIs), produce short and variable adrenaline plasma peaks (100–500 pg/mL) and a less predictable clinical response. | According to current guidelines, IM adrenaline via an auto-injector remains the recommended first-line treatment, but this may not be sufficient in the most critical cases. In such situations, the authors suggest a more effective approach: continuous, controlled intravenous infusions of adrenaline in combination with supportive fluid therapy. However, implementing this strategy in pre-hospital or home settings still presents significant safety and feasibility challenges. | 1 |
| Martelli A. et al. [43] | 2020 | SR without MA | To provide a summary of recent advances in the epidemiology, diagnosis and management of anaphylaxis, paying particular attention to pediatric patients. | Adults and children | New insights into the epidemiology and triggers of anaphylaxis; advances in diagnosis and biomarkers; updates in treatment approaches and guidelines; attention to differences in the pediatric population | In children, food remains the leading cause of anaphylaxis, while in adults, medications and insect stings are more common. Biomarkers such as tryptase have limited diagnostic utility, particularly in children. Timely administration of intramuscular epinephrine remains the cornerstone of treatment. New approaches include personalized action plans, education, and emerging biological therapies (e.g., omalizumab). It is important to recognize biphasic reactions and comorbidities (e.g., asthma). | Effective management of anaphylaxis requires prompt diagnosis, based on an immediate assessment of the patient’s airways, breathing, circulation, and mental state. Treatment must include the immediate intramuscular administration of adrenaline, which is recognized as the essential first-line therapy. Particular attention should be paid to preventing biphasic reactions, which can occur even after the initial symptoms have resolved. Upon discharge, each child must receive a prescription for an adrenaline auto-injector accompanied by detailed instructions on its correct use. Families must also be provided with a personalized action plan, and the school and wider community must be adequately informed and trained to ensure a rapid and safe response in the event of an anaphylactic emergency. | 1 |
| Navalpakam et al. [24] | 2013 | SR without MA | To providing a comprehensive resource for allergists, focusing on the long-term management of anaphylaxis | Adults and children | Long-term management of anaphylaxis by allergists | A specialist allergy assessment is needed for children after an episode of anaphylaxis to identify triggers and establish a personalized plan. Education should focus on patients at higher risk of anaphylaxis recurrence, such as those with a history of severe symptoms or anaphylaxis, those with a trigger of peanuts and/or nuts, those with a history of asthma and those who are female. Patient counselling should involve providing individualized action plans and discussing the correct use, storage and safety of epinephrine auto-injectors. | The article emphasizes the importance of long-term anaphylaxis management, particularly in an outpatient setting. It highlights the central role of allergists in identifying the cause of anaphylactic reactions and comorbidities, such as asthma or mast cell disorders. They are also responsible for personalizing treatment and reducing the risk of recurrence. Great attention is paid to educating patients and caregivers: they must know how to recognize symptoms early, administer auto-injectable adrenaline correctly, and manage emergency situations. For this reason, the article recommends individualized action plans, medical identification tools (such as bracelets or cards) and careful follow-up. | 1 |
| Dribin et al. [19] | 2025 | Consensus statement | To develop a standardized, internationally accepted definition of anaphylaxis, alongside an educational overview and clinical support tools. | Adults and children | To improve the recognition, diagnosis and management of anaphylaxis in different clinical settings by replacing existing inconsistent criteria. | Anaphylaxis is defined as a severe, potentially life-threatening allergic reaction that affects the skin, mucous membranes, respiratory system, cardiovascular system, and gastrointestinal tract. Severe anaphylaxis can affect breathing and/or the heart, even in the absence of skin symptoms. The diagnostic criteria are: -unknown exposure (a probable diagnosis is made if skin and/or mucous membrane symptoms and respiratory and/or cardiovascular involvement occur rapidly); -known exposure (a rapid onset of at least two signs from the skin, respiratory, cardiovascular and/or gastrointestinal systems, or respiratory and/or cardiovascular involvement alone). The treatment of choice is adrenaline (IM 0.01 mg/kg, max. 0.5 mg), which can be repeated every 5–15 min. Administer immediately, even if the criteria are not fully met. Systemic signs include skin, mucous membrane, respiratory and cardiovascular involvement, and gastrointestinal symptoms. In children, examples include “lip licking” in infants. | The GA2LEN 2024 consensus provides a new definition of anaphylaxis (93.5% agreement), an educational overview (97.8%) and a clinical tool (93.5%) to promote clarity in diagnosis and treatment. This intuitive tool is also suitable for pediatric settings with limited experience and encourages the timely use of adrenaline, which can improve clinical outcomes and research consistency globally. | 1 |
| Deschildre et al. [26] | 2024 | Consensus statement | To improve the knowledge and confidence of school staff in recognizing and managing food allergies and severe allergic reactions. Ensure that there are written emergency action plans for each pupil with a food allergy and that staff are trained in the safe administration of adrenaline. | Children | A standardized management framework, improved safety in school environments, emergency preparedness and education and awareness-raising. | It proposes four pillars to ensure safe schools for students with food allergies: (1) staff training through questionnaires and regular courses on allergies, symptoms, anaphylaxis, auto-injectors and psycho-emotional aspects; prevention with clear policies on spaces, meals, cleaning, allergen labelling; (2) identification of at-risk students without stigmatization and avoidance of general bans; (3) emergency preparedness with up-to-date written plans, availability of auto-injectors for general use, adequate storage and accessibility, and legal protection for staff; (4) inclusive culture with adapted educational materials, anti-bullying activities, regular policy review, and a designated coordinator with feedback from students. | The GA2LEN–EFA 2024 consensus sets out minimum guidelines, agreed by over 80 experts, for improving the management of food allergies and anaphylaxis in schools. The four intervention areas propose a flexible, integrated strategy adaptable to different regulatory contexts, aiming to ensure safety, autonomy, and educational continuity, and transform schools into environments that are truly protected and aware. | 1 |
| Anagnostou, K. et al. [22] | 2018 | SR without MA | To synthesize current knowledge to support clinicians in the diagnosis, management and prevention of pediatric anaphylaxis. | Patients aged <18 years | Current knowledge on diagnosing, managing and preventing pediatric anaphylaxis. | Annual incidence: 50–112 episodes per 100,000 people. Rates are higher in the first two years of life (almost three times higher than in other age groups). Anaphylaxis is more common in males up to the age of 10–15; in females, the rate tends to increase after this age. Foods are the main trigger in children, particularly eggs, cow’s milk, and nuts. Risk factors for severe outcomes include uncontrolled asthma, previous biphasic or protracted episodes, repeated administration of epinephrine, wheezing, hypotension, and pharyngeal edema. | The number of cases of anaphylaxis in children is increasing, particularly in the early years of life and among males. Food is the main trigger. Effective management relies on prompt diagnosis, the correct use of auto-injectable adrenaline, the recognition of risk factors (such as uncontrolled asthma), and post-crisis observation categorized according to individual risk. In addition, it is very important that patients and families receive regular training on how to identify anaphylactic episodes and respond appropriately, and this should form part of routine management. | 1 |
| Ball et al. [21] | 2023 | SR without MA | To analyze the changes to the 2021 guidelines and provide an assessment of their practical implications for anaphylaxis treatment, particularly in the pediatric population. | Adults and children | Guidelines for the management of anaphylactic emergencies, with particular attention to the pediatric population | If airway, breathing or circulation problems persist after 5 min, repeat the intramuscular epinephrine injection. The new recommended dose of epinephrine for children under 6 months is 100–150 μg (1 in 1000). Corticosteroids are no longer recommended for the emergency treatment of anaphylaxis. Children under 16 years of age who have received emergency treatment for suspected anaphylaxis should be admitted to hospital. If an auto-injector is required for a child under 12 months of age, it should only be prescribed by a pediatric allergist. In children under 16 years of age after a suspected anaphylactic reaction, tryptase testing is useful if the cause is thought to be related to venom, medication or idiopathic factors. | Analysis of the 2021 RCUK guidelines confirms that the primary treatment for anaphylaxis is intramuscular adrenaline, with a strong recommendation to administer a second dose after five minutes if clinical signs persist. The routine use of corticosteroids has been discontinued, with antihistamines now only recommended for residual skin symptoms. New recommendations include the strategic use of intravenous (IV) fluids, the introduction of a protocol for refractory anaphylaxis involving adrenaline infusion, and a stratified approach to post-event observation. Adopting the GRADE framework ensures transparency and methodological robustness. These changes improve operational clarity, potentially improving survival rates and post-anaphylactic management in healthcare settings. | 1 and 2 |
| Brockow, K. et al. [78] | 2021 | SR without MA | To summarize and analyze the available evidence regarding symptoms of mast cell activation and anaphylactic reactions in children with mastocytosis. | Children with cutaneous mastocytosis | To identify the risk factors, prevalence, clinical manifestations, management strategies and treatment of anaphylaxis in children with mastocytosis. | The incidence of anaphylaxis in children with cutaneous mastocytosis is 1–9%. Risk factors include involvement of more than 90% of the body’s surface area and elevated serum tryptase levels (above 20 ng/mL). Baseline serum tryptase levels should be measured, as very high levels (above 100 ng/mL) may indicate a high mast cell burden and/or secondary mastocytosis. Serum tryptase levels are usually normal or slightly elevated in CM but elevated in most patients with SM and DCM. In children with tryptase levels above 10 µg/L and severe MC symptoms, analyzing extra copies of the alpha-tryptase gene (TPSAB1) can help to understand the genetic basis of these symptoms. | Although mastocytosis in children carries a risk of mast cell-mediated events, anaphylaxis is less common than in adults. Children with severe skin symptoms and high tryptase levels are more likely to experience anaphylaxis and require closer clinical monitoring. A baseline tryptase measurement should be taken 24–48 h after symptoms have resolved to assess risk. Further genetic testing is recommended in cases where tryptase levels are greater than 10 μg/L and there are severe mast cell-mediated symptoms. | 2 |
| Wang J. et al. [36] | 2024 | SR without MA | To update and clarify the clinical recommendations for diagnosing, managing and preventing anaphylaxis in daily practice for adults and children. | Adults and children | Update on the diagnosis, management and prevention of anaphylaxis | The current anaphylaxis criteria can be applied to infants and children to determine whether an allergic reaction is anaphylaxis. After prompt self-administration of epinephrine, hospital admission is not necessary if the patient responds completely and sustainably. Take serum tryptase during an acute event (within two hours of symptom onset) and at a separate time when the patient is in normal health to help diagnose anaphylaxis. Do not rely solely on serum tryptase levels to diagnose underlying mastocytosis. Perform skin tests (percutaneous and intradermal) and/or specific in vitro IgE tests four to six weeks after the event for all potential pharmacological and non-pharmacological agents used during the perioperative period. | The introduction of new, more accurate diagnostic criteria for anaphylaxis includes new biomarkers, such as tryptase. While the use of epinephrine remains central, it is no longer the only method of diagnosis. Management is personalized according to the patient’s age, the clinical context, and their response to treatment, allowing for greater flexibility in the activation of emergency services. Empowering patients is central to this approach, achieved through targeted counselling and shared decision-making. | 2 |
| Giannetti A. et al. [35] | 2021 | SR without MA | To provide an overview of mast cell activation disorders (MCAD), including classification, pathophysiology, clinical manifestations, diagnostic criteria, and treatment options. | Children | Classification and diagnostic criteria of MCAD; pathophysiological mechanisms of mast cell activation; clinical presentation and differential diagnosis; therapeutic approaches and management strategies | Symptoms are caused by the release of mediators, such as histamine, which can affect multiple systems, including the skin, gastrointestinal system, respiratory system and cardiovascular system. There is currently no consensus on specific biomarkers for MCAS. Diagnosis is based on clinical symptoms, elevated serum tryptase levels or other mediator levels, and response to anti-mediator therapy. Treatment includes avoiding triggers, taking antihistamines and mast cell stabilizers, and using corticosteroids. In severe cases, biological drugs such as omalizumab may be required. As mast cell activation syndrome can mimic other conditions, a multidisciplinary approach is essential. | Mast cell activation syndrome (MCAS) is characterized by normal mast cell density, but with functional hyperactivity. In contrast, mastocytosis is characterised by an abnormal accumulation of mast cells in tissues. Currently, the lack of specific and shared biomarkers complicates diagnosis, which is primarily based on clinical evaluation and laboratory tests. Given the absence of definitive treatments, therapeutic management remains predominantly symptomatic, with the aim of controlling signs and symptoms. This review therefore emphasizes the importance of accurate clinical investigation and a better understanding of the characteristics of mast cell activation disorders, with the aim of improving diagnosis and optimizing patient care. | 2 |
| Calvani M. et al. [40] | 2020 | SR without MA | To provide an up-to-date overview of food allergies, focusing on their pathogenesis, clinical characteristics, diagnostic tools, preventive strategies and therapeutic management, particularly in children. | Adults and children | The immunological mechanisms underlying food allergies, advances in diagnostic approaches, and prevention strategies, particularly the early introduction of allergens, as well as current and emerging treatments. | A diagnosis requires a combination of clinical history, specific IgE testing, skin testing and oral food challenge testing. Preventive strategies now support the early introduction of allergenic foods (e.g., peanuts and eggs) in infants at risk. Management includes elimination diets, emergency plans (e.g., epinephrine auto-injectors) and emerging therapies such as oral immunotherapy (OIT). Patient and caregiver education are essential for safety and quality of life. | Food allergy is a condition that is becoming increasingly prevalent, particularly among children, and requires an integrated approach based on early diagnosis, targeted prevention, and personalized treatment strategies. In terms of diagnosis, Component-Resolved Diagnostics (CRD) are emerging as a more accurate tool than traditional tests. They can guide clinical decisions and reducing the need for oral provocation tests. Regarding prevention, the most recent evidence suggests that allergens should be introduced early in at-risk infants, challenging the traditional approach of avoidance. Finally, management strategies are evolving beyond dietary exclusion thanks to emerging therapies such as oral immunotherapy, which show promise in modifying the natural course of the disease. | 3 |
| Yue, D. et al. [39] | 2018 | SR without MA | To summarize the recent advances in understanding, diagnosing and managing food allergies and anaphylaxis, including the latest prevention and treatment strategies. | Adults and children | Diagnosis, management, prevention and treatment strategies for food allergies and anaphylaxis | The diagnosis is based on detailed clinical history; skin tests (SPT); specific IgE dosage; basophil activation tests (BAT) in some cases. Massive release of mediators (histamine, tryptase and PAF) generates the clinical response. Reduced activity of the PAF-acetylhydrolase enzyme is associated with more severe forms. Oral food challenge (OFC) remains the gold standard. Underuse of epinephrine is still common, even in healthcare settings. Emerging evidence (such as that from the LEAP study) supports the early introduction of allergenic foods in high-risk children. | Yue et al. (2018) [39] propose an updated view of allergy-related anaphylaxis. They emphasize that, although most cases are IgE-mediated, anaphylaxis can also have alternative mechanisms with similar clinical implications. The clinical presentation is determined by the massive release of inflammatory and immunological mediators (including PAF). The severity is partly dependent on the activity of the PAF-acetylhydrolase enzyme. The diagnosis remains essentially clinical. However, allergy tests (e.g., skin prick tests, specific IgE assays and oral provocation tests) are essential for identifying the responsible allergen. This is particularly important for preventing recurrence. IM adrenaline is the cornerstone of acute treatment. In the long term, the approach revolves around allergen avoidance. In the future, food immunotherapy protocols may also be used as a promising therapeutic option. | 3 |
| Turner et al. [41] | 2022 | SR with MA | To summarize the most recent evidence on the risk factors for severe allergic reactions to food. | Patients aged under 40 years with an IgE-mediated food allergy or FPIES. | To identify modifiable risk factors in patients with severe food allergies. | Mortality from food anaphylaxis is estimated at 1.81 per million person-years. Near-fatal reactions are approximately ten times more common than deaths but are still rare. Non-predictive factors include previous anaphylaxis, asthma and baseline IgE sensitization. Established risk factors include adolescent/young adult age (12–30 years) and delays in treatment (e.g., epinephrine). Emerging or uncertain factors include specific allergenic components, risk behaviour, concomitant medications, dose and physical exercise. | Currently, there are no reliable clinical or laboratory markers that can accurately predict an individual’s risk of experiencing a severe or fatal reaction to food. However, adolescence and young adulthood, as well as delayed identification and treatment, are well-established risk factors for severe reactions. | 3 and 4 |
| Kattan J. D. and Sicherer S. H. [42] | 2015 | SR without MA | To analyze emerging approaches and future prospects for diagnosing food allergies. | Adults and children | To analyze emerging approaches and future prospects for diagnosing food allergies. | Many individuals who test positive for Skin prick Test (SPT) or sIgE are tolerant to food in oral challenge tests (OCT). For example, only 22% of individuals sensitized to peanuts were clinically allergic. OCT is expensive and labour-intensive, but it is the gold standard and safe in clinical settings (only 1.7% of individuals required epinephrine). CRDs (e.g., Ara h2 and Cor a9/14) offer greater specificity than traditional tests, but often lower sensitivity. Emerging tests, such as epitope binding, can differentiate between tolerant and persistent individuals (e.g., milk) and predict reactivity (e.g., shrimp). T-cell responses can potentially distinguish between individuals who are sensitised but asymptomatic and those who are allergic and symptomatic. Basophil activation is a promising method for predicting tolerance to cooked foods (e.g., cooked milk) and potentially the severity of reactions, but it requires flow cytometry and extensive validation. PAF and PAF AH correlate with anaphylaxis severity, with low PAF AH and high PAF being associated with severe and fatal reactions. | Although traditional tests (SPT and sIgE) are useful as screening tools, they have limitations in terms of specificity and predictability. Sensitization often does not coincide with clinical allergy. Oral food challenge (OFC) remains the gold standard for confirming a diagnosis and has a high rate of negative reactions with manageable risks. Methods such as CRD improve specificity but reduce sensitivity. Emerging tests (epitope binding, basophil/T-cell reactions and PAF) show significant potential for future diagnostics but are currently limited by technical complexity or poor clinical validation. | 4 |
| Martelli A. et al. [43] | 2021 | SR without MA | To explore the role of component-resolved diagnosis (CRD) in improving the accuracy of food allergy diagnosis and risk assessment, with a particular focus on its clinical application in identifying allergenic protein components. | Adults and children | To provide an overview of CRD technology and its clinical utility and covers the identification of specific allergenic components related to severity and persistence, the differentiation between primary sensitization and cross-reactivity, and the use of CRD in predicting the risk of systemic reactions. | CRD enables the precise identification of IgE sensitization to specific protein components, such as storage proteins, lipid transfer proteins and profilins. It can distinguish between genuine food allergies and cross-reactions, such as pollen-food syndrome. Some components, such as Ara h 2 in peanut allergy, are strongly associated with severe reactions. CRD improves the stratification of individual risk, guiding decisions on dietary avoidance and immunotherapy. It is particularly useful in complex cases and for determining when an oral food challenge is necessary. | Molecular diagnosis (CRD) represents a significant advance in the management of food allergies, thanks to its ability to refine diagnosis, assess individual risk profiles and reduce the use of invasive tests. However, its clinical applications continue to be limited by the lack of established diagnostic standards and variability between studies. Until robust, standardized tests are available, CRD should be used as a complementary tool, always integrated with clinical history and, if necessary, confirmed by oral challenge tests. | 4 |
| Cardona V. et al. [2] | 2020 | Guideline | To ensure that the guidelines are aligned with the current state of knowledge regarding anaphylaxis management. | Adults and children | Guidelines for managing anaphylactic emergencies | The guidelines recommend intramuscular adrenaline (0.01 mg/kg, up to a maximum of 0.5 mg) in the anterolateral thigh as the initial treatment. This should be administered immediately and repeated every 5–15 min if necessary. Patients with anaphylaxis should be referred to an allergist/immunologist for advice on identifying and preventing triggers. During acute anaphylaxis, serum tryptase levels increase from 15 min to three hours after the onset of symptoms, peaking between one and two hours afterwards. It is recommended that baseline serum tryptase levels are assessed at least 24 h after the symptoms of anaphylaxis have resolved, even when the concentration of tryptase during the episode remains within normal limits. Allergic sensitization is diagnosed using skin tests, allergen-specific serum IgE and provocation tests. | The WAO 2020 guidelines update the management of anaphylaxis by emphasizing the importance of rapid diagnosis and the optimal use of intramuscular adrenaline, as well as structured long-term interventions such as the prescription of auto-injectors, education involving an action plan, and the involvement of specialists. The guidelines also highlight disparities in access to auto-injectors and propose strategies to overcome these barriers. The guidelines also highlight gaps in education, post-emergency management and epidemiological data collection. | 1, 2, 3 and 4 |
| Santos et al. [3] | 2023 | Guideline | To provide practical recommendations for the diagnosis of severe IgE-mediated food allergy | Adults and children | Practical recommendations for the diagnosis of severe IgE-mediated food allergy | Peanut (Ara h 2, CRD): Sensitivity: 95%; Specificity: 61%. High identification capacity, but risk of false positives. Cow’s milk (whole allergen sIgE): Sensitivity: 53%; Specificity: 88%. Useful for ruling out allergy, but less so for confirming it. Egg (whole sIgE allergen): sensitivity 92%, specificity 58% = high sensitivity with a risk of overdiagnosis. Wheat and soy (whole sIgE allergen): Sensitivity and specificity are between 55% and 73%, indicating less defined diagnostic performance. | Compared to SPT and total sIgE, specific IgE tests, particularly those for peanut allergens such as Ara h 2, improve diagnostic accuracy. However, high sensitivity and variable specificity require clinical interpretation and confirmation with oral food challenge (OFC) to avoid misdiagnosis and unnecessary dietary restrictions. The variability between foods emphasizes the importance of a personalized approach that integrates sIgE, CRD, SPT and BAT, with OFC serving as the gold standard for diagnosis when necessary. | 3 and 4 |
| Authors | Year | Type of Study | Objectives | Population (General Characteristics) | Sample Size | Outcome | Results (Quantitative Elements) | Results (Description) | Limitations | Pico |
|---|---|---|---|---|---|---|---|---|---|---|
| Moral et al. [53] | 2022 | CS, R, M | To describe the clinical characteristics of pediatric patients with a presumed positive or inconclusive drug provocation test (DPT) for hypersensitivity to beta-lactam antibiotics (BLA), to evaluate the decision to repeat the DPT, and describe the outcome. | Patients (<15 years of age) who attended one of the six participating pediatric allergy clinics between January 2017 and December 2019. | 439 children underwent beta-lactam challenge testing; 26 of them (5.9%) with presumed positive or inconclusive results were included in the study. | Interpretation of initial DPT results, decision to repeat the DPT, and outcome of the repeated DPT | Only 5.9% of patients evaluated for suspected BLA allergy had a potentially positive DPT. However, more than half of patients with a positive or inconclusive DPT-1 tolerated BLA during DPT-2, most of which was performed within 6 months of DPT-1. | This study concludes that a DPT positive for BLA should preferably be confirmed with a second DPT, to be performed within a few weeks or months. | Retrospective study, small sample size and heterogeneous practices in participating centres. | 1 |
| Goh et al. [49] | 2021 | C, R, S | To evaluate the results of oral provocation tests (OPT) in children with suspected hypersensitivity reactions to beta-lactam antibiotics | Children (<18 years) treated at KK Women’s and Children’s Hospital, Singapore, between August 2016 and December 2017 | 120 | The proportion of negative OPT results; the frequency of mild adverse reactions; the diagnostic accuracy of OPT | 93% of OPTs were negative (110/118): 92% negative for the penicillin group (96/104), 100% negative for cephalosporins (14/14). | All reactions were mild | Retrospective design. Limited assessment of hypersensitivity to clavulanic acid. Small sample size for some drug groups. | 1 |
| Labrosse et al. [52] | 2020 | C, P, S | To evaluate the sensitivity and specificity of the double-blind penicillin skin test (PST) with penicilloyl-polylysine (PPL) and benzylpenicillin (BP) in predicting reactions to oral provocation test with amoxicillin in children with a history of non-life-threatening penicillin allergy | Children aged between 0.7 and 18.1 years with a history of non-severe penicillin allergy, referred to a tertiary allergy centre. | 158 | Sensitivity and specificity of PST in predicting reactions to oral provocation with amoxicillin; positive and negative predictive values; proportion of patients with positive PST and subsequent reactions | Sensitivity: 20% (95% CI: 0.5–71.6%). Specificity: 90% (95% CI: 84.4–94.4%). Positive predictive value: 6.3% (95% CI: 0.4–26.3%) | 3.2% of participants had an immediate or accelerated reaction to the amoxicillin challenge; all reactions were mild and resolved with antihistamines. | Low incidence of events, resulting in wide confidence intervals. Exclusion of accelerated reactions led to an estimated sensitivity of 33% (95% CI: 0.8–90.5%). Lack of intradermal testing with amoxicillin due to its unavailability in Canada. Possible variability in skin test results that may affect reliability. | 1 |
| Mill et al. [57] | 2016 | C, P, S | To evaluate the diagnostic accuracy of a graded oral challenge (OC) in diagnosing both immediate and non-immediate allergic reactions to amoxicillin in children | Children (between 1 and 3.9 years old) with suspected reaction to amoxicillin | 818 | The proportion of children who experienced immediate and non-immediate reactions to graded OC. Measures of the diagnostic accuracy (sensitivity, specificity and positive and negative predictive values) of graded OC associated with immediate and non-immediate reactions | 94.1% of children tolerated the graduated OC. 2.1% had immediate reactions (all mild). 3.8% had non-immediate reactions (all mild). The graduated OC showed 100% specificity, 100% positive predictive value, and 89.1% negative predictive value. | Factors associated with immediate reactions: history of reaction within 5 min of exposure. Factors associated with non-immediate reactions: rash lasting more than 7 days and family (parental) history of drug allergy. | It is impossible to determine the sensitivity of the graded OC due to the study design. There is a possibility of classification bias due to reliance on parental reports. The results are limited in their generalisability to populations other than the one studied. | 1 |
| Authors | Year | Systematic Review with/Without Meta-Analysis | Objectives | Population | Outcome | Results (Quantitative Elements) | Results (Description) | Pico |
|---|---|---|---|---|---|---|---|---|
| Paño-Pardo et al. [48] | 2023 | Consensus document | To formulate evidence-based recommendations aimed at improving the management of patients with suspected or confirmed antibiotic allergy, as well as standardizing the approach of both clinicians in prescribing antimicrobial agents to patients labelled as allergic and allergists in confirming or excluding the label of antibiotic allergy. | These guidelines are not limited to patients of a specific gender or age group. Instead, they take a comprehensive approach that includes allergies to all classes of antibiotics. | Epidemiology of antibiotic allergies; Risk assessment in patients labelled as allergic to antibiotics; Assessment of patients with suspected antibiotic allergies through additional testing; Choice of antibiotic in patients with reported allergies to penicillin or cephalosporins. | Eleven recommendations were formulated, all with a strength rating of A: solid evidence supporting a recommendation for or against use. | Epidemiology of antibiotic allergies; Risk assessment in patients labelled as allergic to antibiotics; Assessment of patients with suspected antibiotic allergies through additional testing; Choice of antibiotic in patients with reported allergies to penicillin or cephalosporins. | 1 |
| Sousa-Pinto et al. [46] | 2021 | SR with MA | To evaluate the effectiveness of skin tests and the quantification of specific IgE in diagnosing patients with allergies to penicillin or other β-lactams. | Patients with reported allergy to penicillin or β-lactams, in whom the results of the DPT were compared with those of skin tests (prick test and/or intradermal test) and/or with the quantification of specific IgE (sIgE). | Sensitivity and specificity of skin tests and specific IgE quantification; predictive values (positive and negative) of these tests | Skin tests: sensitivity 30.7%, specificity 96.8%; specific IgE: sensitivity 19.3%, specificity 97.4% | Both tests show low sensitivity and high specificity. | 1 |
| Felix et al. [50] | 2020 | SR | To evaluate the safety and efficacy of direct OPT in the diagnosis of beta-lactam allergy in children with mild skin reactions. | Children with a history of mild, immediate and non-immediate skin reactions to beta-lactams | Proportion of negative OPT results; frequency of mild adverse reactions; safety of direct OPT | High proportion of negative results in OPT; most patients tolerated OPT without serious reactions. | 1 | |
| Mori et al. [54] | 2019 | Reviews | To provide a comprehensive overview of the management of suspected reactions to antibiotics in children, including diagnostic approaches and treatment strategies. | Children suspected of having an allergic reaction to antibiotics. | Diagnostic strategies for antibiotic hypersensitivity in children; management approaches for suspected antibiotic reactions | Emphasis on the importance of a detailed clinical history in the diagnosis of antibiotic hypersensitivity; discussion of various diagnostic tests, including skin tests and drug challenge tests; recommendations for management strategies based on the type of reaction and the suspected antibiotic. | Diagnostic strategies for antibiotic hypersensitivity in children; management approaches for suspected antibiotic reactions | 1 |
| Calamelli et al. [55] | 2019 | Review | Provide a practical approach to managing children who have experienced adverse reactions to antibiotics, emphasizing the importance of accurate diagnosis and appropriate management strategies. | Children who have experienced an adverse reaction to antibiotics | Overview of diagnostic tools and procedures used to identify antibiotic allergies in pediatric patients; guidance on how to manage and treat children with suspected antibiotic-related allergic reactions. | It emphasizes the key role of taking an accurate clinical history to distinguish true allergic reactions from non-allergic side effects; it describes the use of skin tests and oral provocation as essential methods for confirming or ruling out an allergy; it offers practical recommendations tailored to the type and severity of the reaction, with the aim of unnecessary avoidance of antibiotics and optimizing treatment options. | Overview of diagnostic tools and procedures used to identify antibiotic allergies in pediatric patients; guidance on how to manage and treat children with suspected antibiotic-related allergic reactions. | 1 |
| Romano et al. [4] | 2020 | Position paper | To update and to standardize the diagnostic approach to beta-lactam hypersensitivity, integrating evidence-based guidelines and expert consensus. | Development of algorithms for risk stratification. Standardization of protocols for skin testing (ST) and DPT. Recommendations for the administration of alternative beta-lactams in allergic individuals. | Introduction of risk stratification based on morphology, timing and severity of the reaction. Emphasis on the use of ST and DPT in the diagnosis of hypersensitivity to beta-lactams. Recommendations for the administration of alternative beta-lactams in allergic individuals. | Development of algorithms for risk stratification. Standardization of protocols for ST and DPT. Recommendations for the administration of alternative beta-lactams in allergic individuals. | 1 | |
| Caffarelli et al. [47] | 2018 | Position paper | To provide guidelines for managing suspected allergies to penicillin and β-lactam antibiotics, including diagnostic and therapeutic strategies. | Recommendations for performing DPTs in children with suspected penicillin/β-lactam antibiotic allergy. Guidelines for managing immediate and delayed reactions to penicillin/β-lactam antibiotics in pediatric patients. | The DPT is identified as the most reliable method for confirming or ruling out β-lactam allergy in children. The document provides detailed, step-by-step protocols for performing DPTs safely, including dosing schedules and monitoring measures. The critical role of a thorough medical history and proper risk assessment in determining, when DPT, is appropriate is emphasized. The guidelines also offer strategies for managing any allergic reactions that may occur during or after the test. | Recommendations for performing DPTs in children with suspected penicillin/β-lactam antibiotic allergy. Guidelines for managing immediate and delayed reactions to penicillin/β-lactam antibiotics in pediatric patients. | 1 | |
| Brockow et al. [56] | 2025 | Position paper | Provide guidelines for managing suspected allergies to penicillin and β-lactam antibiotics, including diagnostic and therapeutic strategies. | Recommendations for performing DPTs in children with suspected penicillin/β-lactam antibiotic allergy. Guidelines for managing immediate and delayed reactions to penicillin/β-lactam antibiotics in pediatric patients. | The DPT is identified as the most reliable method for confirming or ruling out β-lactam allergy in children. The document provides detailed, step-by-step protocols for performing DPTs safely, including dosing schedules and monitoring measures. The critical role of a thorough medical history and proper risk assessment in determining when DPT is appropriate is emphasized. The guidelines also offer strategies for managing any allergic reactions that may occur during or after the test. | Recommendations for performing DPTs in children with suspected penicillin/β-lactam antibiotic allergy. Guidelines for managing immediate and delayed reactions to penicillin/β-lactam antibiotics in pediatric patients. | 1 |
| Authors | Year | Type of Study | Objectives | Population (General Characteristics) | Sample Size | Outcome | Results (Quantitative Elements) | Results (Description) | Limitations | Pico |
|---|---|---|---|---|---|---|---|---|---|---|
| Worm et al. [60] | 2014 | CS, P, M | The aim of the NORA study was to collect and analyze prospective data on cases of anaphylaxis treated in European specialist centres in order to describe their causes, clinical characteristics, therapeutic management and differences between pediatric and adult populations. | The study analyzed a very large and diverse population (26.7% of cases under the age of 18, 73.3% adults) of patients suffering from severe allergic reactions (anaphylaxis) in several European countries. | 3333 cases of anaphylaxis collected via an online questionnaire based on clinical and diagnostic data between June 2011 and March 2014. | The study highlighted significant differences in the triggers and clinical presentation of anaphylaxis in children and adults. It emphasized the frequent underuse of adrenaline as an emergency treatment and confirmed the need to improve the early identification and management of the condition across Europe. | 59 allergy/dermatology/pediatrics centres in 10 European countries. 3333 episodes of anaphylaxis recorded. 26.7% of patients were aged < 18 years; 73.3% were adults. In children, the most frequent cause of anaphylaxis (64.9%) was food; in adults, the most frequent cause (47.4%) was medication. Hymenoptera venom was the cause of anaphylaxis in children in 20.2% of cases. 80.5% of cases occurred within 30 min of exposure; only 6.7% had a delay of more than 4 h (mainly in cases involving drugs). Skin symptoms were present in 84.1% of cases. 34.2% had already had a reaction, usually milder, to the same allergen. Among the treatments administered by professionals as first aid, 60.4% were corticosteroids and 52.8% were antihistamines. Adrenaline was administered at the site of the event in 13.7% of cases due to food and in 27.6% of cases due to hymenoptera venom. | The NORA study described 3333 cases of anaphylaxis in Europe, highlighting how the causes, clinical manifestations and management vary significantly between children and adults. Food is the main cause in children, while drugs and insect venom predominate in adults. Respiratory symptoms are more common in children than in adults, while cardiovascular symptoms are more common in adults. In both children and adults, skin involvement is the predominant clinical manifestation. Despite clinical recommendations, adrenaline has been underused as an emergency treatment, indicating a need for improved medical education and early response to anaphylaxis. | It is not exclusively focused on pediatric populations. It is based on observational and non-experimental data collection from spontaneous reports, which may lead to overestimation or underestimation of the data. | 1 |
| Quercia et al. [58] | 2014 | CS, R, S | To assess the actual prevalence and incidence of reactions to hymenoptera venom in the pediatric population, with particular attention to systemic reactions and extensive local reactions. To estimate the annual incidence of new allergic reactions in that population during the 2 years following the initial survey. To investigate the clinical management of children who had experienced significant reactions. | 1035 children aged between 6 and 14 in the town of Cotignola (RA). Study conducted in the town’s state schools. | 1035 children | The study results do not confirm recent reports of an increase in the prevalence of hymenoptera venom allergy in children. The study reduces the perception of the risk of severe systemic reactions to hymenoptera stings in children and suggests that the prevalence is much lower than previously estimated, the incidence of new reactions is statistically negligible, clinical management is often inadequate and needs to be improved, especially with regard to allergy diagnosis and the use of immunotherapy. | Of a population of 1035 children, 173 (16.7%) had been stung by hymenopterans at least once. Of these, five (0.5%) had a systemic reaction (SR) and nine (0.9%) had an extensive local reaction (LLR). Only one reaction was severe. Of the 14 subjects with an SR or LLR, five (35.7%) underwent diagnostic evaluation, and one (7.1%) received venom immunotherapy. The incidence of SR was 0.09% in the first year and 0.08% in the second year. | The results of the study suggest that severe reactions to hymenopteran stings in children are rarer than previously estimated, and emphasize the importance of improving the diagnostic and therapeutic pathways for clinically relevant cases. | Observational and non-experimental data collection based on questionnaires, with possible overestimation or underestimation of data. Limited number of reactions observed. Data comes from a single city in Italy and may not be generalizable to other populations or geographical contexts. | 1 |
| Graif et al. [79] | 2009 | CS, R, M | This study aimed to assess the prevalence and severity of allergic reactions to insect stings in children with atopy, compared to those without, to determine whether atopy is a risk factor for a higher incidence and severity of hymenoptera venom reactions. | Israeli teenagers aged between 13 and 14 | 10,021 teenagers | Children with atopic diseases reported a significantly higher frequency of allergic reactions than non-atopic children. The reactions were more severe in children with atopy. | A total of 10,021 questionnaires were useful for analysis. Among children who reported insect bites (56.3%), the prevalence of current asthma was 6.0%, allergic rhinitis 10.5% and atopic eczema 8.7%, with no significant differences compared to the entire study population. Among children with any of the atopic diseases, 36.9% reported an allergic reaction to insect bites, compared with 24.8% of non-atopic children (p < 0.0001). In the multivariate analysis, asthma, allergic rhinitis, and atopic eczema were significant risk factors for allergic reactions of any severity. Children in the atopic group had a significantly higher rate of severe allergic reactions than non-atopic children, as well as relatively higher rates of mild reactions (p < 0.0001). Asthmatic patients with severe allergic reactions had more parameters of severe asthma than asthmatic patients with mild or no reactions. | The data showed that more than half of the children (56.3%) had reported at least one insect sting during their lifetime. Among these, a significant proportion developed allergic reactions, classified into three levels: extensive local reactions (LLR), mild systemic reactions (MSR) and severe systemic reactions (SSR). Children with atopic diseases showed a higher frequency of allergic reactions than their non-atopic peers. The severity of reactions was also higher in atopic subjects. In particular, these children reported more intense systemic reactions, such as widespread urticaria, breathing difficulties and extensive edema. | The data were collected through observational and non-experimental methods based on questionnaires. There is a possibility of overestimation or underestimation of the data, and there is a lack of clinical confirmation of atopic conditions. The data were collected in a single country (Israel) and may not be generalizable to other populations or geographical contexts. | 2 |
| Tischler et al. [76] | 2025 | CS, R, S | To assess whether the quantitative ratio of bee (BV) and Vespula (VV) venom specific IgE can identify the responsible insect in patients with dual sensitization and determine whether dual immunotherapy is necessary in cases of dual sensitization to specific components. | patients (adult and pediatric population) with dual sensitization to bee and Vespula venom, confirmed through clinical and serological tests | 1069 consecutive patients with suspected insect venom allergy (bee or wasp) and 490 non-allergic subjects, used for statistical comparisons. | A specific IgE ratio ≥ 5:1 is a strong indicator of the ‘culprit’ venom in double-sensitized patients. Double immunotherapy is not necessary in cases where a dominant venom is clearly identified. | Patients with dual sensitization (to both bee and Vespula venom) 459; among these, 239 patients (52.1%) had a specific BV/VV IgE ratio of at least 5:1 (dominance > 5:1 towards one venom). Of these patients, 232 (97.1%) were found to be monoallergic to the dominant venom. | A specific IgE ratio ≥ 5:1 (favouring BV or VV) is a highly reliable indicator of the venom responsible in patients with dual sensitisation. In most cases (97.1%), this parameter allows the culprit to be correctly identified, avoiding unnecessarily prolonged treatment. | Retrospective study based on previously collected clinical data, which may be subject to selection bias and data incompleteness. Data collected at a single centre. Population not exclusively pediatric. Use of an IgE threshold that is not universally standardized. Results not confirmed by provocation tests. | 2 |
| Baker et al. [71] | 2016 | CS, R, S | To evaluate the accuracy with which allergists can identify stinging insects, and analyze their standard procedures for assessing individuals suspected of being hypersensitive to insect stings. | Allergists attending the 2013 American College of Allergy, Asthma, and Immunology conference were invited to participate in the study. | 79, of whom 36 were specialist allergists and 43 were non-specialists | The primary outcome is the accuracy of identifying the biting insect, measured using a standardized test, while secondary outcomes concern the clinical diagnostic habits of specialists. | Allergists are collectively more skilled at identifying insects than non-allergists. Overall, the average number of correct answers for non-allergists was 5.4 (2.0) out of a total of 10. This score was significantly lower than that of allergists (6.1 [2.0]; p = 0.01) who participated in the study. Most allergists (78.5%) test for all stinging insects and use skin testing (69.5%) as their first test of choice in evaluating individuals with insect hypersensitivity. | Allergists demonstrated a greater ability to correctly identify stinging insects than non-specialists, with an average score of 6.1 out of 10 compared to 5.4 for non-specialists, but even they can confuse some species of stinging insects. Most allergists report testing for all stinging insects when evaluating patients with suspected sting allergy, demonstrating a comprehensive diagnostic approach. Skin testing is the most frequently used initial diagnostic method. | The sample size is limited. The study was conducted at a single location and the sample may not be sufficiently representative. | 2 |
| Kokcu Karadag et al. [75] | 2024 | CS, P, S | The main objective of the study is to demonstrate the applicability and effectiveness of the Basophil Activation Test (BAT) in detecting hypersensitivity to hymenoptera venom. In particular, the study provides a comparative evaluation of this test with skin prick tests and allergen-specific serum IgE measurements, both in terms of clinical sensitivity and positive predictive values. | This study included a total of 43 patients (General population. 14% under 10 years of age, 55.8% between 10 and 20 years of age; 30.2% over 20 years of age) who experienced systemic allergic reactions following stings from Apis mellifera and Vespula vulgaris, and who were subsequently treated with venom immunotherapy (VIT). | 43 patients | The study examines the advantages and limitations of three different diagnostic methods (basophil activation test, skin prick test and specific IgE measurement) and assesses the potential contribution of BAT in the diagnosis of hymenoptera venom allergy. The study highlights the need for updated approaches for more accurate and effective detection and management of hypersensitivity conditions resulting from hymenoptera stings. | The study determined that the overall clinical sensitivities of the BAT, specific serum IgE (sIgE) and skin prick test (SPT) for Apis mellifera were 95.5%, 95.7%, and 48.4%, respectively, while for Vespula vulgaris they were 83.3%, 100%, and 33.3%. Based on these results, the ability to predict systemic reactions to bee stings is ranked as: spIgE > BAT > SPT. Furthermore, skin tests in the early stages showed a sensitivity of 67% and a specificity of 50% with a cut-off value of 1.5 mm, and a sensitivity of 33% and specificity of 83% with a cut-off of 2.5 mm. | The data suggest that, in terms of predicting systemic reactions to bee stings, specific IgE testing is the most reliable method, followed by BAT and finally skin testing. Furthermore, analysis of skin tests in the early stages showed that a cut-off value of 1.5 mm ensures good sensitivity (67%) but relatively low specificity (50%). Conversely, increasing the cut-off to 2.5 mm decreases sensitivity (33%), while specificity improves significantly (83%). This indicates that the choice of cut-off significantly influences the diagnostic performance of the skin test. | Not only pediatric population. Small sample size. Probably single-centre. | 2 |
| Vos et al. [74] | 2013 | CS, R, S | Evaluate whether adding the recombinant molecular component rVes v 5 to the natural Vespula venom extract used in diagnostic tests for determining specific IgE can improve the sensitivity of these tests in patients with a confirmed allergy to Vespula venom. | Adult patients (adult population) with confirmed systemic allergy to Vespula venom | 308 | Adding the molecular component rVes v 5 to the Vespula extract used in IgE tests significantly improves diagnostic sensitivity. This allows to find a significant proportion of allergic patients who would have tested ‘negative’ with the standard test to be identified, without compromising specificity. | The standard test (natural extract) detected specific IgE in 257 out of 308 patients (approximately 83.4%). Using molecular components (rVes v1 and rVes v5) increased diagnostic sensitivity to around 96%. | It drastically reduces the number of false negatives, ensuring greater reliability in the diagnosis of Vespula venom allergy, especially in contexts where early diagnosis is essential for the initiation of specific immunotherapy. | It does not concern the pediatric population. It is retrospective and monocentric. It analyses only one component rVes v 5. It does not provide clinical data on patients. | 2 |
| Author | Years | Systematic Review with/Without Meta-Analysis | Objectives | Population | Outcome | Results (Quantitative Elements) | Results (Description) | Pico |
|---|---|---|---|---|---|---|---|---|
| Tomei L. et al. [64] | 2025 | SR con MA | It identifies and summarizes the main risk factors for systemic reactions (SR) to hymenoptera venom in children, through a systematic review of the literature. | Pediatric population < 18 years of age, affected by hymenoptera stings who have experienced systemic reactions. | A history of previous systemic reactions and the number of stings are considered significant risk factors for the development of severe reactions. Greater exposure to a specific allergen, often linked to environmental factors and lifestyle, appears to increase the risk of sensitization and subsequent severe reactions. No conclusive data on genetic predisposition in pediatric patients have been reported; ethnicity appears to influence the risk of systemic reactions more in relation to the lifestyle of the population studied than to genetic makeup. With regard to gender, the available data are inconsistent. Asthma has been indicated in two studies as a possible risk factor for systemic reactions to hymenoptera in children. Advanced age in children is associated with an increased risk of systemic reactions. No definitive associations have emerged between eosinophilia or other atopic diseases and the risk of systemic reactions to hymenoptera venom. | Not applicable | In children, there is no evidence that gender or atopic diseases (e.g., allergic rhinitis, atopic dermatitis) are risk factors for systemic reactions to hymenoptera; only asthma has been associated with an increased risk in two studies. No significant correlations between reaction severity and diagnostic findings have emerged. Children allergic to bees are younger on average and have a higher risk of systemic reactions, although there are no statistically significant differences compared to wasp allergy. | 1 |
| Stoevesandt et al. [76] | 2020 | Reviews | Review of the literature on risk factors and clinical indicators associated with severe systemic reactions induced by hymenoptera stings. It seeks to identify and describe demographic, anamnestic and biological parameters that can help stratify the risk of developing severe or potentially fatal anaphylactic events in individuals sensitized to hymenoptera venom in order to improve the clinical management and prevention of such reactions in the field of allergology. | General population | Qualitative assessment of the frequency and severity of allergic reactions in relation to various factors, identifying risk factors associated with severe reactions (e.g., age, gender, comorbidities, previous stings, type of hymenoptera), identifying predictors of severe reactions for adequate risk stratification and clinical management. | Not applicable | Advanced age is associated with a significantly higher risk of developing severe anaphylactic reactions, with a higher prevalence in adults than in children. In addition, a slight male predominance in severe reactions has been observed, suggesting possible biological or exposure differences between the sexes. Cardiovascular comorbidities represent an important risk factor, especially in the presence of drug therapies with beta-blockers and ACE inhibitors, which can amplify the severity of the allergic response. Elevated baseline serum tryptase levels are identified as a predictive marker of severity, especially in patients with mast cell conditions or systemic mast cell hyperplasia. A history of previous systemic reactions is a strong prognostic indicator, suggesting a higher likelihood of recurrence with increased severity. Furthermore, the frequency and severity of reactions appear to be related to the intensity and repetition of exposure to hymenoptera venom. Wasp venom is associated with more severe reactions than bee venom. Occupational or recreational activities that increase exposure to hymenoptera contribute significantly to the overall risk of severe systemic reactions. | 1 |
| Giovannini et al. [70] | 2024 | Reviews | Critical and up-to-date summary of knowledge regarding hymenoptera venom allergy in children, with particular attention to epidemiology and clinical pictures, pathophysiology, diagnosis, clinical management and treatment. | Pediatric population | This recent review provides a comprehensive and in-depth overview of hymenoptera venom allergy in children. It addresses epidemiological aspects, clinical manifestations with relative pathogenesis (immunological or non-immunological) and classifies reactions into: local reactions, extensive local reactions, systemic reactions, toxic reactions and unusual reactions. The tools available to clinicians for diagnosis and patient management based on the type of reaction are analyzed in detail, as are therapeutic strategies, in particular venom immunotherapy (VIT). | Not applicable | Allergy to hymenoptera venom in children has an estimated prevalence of sensitization of around 3.7% in Italy. Allergic reactions mainly manifest as extensive local reactions (0.9–11.5%) and systemic reactions (less than 1% in Italy, up to 6.5% in other geographical areas). Severe systemic reactions are rarer in children than in adults and are associated with risk factors such as mastocytosis, cardiovascular comorbidities and the use of beta-blockers or ACE inhibitors. Diagnosis is based on a combination of medical history, skin tests, specific IgE dosage and molecular tests, while treatment includes acute therapy with adrenaline and VIT, which has been shown to be safe and effective even in children. | 1, 2 |
| Blank et al. [73] | 2020 | Reviews | This review analyses various endotypes of sting reactions, such as IgE-mediated allergy, asymptomatic sensitization or the simultaneous presence of venom allergy and mast cell disorders, including specific considerations for diagnosis and treatment. The clinical phenotypes of venom allergy are also described, such as differences in age of onset and disease severity, polysensitization, or patients unresponsive to therapy. Finally, biomarkers and diagnostic strategies that may reflect the patient’s immunological status and their value in guiding treatment are discussed. | General population | Diagnostic accuracy of molecular tests and immunological biomarkers. Identification of different response profiles (endotypes/phenotypes) related to severity and risk of reactions. Effectiveness and personalisation of VIT in relation to the patient’s profile. | Not applicable | It is highlighted that a more in-depth understanding and classification of endotypes and phenotypes in hymenoptera venom allergy may represent a promising approach to better understand the disease and strengthen personalized treatment strategies. Furthermore, greater knowledge of the immunological processes that occur during venom immunotherapy (VIT) could contribute to improving the management of this condition. Currently, the availability of molecular-based diagnostics has facilitated the distinction between primary allergy and cross-reactivity, thus supporting therapeutic decisions in polysensitized patients. | 2 |
References
- Field, M.J.; Lohr, K.N. Clinical Practice Guidelines: Directions for a New Program, 1st ed.; Field, M.J., Lohr, K.N., Eds.; National Academy Press: Washington, DC, USA, 1990; ISSN 0309560012. [Google Scholar]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Fernandez Rivas, M.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Sanchez Borges, M.; et al. World Allergy Organization Anaphylaxis Guidance 2020. World Allergy Organ. J. 2020, 70, 314. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Riggioni, C.; Agache, I.; Akdis, C.A.; Akdis, M.; Alvarez-Perea, A.; Alvaro-Lozano, M.; Ballmer-Weber, B.; Barni, S.; Beyer, K.; et al. EAACI Guidelines on the Diagnosis of IgE-Mediated Food Allergy. Allergy Eur. J. Allergy Clin. Immunol. 2023, 78, 3057–3076. [Google Scholar] [CrossRef]
- Romano, A.; Atanaskovic-Markovic, M.; Barbaud, A.; Bircher, A.J.; Brockow, K.; Caubet, J.C.; Celik, G.; Cernadas, J.; Chiriac, A.M.; Demoly, P.; et al. Towards a More Precise Diagnosis of Hypersensitivity to Beta-Lactams—An EAACI Position Paper. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 1300–1315. [Google Scholar] [CrossRef]
- Sturm, G.J.; Arzt-Gradwohl, L. An Algorithm for the Diagnosis and Treatment of Hymenoptera Venom Allergy, 2024 Update. Allergy Eur. J. Allergy Clin. Immunol. 2024, 79, 2298–2301. [Google Scholar] [CrossRef]
- Wang, Y.; Allen, K.J.; Suaini, N.H.A.; McWilliam, V.; Peters, R.L.; Koplin, J.J. The Global Incidence and Prevalence of Anaphylaxis in Children in the General Population: A Systematic Review. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 1063–1080. [Google Scholar] [CrossRef]
- Dribin, T.E.; Neuman, M.I.; Schnadower, D.; Sampson, H.A.; Porter, J.J.; Michelson, K.A. Trends and Variation in Pediatric Anaphylaxis Care from 2016 to 2022. J. Allergy Clin. Immunol. Pract. 2023, 11, 1184–1189. [Google Scholar] [CrossRef]
- Bilò, M.B.; Pravettoni, V.; Bignardi, D.; Bonadonna, P.; Mauro, M.; Novembre, E.; Quercia, O.; Cilia, M.; Cortellini, G.; Costantino, M.T.; et al. Hymenoptera Venom Allergy: Management of Children and Adults in Clinical Practice. J. Investig. Allergol. Clin. Immunol. 2019, 29, 180–205. [Google Scholar] [CrossRef]
- Turner, P.J.; Jerschow, E.; Umasunthar, T.; Lin, R.; Campbell, D.E.; Boyle, R.J. Fatal Anaphylaxis: Mortality Rate and Risk Factors. J. Allergy Clin. Immunol. Pract. 2017, 5, 1169–1178. [Google Scholar] [CrossRef]
- Bilò, M.B.; Corsi, A.; Martini, M.; Penza, E.; Grippo, F.; Bignardi, D. Fatal Anaphylaxis in Italy: Analysis of Cause-of-Death National Data, 2004–2016. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 2644–2652. [Google Scholar] [CrossRef]
- Sousa-Pinto, B.; Fonseca, J.A.; Gomes, E.R. Frequency of Self-Reported Drug Allergy: A Systematic Review and Meta-Analysis with Meta-Regression. Ann. Allergy Asthma Immunol. 2017, 119, 362–373.e2. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.5; The Cochrane Collaboration: London, UK, 2011; Available online: https://training.cochrane.org/handbook/current (accessed on 1 October 2025).
- Mann, C.J. Observational Research Methods. Research Design II: Cohort, Cross Sectional, and Case-Control Studies. Emerg. Med. J. 2003, 20, 54–60. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Environmental Health Sciences. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration; National Institute of Environmental Health Sciences: Durham, NC, USA, 2019. [Google Scholar]
- Eick, S.M.; Goin, D.E.; Chartres, N.; Lam, J.; Woodruff, T.J. Assessing Risk of Bias in Human Environmental Epidemiology Studies Using Three Tools: Different Conclusions from Different Tools. Syst. Rev. 2020, 9, 249. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing: Reference Index; R Foundation for Statistical Computing: Vienna, Austria, 2010; ISBN 3900051070. [Google Scholar]
- Dribin, T.E.; Muraro, A.; Camargo, C.A.; Turner, P.J.; Wang, J.; Roberts, G.; Anagnostou, A.; Halken, S.; Liebermann, J.; Worm, M.; et al. Anaphylaxis Definition, Overview, and Clinical Support Tool: 2024 Consensus Report—A GA2LEN Project. J. Allergy Clin. Immunol. 2025, 156, 406–417.e6. [Google Scholar] [CrossRef]
- Martelli, A.; Ippolito, R.; Votto, M.; De Filippo, M.; Brambilla, I.; Calvani, M.; Cardinale, F.; Chiappini, E.; Duse, M.; Manti, S.; et al. What Is New in Anaphylaxis? Acta Biomed. 2020, 91, e2020005. [Google Scholar]
- Ball, E.; Purchase, T.; Morgan, J. Review of the 2021 Resuscitation Council United Kingdom Guideline for the Emergency Treatment of Anaphylaxis. Arch. Dis. Child.-Educ. Pract. 2023, 108, 296–300. [Google Scholar] [CrossRef]
- Anagnostou, K. Anaphylaxis in Children: Epidemiology, Risk Factors and Management. Curr. Pediatr. Rev. 2018, 14, 180–186. [Google Scholar] [CrossRef]
- Khojah, A.; Bukhari, A.; Alibrahim, I.; AlSulami, M.; Alotaibi, T.; Alotaibi, R.; Bahareth, E.; Abulreish, I.; Alsuruji, S.; Rajab, R.; et al. Possession of Injectable Epinephrine Among Children with Parent-Reported Food Allergies in Saudi Arabia. J. Clin. Med. 2025, 14, 5274. [Google Scholar] [CrossRef]
- Navalpakam, A.; Thanaputkaiporn, N.; Poowuttikul, P. Anaphylaxis: Long-Term Management and Resources. Allergy Asthma Proc. 2023, 44, 35–44. [Google Scholar] [CrossRef]
- Umasunthar, T.; Procktor, A.; Hodes, M.; Smith, J.G.; Gore, C.; Cox, H.E.; Marrs, T.; Hanna, H.; Phillips, K.; Pinto, C.; et al. Patients’ Ability to Treat Anaphylaxis Using Adrenaline Autoinjectors: A Randomized Controlled Trial. Allergy Eur. J. Allergy Clin. Immunol. 2015, 70, 855–863. [Google Scholar] [CrossRef]
- Deschildre, A.; Alvaro-Lozano, M.; Muraro, A.; Podesta, M.; de Silva, D.; Giovannini, M.; Barni, S.; Dribin, T.E.; Sandoval-Ruballos, M.; Anagnostou, A.; et al. Towards a Common Approach for Managing Food Allergy and Serious Allergic Reactions (Anaphylaxis) at School. GA2LEN and EFA Consensus Statement. Clin. Transl. Allergy 2025, 15, e70013. [Google Scholar] [CrossRef]
- Burrell, S.; Patel, N.; Vazquez-Ortiz, M.; Campbell, D.E.; DunnGalvin, A.; Turner, P.J. Self-Administration of Adrenaline for Anaphylaxis during in-Hospital Food Challenges Improves Health-Related Quality of Life. Arch. Dis. Child. 2020, 69, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.; Sharma, V.; Li, K.; Gowland, M.H.; Garcez, T.; Shilladay, C.; Pumphrey, R.; Patel, N.; Turner, P.J.; Boyle, R.J. Adrenaline Auto-Injectors for Preventing Fatal Anaphylaxis. Clin. Exp. Allergy 2025, 55, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Prince, B.T.; Mikhail, I.; Stukus, D.R. Underuse of Epinephrine for the Treatment of Anaphylaxis: Missed Opportunities. J. Asthma Allergy 2018, 11, 143–151. [Google Scholar] [CrossRef]
- Ellis, A.K.; Casale, T.B.; Kaliner, M.; Oppenheimer, J.; Spergel, J.M.; Fleischer, D.M.; Bernstein, D.; Camargo, C.A.; Lowenthal, R.; Tanimoto, S. Development of Neffy, an Epinephrine Nasal Spray, for Severe Allergic Reactions. Pharmaceutics 2024, 16, 811. [Google Scholar] [CrossRef] [PubMed]
- Ebisawa, M.; Takahashi, K.; Takahashi, K.; Yanagida, N.; Sato, S.; Lieberman, J.; Pistiner, M.; Spergel, J.M.; Lowenthal, R.; Tanimoto, S. Epinephrine Nasal Spray Improves Allergic Symptoms in Patients Undergoing Oral Food Challenge, Phase 3 Trial. J. Allergy Clin. Immunol. Pract. 2025, 13, 2787–2794. [Google Scholar] [CrossRef]
- Clark, E.; Kase Tanno, L.; Vo, T.; Blanc, B.; Demoly, P.; Caimmi, D. Anaphylaxis Management in a French Pediatric Emergency Department: Lessons from the ANA-PED Study. Clin. Transl. Allergy 2023, 13, e12289. [Google Scholar] [CrossRef]
- De Schryver, S.; Halbrich, M.; Clarke, A.; La Vieille, S.; Eisman, H.; Alizadehfar, R.; Joseph, L.; Morris, J.; Ben-Shoshan, M. Tryptase Levels in Children Presenting with Anaphylaxis: Temporal Trends and Associated Factors. J. Allergy Clin. Immunol. 2016, 137, 1138–1142. [Google Scholar] [CrossRef]
- Cavkaytar, O.; Karaatmaca, B.; Arik Yilmaz, E.; Sahiner, U.M.; Sackesen, C.; Sekerel, B.E.; Soyer, O. Basal Serum Tryptase Is Not a Risk Factor for Immediate-Type Drug Hypersensitivity during Childhood. Pediatr. Allergy Immunol. 2016, 27, 736–742. [Google Scholar] [CrossRef]
- Giannetti, A.; Filice, E.; Caffarelli, C.; Ricci, G.; Pession, A. Mast Cell Activation Disorders. Medicina 2021, 57, 124. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lieberman, J.A.; Wallace, D.V.; Waserman, S.; Golden, D.B.K. Anaphylaxis in Practice: A Guide to the 2023 Practice Parameter Update. J. Allergy Clin. Immunol. Pract. 2024, 12, 2325–2336. [Google Scholar] [CrossRef]
- Lyons, J.J.; Chovanec, J.; O’Connell, M.P.; Liu, Y.; Šelb, J.; Zanotti, R.; Bai, Y.; Kim, J.; Le, Q.T.; DiMaggio, T.; et al. Heritable Risk for Severe Anaphylaxis Associated with Increased α-Tryptase–Encoding Germline Copy Number at TPSAB1. J. Allergy Clin. Immunol. 2021, 147, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Vetander, M.; Protudjer, J.L.P.; Lilja, G.; Kull, I.; Hedlin, G.; van Hage, M.; Östblom, E.; Bergström, A.; Wickman, M. Anaphylaxis to Foods in a Population of Adolescents: Incidence, Characteristics and Associated Risks. Clin. Exp. Allergy 2016, 46, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Ciccolini, A.; Avilla, E.; Waserman, S. Food Allergy and Anaphylaxis. J. Asthma Allergy 2018, 11, 111–120. [Google Scholar] [CrossRef]
- Calvani, M.; Anania, C.; Caffarelli, C.; Martelli, A.; del Giudice, M.M.; Cravidi, C.; Duse, M.; Manti, S.; Tosca, M.A.; Cardinale, F.; et al. Food Allergy: An Updated Review on Pathogenesis, Diagnosis, Prevention and Management. Acta Bio Med. Atenei Parm. 2020, 91, e2020012. [Google Scholar]
- Turner, P.J.; Arasi, S.; Ballmer-Weber, B.; Baseggio Conrado, A.; Deschildre, A.; Gerdts, J.; Halken, S.; Muraro, A.; Patel, N.; Van Ree, R.; et al. Risk Factors for Severe Reactions in Food Allergy: Rapid Evidence Review with Meta-Analysis. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 2634–2652. [Google Scholar] [CrossRef]
- Kattan, J.D.; Sicherer, S.H. Optimizing the Diagnosis of Food Allergy. Immunol. Allergy Clin. North Am. 2015, 35, 61–76. [Google Scholar] [CrossRef]
- Martelli, A.; Calvani, M.; Foiadelli, T.; Tosca, M.; Pingitore, G.; Licari, A.; Marseglia, A.; Ciprandi, G.; Caffarelli, C. Component Resolved Diagnosis and Risk Assessment in Food Allergy. Acta Biomed. 2021, 92, e2021528. [Google Scholar]
- Kukkonen, A.K.; Pelkonen, A.S.; Mäkinen-Kiljunen, S.; Voutilainen, H.; Mäkelä, M.J. Ara h 2 and Ara 6 Are the Best Predictors of Severe Peanut Allergy: A Double-Blind Placebo-Controlled Study. Allergy Eur. J. Allergy Clin. Immunol. 2015, 70, 1239–1245. [Google Scholar] [CrossRef]
- van Veen, L.N.; Heron, M.; Batstra, M.; van Haard, P.M.M.; de Groot, H. The Diagnostic Value of Component-Resolved Diagnostics in Peanut Allergy in Children Attending a Regional Pediatric Allergology Clinic. BMC Pediatr. 2016, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Pinto, B.; Tarrio, I.; Blumenthal, K.G.; Araújo, L.; Azevedo, L.F.; Delgado, L.; Fonseca, J.A. Accuracy of Penicillin Allergy Diagnostic Tests: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. 2021, 147, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Caffarelli, C.; Franceschini, F.; Caimmi, D.; Mori, F.; Diaferio, L.; Di Mauro, D.; Mastrorilli, C.; Arasi, S.; Barni, S.; Bottau, P.; et al. SIAIP Position Paper: Provocation Challenge to Antibiotics and Non-Steroidal Anti-Inflammatory Drugs in Children. Ital. J. Pediatr. 2018, 44, 147. [Google Scholar] [CrossRef]
- Paño-Pardo, J.R.; Moreno Rodilla, E.; Cobo Sacristan, S.; Cubero Saldaña, J.L.; Periañez Párraga, L.; Del Pozo León, J.L.; Retamar-Gentil, P.; Rodríguez Oviedo, A.; Torres Jaén, M.J.; Vidal-Cortes, P.; et al. Management of Patients with Suspected or Confirmed Antibiotic Allergy: Executive Summary of Guidelines from the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), the Spanish Society of Allergy and Clinical Immunology (SEAIC), the Spanish Society of Hospital Pharmacy (SEFH) and the Spanish Society of Intensive Medicine and Coronary Care Units (SEMICYUC). J. Investig. Allergol. Clin. Immunol. 2023, 33, 95–101. [Google Scholar] [CrossRef]
- Goh, S.H.; Chong, K.W.; Chiang, W.C.; Goh, A.; Loh, W. Outcome of Drug Provocation Testing in Children with Suspected Beta-Lactam Hypersensitivity. Asia Pac. Allergy 2021, 11, e3. [Google Scholar] [CrossRef]
- Felix, M.M.R.; Kuschnir, F.C. Direct Oral Provocation Test Is Safe and Effective in Diagnosing Beta-Lactam Allergy in Low-Risk Children with Mild Cutaneous Reactions. Front. Pharmacol. 2020, 11, 1223. [Google Scholar] [CrossRef]
- Cravidi, C.; Caimmi, S.; De Filippo, M.; Martelli, A.; Caffarelli, C.; Del Giudice, M.M.; Calvani, M.; Tosca, M.A.; Cardinale, F.; Marseglia, G.L.; et al. Drug Allergy in Children: Focus on Beta-Lactams and NSAIDs. Acta Biomed. 2020, 91, e2020008. [Google Scholar]
- Labrosse, R.; Paradis, L.; Samaan, K.; Lacombe-Barrios, J.; Paradis, J.; Bégin, P.; Des Roches, A. Sensitivity and Specificity of Double-Blinded Penicillin Skin Testing in Relation to Oral Provocation with Amoxicillin in Children. Allergy Asthma Clin. Immunol. 2020, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Moral, L.; Latorre, S.; Toral, T.; Marco, N.; Canals, F.; Forniés, M.J.; González, M.C.; García-Avilés, B. Positive Drug Provocation with Beta-Lactam Antibiotics in Children: A Single Test May Not Be Enough. Allergol. Immunopathol. 2022, 50, 148–152. [Google Scholar] [CrossRef]
- Mori, F.; Liccioli, G.; Barni, S.; Novembre, E. Management of Suspected Antibiotic Reactions in Childrena. Pediatr. Infect. Dis. J. 2019, 38, E149–E152. [Google Scholar] [CrossRef]
- Calamelli, E.; Caffarelli, C.; Franceschini, F.; Saretta, F.; Cardinale, F.; Bernardini, R.; Liotti, L.; Mori, F.; Crisafulli, G.; Caimmi, S.; et al. A Practical Management of Children with Antibiotic Allergy. Acta Biomed. 2019, 90, 11–19. [Google Scholar]
- Brockow, K.; Pfützner, W.; Wedi, B.; Wurpts, G.; Trautmann, A.; Kreft, B.; Buhl, T.; Sulk, M.; Recke, A.; Scherer, K.; et al. Recommendations on How to Proceed in Case of Suspected Allergy to Penicillin/β-Lactam Antibiotics Position Paper of the German Society for Allergology and Clinical Immunology (DGAKI) in Cooperation with the German Society for Pediatric Allergology (GPA), Austrian Society for Allergology and Immunology (ÖGAI) and the Swiss Society for Allergology and Immunology (SGAI). Allergologie 2025, 9, 28–39. [Google Scholar] [CrossRef]
- Mill, C.; Primeau, M.N.; Medoff, E.; Lejtenyi, C.; O’Keefe, A.; Netchiporouk, E.; Dery, A.; Ben-Shoshan, M. Assessing the Diagnostic Properties of a Graded Oral Provocation Challenge for the Diagnosis of Immediate and Nonimmediate Reactions to Amoxicillin in Children. JAMA Pediatr. 2016, 170, e160033. [Google Scholar] [CrossRef] [PubMed]
- Quercia, O.; Incorvaia, C.; Marseglia, G.L.; Puccinelli, P.; Dell’albani, I.; Emiliani, F.; Frati, F.; Stefanini, G. Prevalence and Incidence of Reactions to Insect Stings in Children: A Reappraisal. Minerva Pediatr. 2014, 66, 257–260. [Google Scholar]
- Graif, Y.; Romano-Zelekha, O.; Livne, I.; Green, M.S.; Shohat, T. Allergic Reactions to Insect Stings: Results from a National Survey of 10,000 Junior High School Children in Israel. J. Allergy Clin. Immunol. 2006, 117, 1435–1439. [Google Scholar] [CrossRef]
- Worm, M.; Moneret-Vautrin, A.; Scherer, K.; Lang, R.; Fernandez-Rivas, M.; Cardona, V.; Kowalski, M.L.; Jutel, M.; Poziomkowska-Gesicka, I.; Papadopoulos, N.G.; et al. First European Data from the Network of Severe Allergic Reactions (NORA). Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 1397–1404. [Google Scholar] [CrossRef]
- Novembre, E.; Giovannini, M.; Mori, F.; BArni, S.; Saretta, F.; Arasi, S.; Castagnoli, R.; Liotti, L.; Mastrorilli, C.; Pecoraro, L.; et al. Allergia al Veleno Di imenotteri in Età Pediatrica. Riv. Immunol. Allergol. Pediatr. 2020, 34, I–XX. [Google Scholar]
- Ruëff, F.; Bauer, A.; Becker, S.; Brehler, R.; Brockow, K.; Chaker, A.M.; Darsow, U.; Fischer, J.; Fuchs, T.; Gerstlauer, M.; et al. Diagnosis and Treatment of Hymenoptera Venom Allergy. Allergol. Sel. 2023, 7, 154–190. [Google Scholar] [CrossRef]
- Sturm, G.J.; Schadelbauer, E.; Marta, G.; Bonadonna, P.; Kosnik, M. Risk Factors for Severe Sting Reactions and Side Effects During Venom Immunotherapy. J. Allergy Clin. Immunol. Pract. 2025, 13, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Tomei, L.; Bettini, I.; D’Addio, E.; Licari, A.; Klain, A.; Galletta, F.; Manti, S.; Senatore, A.A.; Del Giudice, M.M.; Indolfi, C.; et al. Risk Factors for Severe Systemic Reactions to Hymenoptera Venom in Children. Ital. J. Pediatr. Allergy Immunol. 2025, 39, 18–21. [Google Scholar] [CrossRef]
- Jennings, A.; Duggan, E.; Perry, I.J.; Hourihane, J.O.B. Epidemiology of Allergic Reactions to Hymenoptera Stings in Irish School Children. Pediatr. Allergy Immunol. 2010, 21, 1166–1170. [Google Scholar] [CrossRef]
- Arikan-Ayyildiz, Z.; Isik, S.; Babus, S.; Ucku, R.; Caglayan-Sozmen, S.; Karaman, O.; Uzuner, N. Allergic Reactions to Hymenoptera Stings in Turkish School Children. Allergol. Immunopathol. 2016, 44, 41–45. [Google Scholar] [CrossRef]
- Yavuz, T.; Ozdemir, I.; Sencan, I.; Arbak, P.; Behçet, M.; Sert, E. Seroprevalence of Varicella, Measles and Hepatitis B among Female Health Care Workers of Childbearing Age. Jpn. J. Infect. Dis. 2005, 58, 383–386. [Google Scholar] [CrossRef]
- Bilò, B.M.; Bonifazi, F. Hymenoptera Venom Immunotherapy. Immunotherapy 2011, 3, 229–246. [Google Scholar] [CrossRef]
- Krishna, M.T.; Ewan, P.W.; Diwakar, L.; Durham, S.R.; Frew, A.J.; Leech, S.C.; Nasser, S.M. Diagnosis and Management of Hymenoptera Venom Allergy: British Society for Allergy and Clinical Immunology (BSACI) Guidelines. Clin. Exp. Allergy 2011, 41, 1201–1220. [Google Scholar] [CrossRef]
- Giovannini, M.; Mori, F.; Barni, S.; Saretta, F.; Arasi, S.; Castagnoli, R.; Liotti, L.; Mastrorilli, C.; Pecoraro, L.; Caminiti, L.; et al. Hymenoptera Venom Allergy in Children. Ital. J. Pediatr. 2024, 50, 262. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.W.; Forester, J.P.; Johnson, M.L.; Sikora, J.M.; Stolfi, A.; Stahl, M.C. Stinging Insect Identification: Are the Allergy Specialists Any Better than Their Patients? Ann. Allergy Asthma Immunol. 2016, 116, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Dramburg, S.; Hilger, C.; Santos, A.F.; de las Vecillas, L.; Aalberse, R.C.; Acevedo, N.; Aglas, L.; Altmann, F.; Arruda, K.L.; Asero, R.; et al. EAACI Molecular Allergology User’s Guide 2.0. Pediatr. Allergy Immunol. 2023, 34, e13854. [Google Scholar] [CrossRef] [PubMed]
- Blank, S.; Grosch, J.; Ollert, M.; Bilò, M.B. Precision Medicine in Hymenoptera Venom Allergy: Diagnostics, Biomarkers, and Therapy of Different Endotypes and Phenotypes. Front. Immunol. 2020, 11, 579409. [Google Scholar] [CrossRef]
- Vos, B.; Köhler, J.; Müller, S.; Stretz, E.; Ruëff, F.; Jakob, T. Spiking Venom with RVes v 5 Improves Sensitivity of IgE Detection in Patients with Allergy to Vespula Venom. J. Allergy Clin. Immunol. 2013, 131, 1225–1227.e1. [Google Scholar] [CrossRef]
- Karadag, S.I.K.; Çökelez, S.Ö.; Çepniler, E.B.; Abdullayev, E.; Terzi, Ö.; Özçeker, D.; Sancak, R.; Yıldıran, A. The Role of Basophil Activation Test in Venom Immunotherapy: Comparative Evaluation with Specific IgE and Skin Prick Tests, Innovative Approaches. Eur. Ann. Allergy Clin. Immunol. 2025, 57, 228–235. [Google Scholar] [CrossRef]
- Tischler, S.; Trautmann, A.; Goebeler, M.; Stoevesandt, J. Bee/Vespula Venom-Specific IgE Ratio Greater Than 5:1 Indicates Culprit Insect in Double-Sensitized Patients. J. Allergy Clin. Immunol. Pract. 2025, 13, 79–88.e4. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses; The Ottawa Hospital Research Institute: Ottawa, ON, Canada; Available online: https://ohri.ca/en/who-we-are/core-facilities-and-platforms/ottawa-methods-centre/newcastle-ottawa-scale (accessed on 30 December 2025).
- Le, M.; Gabrielli, S.; Clarke, A.; Eisman, H.; Morris, J.; Gravel, J.; Chan, E.; Lim, R.; O’Keefe, A.; Shand, G.; et al. Emergency Management of Anaphylaxis Due to an Unknown Trigger: An 8-Year Follow-Up Study in Canada. J. Allergy Clin. Immunol. 2019, 4, 1166–1173.e1. [Google Scholar] [CrossRef]
- Graif, Y.; Romano-Zelekha, O.; Livne, I.; Green, M.; Shohat, T. Increased rate and greater severity of allergic reactions to insect sting among schoolchildren with atopic diseases. Pediatr. Allergy Immunol. 2009, 20, 757–762. [Google Scholar] [CrossRef] [PubMed]
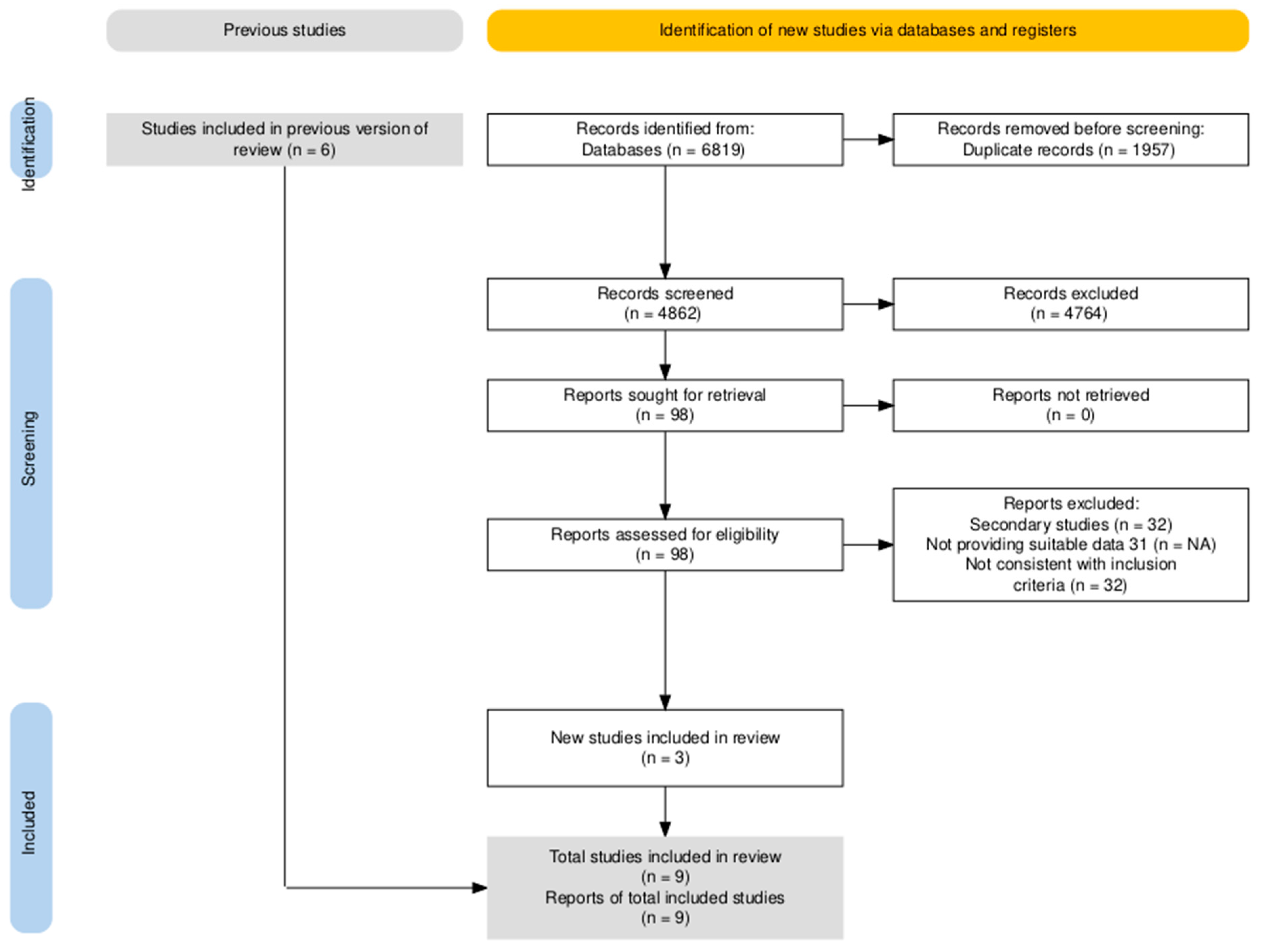
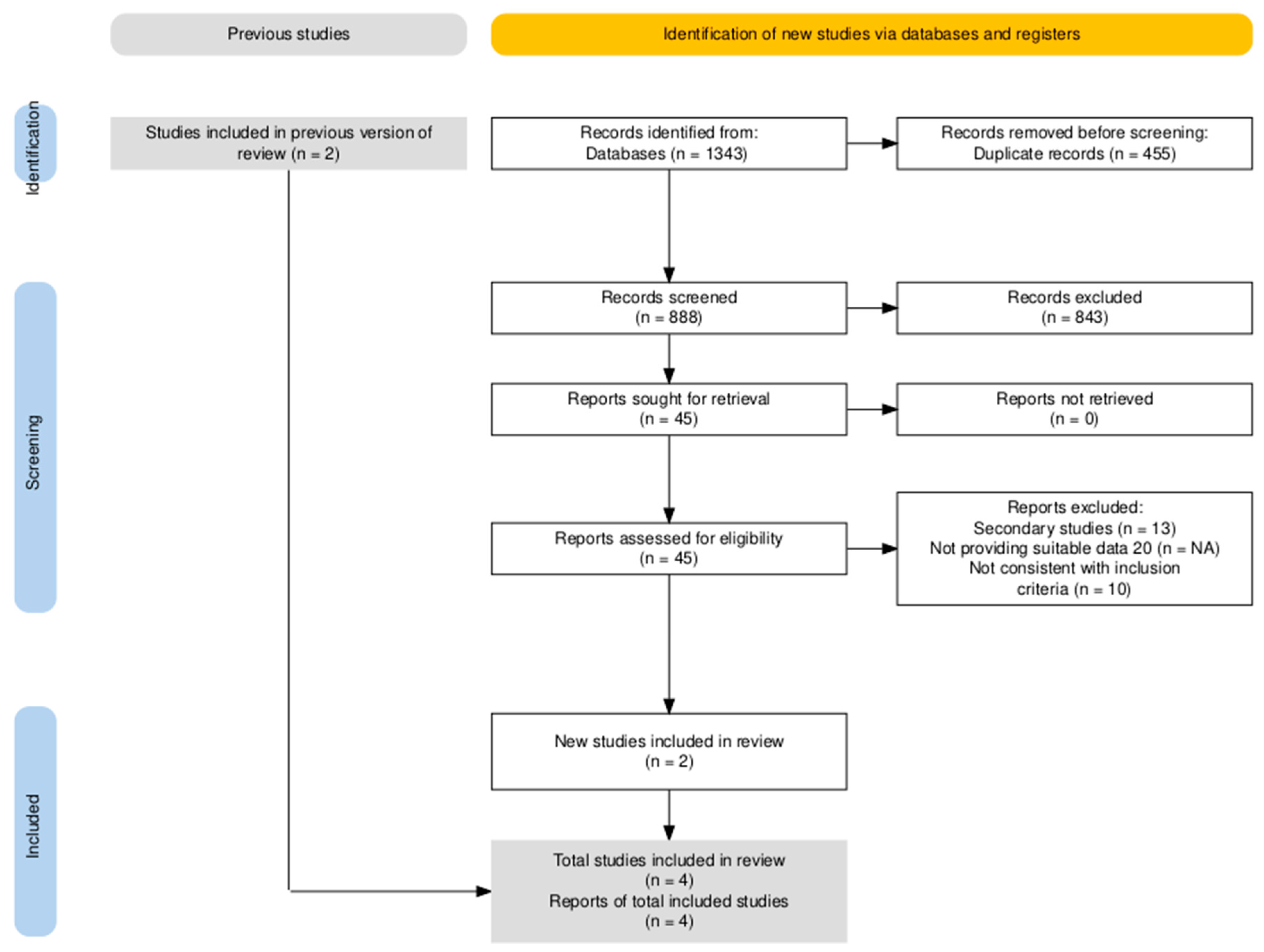
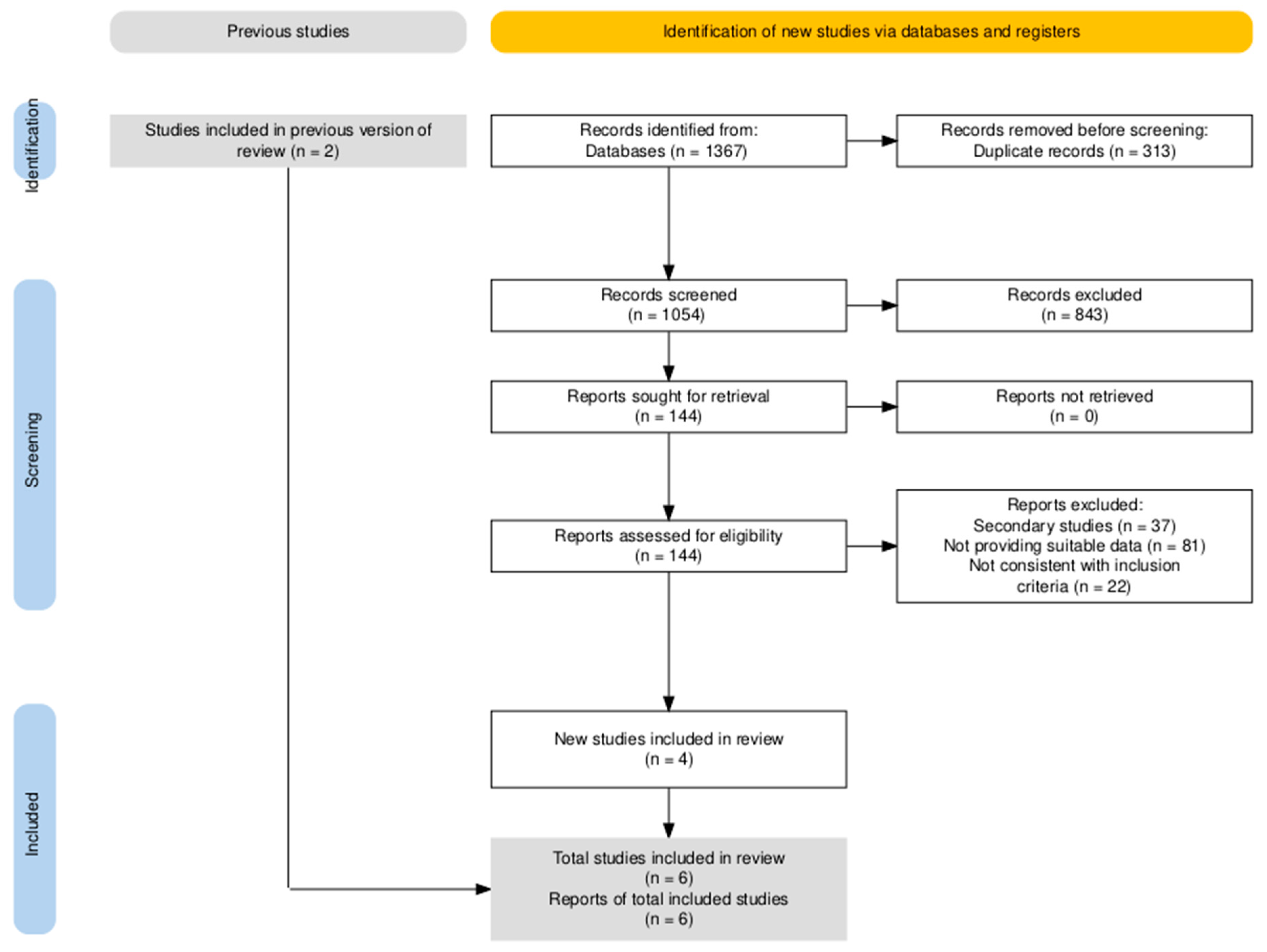
| Quality of Evidence (GRADE) | Strength of Recommendation | Balance of Benefits and Risks | Values and Preferences | Resource Use Implementation Impact |
|---|---|---|---|---|
| Moderate | Strong recommendation for referral to a specialist in deferred urgency | Benefits: identification of the trigger, AAI prescription and training, and provision of an action plan. Risks: cost and access time. The benefit–risk balance is clearly favourable. | Families place a high value on preventing recurrence and safety; overall acceptability is high. | It requires an established allergy referral network and access to training materials; it may reduce emergency department visits and recurrence-related costs. |
| Quality of Evidence (GRADE) | Strength of Recommendation | Balance of Benefits and Risks | Values and Preferences | Resource Use Implementation Impact |
|---|---|---|---|---|
| Moderate | Strong recommendation to measure serum tryptase (acute + baseline) | Benefits: supports objective diagnosis, allows risk stratification, and may reveal underlying mastocytosis or Mast Cell Activation Syndrome (MCAS). Risks: low cost and need for precise timing. | High value for diagnostic confirmation and management planning; good acceptability (blood test). | Available in most laboratories; requires logistical coordination for time-sensitive sampling; modest economic impact. |
| Quality of Evidence (GRADE) | Strength of Recommendation | Balance of Benefits and Risks | Values and Preferences | Resource Use Implementation Impact |
|---|---|---|---|---|
| Moderate | Weak/conditional recommendation in favour of SPT (extracts + fresh food) when indicated | Benefits: improved diagnostic accuracy and avoidance of unnecessary diets. Risks: false positives, requires expert interpretation. | Families seek to clarify food-related responsibilities and reduce dietary restrictions; high value on quality of life (QoL). | Low-cost and widely available tests; expertise is required for the selection of extracts/fresh food and interpretation. |
| Quality of Evidence (GRADE) | Strength of Recommendation | Balance of Benefits and Risks | Values and Preferences | Resource Use Implementation Impact |
|---|---|---|---|---|
| Low to Moderate | Weak/conditional recommendation in favour of specific IgE measurement using CRD | Benefits: risk profiling (e.g., storage proteins/profilins/lipid transfer proteins), personalized prevention and AAI use. Risks: cost, potential for over-interpretation. | Clinicians and families value risk stratification and personalized advice; good acceptability. | Variable availability; higher cost than standard tests; implementation in specialized centres with experience. |
| Quality of Evidence (GRADE) | Strength | Balance of Benefits/Risks | Values and Preferences | Impact on Resources/Implementation |
|---|---|---|---|---|
| Moderate | Strongly recommended use of diagnostic tests over medical history alone | Significantly greater benefits (improved diagnostic accuracy, fewer misdiagnoses, reduced use of alternative antibiotics) | Parents and clinicians supportive, although OPT may cause apprehension | Requires centres equipped for tests/OPT; higher initial cost but cost-effective in the long term |
| Quality of Evidence (GRADE) | Strength | Balance of Benefits/Risks | Values and Preferences | Impact on Resources/Implementation |
|---|---|---|---|---|
| N.A. | N.A. | Benefits possibly outweigh risks (improved diagnostic accuracy, fewer incorrect diagnoses, reduced use of alternative antibiotics) | Parents and clinicians supportive | Requires centres equipped with specific consultations; possible delay in application of therapy but probably sustainable |
| Quality of Evidence (GRADE) | Strength of Recommendation | Balance of Benefits and Risks | Values and Preferences | Resource Use/Implementation Impact |
|---|---|---|---|---|
| Moderate-high | Strong in favour of specialist evaluation | Although greater use of specialist resources compared with management by a pediatrician alone, it is highly cost-effective for the prevention of acute events and hospitalizations. | Families and clinicians place great importance on anaphylaxis prevention and safety. High acceptability among families and pediatricians, strong endorsement from clinicians and the healthcare system. | Integration into regional care pathways and improvement in the availability of pediatric allergy centres are required. |
| Quality of Evidence (GRADE) | Strength of Recommendation | Balance of Benefits and Risks | Values and Preferences | Resource Use Implementation Impact |
|---|---|---|---|---|
| Moderate | Strong recommendation in favour of specific diagnostic tests | Balance in favour of the use of specific diagnostic tests Minimal risks: local reactions to skin tests; anxiety related to the investigation; risk of false positives/negatives | Families and doctors tend to prefer tests that provide diagnostic clarity and certainty in treatment decisions Generally high acceptability by families and clinicians | Easily implementable in allergy centres; requires shared pathways and pediatric training. Additional costs for diagnostic tests but largely justified by improved treatment targeting and reduction in adverse events. Possible disparities in access to specialized laboratories and advanced tests (e.g., BAT—basophil activation test). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Fainardi, V.; Riccò, M.; Antignani, R.; Bellodi, S.; Buono, E.V.; Calvani, M.; Carbone, R.; Cardinale, F.; Chiappini, E.; Crivellaro, M.A.; et al. Italian Evidence-Based Clinical Recommendations on the Appropriateness of Prescriptions and Diagnostic Tests in Pediatric Allergology: Focus on Anaphylaxis, Drug Allergy and Hymenoptera Venom Allergy. J. Clin. Med. 2026, 15, 678. https://doi.org/10.3390/jcm15020678
Fainardi V, Riccò M, Antignani R, Bellodi S, Buono EV, Calvani M, Carbone R, Cardinale F, Chiappini E, Crivellaro MA, et al. Italian Evidence-Based Clinical Recommendations on the Appropriateness of Prescriptions and Diagnostic Tests in Pediatric Allergology: Focus on Anaphylaxis, Drug Allergy and Hymenoptera Venom Allergy. Journal of Clinical Medicine. 2026; 15(2):678. https://doi.org/10.3390/jcm15020678
Chicago/Turabian StyleFainardi, Valentina, Matteo Riccò, Rachele Antignani, Simona Bellodi, Enrico Vito Buono, Mauro Calvani, Roberta Carbone, Fabio Cardinale, Elena Chiappini, Maria Angiola Crivellaro, and et al. 2026. "Italian Evidence-Based Clinical Recommendations on the Appropriateness of Prescriptions and Diagnostic Tests in Pediatric Allergology: Focus on Anaphylaxis, Drug Allergy and Hymenoptera Venom Allergy" Journal of Clinical Medicine 15, no. 2: 678. https://doi.org/10.3390/jcm15020678
APA StyleFainardi, V., Riccò, M., Antignani, R., Bellodi, S., Buono, E. V., Calvani, M., Carbone, R., Cardinale, F., Chiappini, E., Crivellaro, M. A., Cunico, D., Esposito, M., Licari, A., Miraglia Del Giudice, M., Marsella, M., Neri, I., Nocerino, R., Peroni, D., Piersantelli, C., ... Esposito, S. (2026). Italian Evidence-Based Clinical Recommendations on the Appropriateness of Prescriptions and Diagnostic Tests in Pediatric Allergology: Focus on Anaphylaxis, Drug Allergy and Hymenoptera Venom Allergy. Journal of Clinical Medicine, 15(2), 678. https://doi.org/10.3390/jcm15020678










