A Proposed Model of a Pragmatic Surgical Approach in Women Affected by Uterine Fibroids Undergoing IVF: A “Real Practice” Experience
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IVF | In Vitro Fertilization |
| ART | Assisted Reproductive Technologies |
| ICSI | Intracytoplasmic Sperm Injection |
| WHO | World Health Organization |
| ESHRE | European Society of Human Reproduction and Embryology |
| NICE | National Institute for Health and Care Excellence |
| ASRM | American Society Reproductive Medicine |
| FIGO | International Federation of Gynecology and Obstetrics |
| AGUI | Italian University Gynaecologist Association |
References
- Giuliani, E.; As-Sanie, S.; Marsh, E.E. Epidemiology and management of uterine fibroids. Int. J. Gynaecol. Obstet. 2020, 149, 3–9. [Google Scholar] [PubMed]
- Peddada, S.D.; Laughlin, S.K.; Miner, K.; Guyon, J.P.; Haneke, K.; Vahdat, H.L.; Semelka, R.C.; Kowalik, A.; Armao, D.; Davis, B.; et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc. Natl. Acad. Sci. USA 2008, 105, 19887–19892. [Google Scholar] [PubMed]
- Bulun, S.E.; Yin, P.; Wei, J.; Zuberi, A.; Iizuka, T.; Suzuki, T.; Saini, P.; Goad, J.; Parker, J.B.; Adli, M.; et al. Uterine Fibroids. Physiol. Rev. 2025, 105, 1947–1988. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ciebiera, M.; Bariani, M.V.; Ali, M.; Elkafas, H.; Boyer, T.G.; Al-Hendy, A. Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment. Endocr. Rev. 2022, 43, 678–719. [Google Scholar]
- Vannuccini, S.; Petraglia, F.; Carmona, F.; Calaf, J.; Chapron, C. The modern management of uterine fibroids-related abnormal uterine bleeding. Fertil. Steril. 2024, 122, 20–30. [Google Scholar] [CrossRef]
- Miriello, D.; Galanti, F.; Meneghini, C.; Fabiani, C.; Dal Lago, A.; Schiavi, M.C.; Rago, R. Management of women with uterine fibroids in reproductive center: Retrospective analysis of clinical and reproductive outcomes. Minerva Obstet. Gynecol. 2022, 74, 130–136. [Google Scholar] [CrossRef]
- Donnez, J.; Donnez, O.; Matule, D.; Ahrendt, H.J.; Hudecek, R.; Zatik, J.; Kasilovskiene, Z.; Dumitrascu, M.C.; Fernandez, H.; Barlow, D.H.; et al. Long-term medical management of uterine fibroids with ulipristal acetate. Fertil. Steril. 2016, 105, 165–173. [Google Scholar] [CrossRef]
- Miriello, D.; Galanti, F.; Cignini, P.; Antonaci, D.; Schiavi, M.C.; Rago, R. Uterine fibroids treatment: Do we have new valid alternative? Experiencing the combination of vitamin D plus epigallocatechin gallate in childbearing age affected women. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2843–2851. [Google Scholar]
- Gil, Y.; Badeghiesh, A.; Suarthana, E.; Mansour, F.; Capmas, P.; Volodarsky-Perel, A.; Tulandi, T. Risk of uterine rupture after myomectomy by laparoscopy or laparotomy. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101843. [Google Scholar] [CrossRef]
- Catanese, A.; Siesto, G.; Cucinella, G.; Chiantera, V.; Culmone, S.; Schiattarella, A.; Calagna, G.; Vitobello, D. Factors influencing surgical outcomes of laparoscopic myomectomy. A propensity-score matched analysis. Przegląd Menopauzalny 2022, 21, 149–156. [Google Scholar] [CrossRef]
- Cezar, C.; Becker, S.; di Spiezio Sardo, A.; Herrmann, A.; Larbig, A.; Tanos, V.; de la Roche, L.A.T.; Verhoeven, H.C.; Wallwiener, M.; De Wilde, R.L. Laparoscopy or laparotomy as the way of entrance in myoma enucleation. Arch. Gynecol. Obstet. 2017, 296, 709–720. [Google Scholar] [CrossRef] [PubMed]
- El-Balat, A.; DeWilde, R.L.; Schmeil, I.; Tahmasbi-Rad, M.; Bogdanyova, S.; Fathi, A.; Becker, S. Modern Myoma Treatment in the Last 20 Years: A Review of the Literature. Biomed. Res. Int. 2018, 2018, 4593875. [Google Scholar] [CrossRef] [PubMed]
- Alkhrait, S.; Malasevskaia, I.; Madueke-Laveaux, O.S. Fibroids and Fertility. Obstet. Gynecol. Clin. N. Am. 2023, 50, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Don, E.E.; Mijatovic, V.; Huirne, J.A.F. Infertility in patients with uterine fibroids: A debate about the hypothetical mechanisms. Hum. Reprod. 2023, 38, 2045–2054. [Google Scholar] [CrossRef]
- Lisiecki, M.; Paszkowski, M.; Woźniak, S. Fertility impairment associated with uterine fibroids—A review of literature. Menopause Rev. 2017, 16, 137–140. [Google Scholar] [CrossRef]
- Ng, E.H.; Ho, P.C. Doppler ultrasound examination of uterine arteries on the day of oocyte retrieval in patients with uterine fibroids undergoing IVF. Hum. Reprod. 2002, 17, 765–770. [Google Scholar] [CrossRef]
- Ishikawa, H.; Goto, Y.; Hirooka, C.; Katayama, E.; Baba, N.; Kaneko, M.; Saito, Y.; Kobayashi, T.; Koga, K. Role of inflammation and immune response in the pathogenesis of uterine fibroids: Including their negative impact on reproductive outcomes. J. Reprod. Immunol. 2024, 165, 104317. [Google Scholar] [CrossRef]
- Casini, M.L.; Rossi, F.; Agostini, R.; Unfer, V. Effects of the position of fibroids on fertility. Gynecol. Endocrinol. 2006, 22, 106–109. [Google Scholar] [CrossRef]
- Pritts, E.A.; Parker, W.H.; Olive, D.L. Fibroids and infertility: An updated systematic review of the evidence. Fertil. Steril. 2009, 91, 1215–1223. [Google Scholar] [CrossRef]
- Sunkara, S.K.; Khairy, M.; El-Toukhy, T.; Khalaf, Y.; Coomarasamy, A. The effect of intramural fibroids without uterine cavity involvement on the outcome of IVF treatment: A systematic review and meta-analysis. Hum. Reprod. 2010, 25, 418–429. [Google Scholar] [CrossRef]
- Van den Bosch, T.; Dueholm, M.; Leone, F.P.; Valentin, L.; Rasmussen, C.K.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installé, A.J.; Guerriero, S.; et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: A consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G.; Critchley, H.O.D.; Fraser, I.S. The FIGO classification of causes of abnormal uterine bleeding: Malcolm G. Munro, Hilary O.D. Crithcley, Ian S. Fraser, for the FIGO Working Group on Menstrual Disorders. Int. J. Gynaecol. Obstet. 2011, 113, 2204–2208. [Google Scholar] [CrossRef] [PubMed]
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting Alpha scientists in reproductive medicine and ESHRE special interest group of embryology. Hum. Reprod. 2011, 26, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, S.; Vercellini, P.; Daguati, R.; Pasin, R.; De Giorgi, O.; Crosignani, P.G. Fibroids and female reproduction: A critical analysis of the evidence. Hum. Reprod. Update 2007, 13, 465–476. [Google Scholar] [CrossRef]
- DE Angelis, M.C.; DI Spiezio Sardo, A.; Carugno, J.; Manzi, A.; Sorrentino, F.; Nappi, L. Fertility outcomes after hysteroscopic removal of intrauterine leiomyomas and polyps. Minerva Obstet. Gynecol. 2022, 74, 3–11. [Google Scholar] [CrossRef]
- Oliveira, F.G.; Abdelmassih, V.G.; Diamond, M.P.; Dozortsev, D.; Melo, N.R.; Abdelmassih, R. Impact of subserosal and intramural uterine fibroids that do not distort the endometrial cavity on the outcome of in vitro fertilization-intracytoplasmic sperm injection. Fertil. Steril. 2004, 81, 582–587. [Google Scholar] [CrossRef]
- Yan, L.; Ding, L.; Li, C.; Wang, Y.; Tang, R.; Chen, Z.J. Effect of fibroids not distorting the endometrial cavity on the outcome of in vitro fertilization treatment: A retrospective cohort study. Fertil. Steril. 2014, 101, 716–721.e6. [Google Scholar] [CrossRef]
- Christopoulos, G.; Vlismas, A.; Salim, R.; Islam, R.; Trew, G.; Lavery, S. Fibroids that do not distort the uterine cavity and IVF success rates: An observational study using extensive matching criteria. BJOG 2017, 124, 615–621. [Google Scholar] [CrossRef]
- Yan, L.; Yu, Q.; Zhang, Y.N.; Guo, Z.; Li, Z.; Niu, J.; Ma, J. Effect of type 3 intramural fibroids on in vitro fertilization-intracytoplasmic sperm injection outcomes: A retrospective cohort study. Fertil. Steril. 2018, 109, 817–822. [Google Scholar] [CrossRef]
- Bulletti, C.; DEZiegler, D.; Levi Setti, P.; Cicinelli, E.; Polli, V.; Stefanetti, M. Myomas, pregnancy outcome, and in vitro fertilization. Ann. N. Y. Acad. Sci. 2004, 1034, 84–92. [Google Scholar] [CrossRef]
- Sarıdoğan, E.; Sarıdoğan, E. Management of fibroids prior to in vitro fertilization/intracytoplasmic sperm injection: A pragmatic approach. J. Turk.-Ger. Gynecol. Assoc. 2019, 20, 55–59. [Google Scholar] [PubMed]
- Favilli, A.; Etrusco, A.; Chiantera, V.; Laganà, A.S.; Cicinelli, E.; Gerli, S.; Vitagliano, A. Impact of FIGO type 3 uterine fibroids on in vitro fertilization outcomes: A systematic review and meta-analysis. Int. J. Gynecol. Obstet. 2023, 163, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Erden, M.; Uyanik, E.; Polat, M.; Ozbek, I.Y.; Yarali, H.; Mumusoglu, S. The effect of ≤6 cm sized noncavity-distorting intramural fibroids on in vitro fertilization outcomes: A systematic review and meta-analysis. Fertil. Steril. 2023, 119, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Guven, S.; Kart, C.; Unsal, M.A.; Odaci, E. Intramural leoimyoma without endometrial cavity distortion may negatively affect the ICSI-ET outcome. Reprod. Biol. Endocrinol. 2013, 11, 102. [Google Scholar] [CrossRef]
- Deger, U.; Altinbas, E.; Karabay, M.; Karatas, Y.; Deniz, Z.; Buyuker, C.; Yildirim Kopuk, S.; Tiras, B.; Cakiroglu, Y. Effects of Non-Cavity-Distorting Intramural Fibroids on IVF Outcomes in Patients with Recurrent IVF Failure: Does Myomectomy Change IVF Outcomes? J. Obstet. Gynaecol. India 2023, 73, 322–328. [Google Scholar] [CrossRef]
- Bonanni, V.; Reschini, M.; La Vecchia, I.; Castiglioni, M.; Muzii, L.; Vercellini, P.; Somigliana, E. The impact of small and asymptomatic intramural and subserosal fibroids on female fertility: A case-control study. Hum. Reprod. Open 2022, 2023, hoac056. [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Removal of myomas in asymptomatic patients to improve fertility and/or reduce miscarriage rate: A guideline. Fertil. Steril. 2017, 108, 416–425. [Google Scholar] [CrossRef]
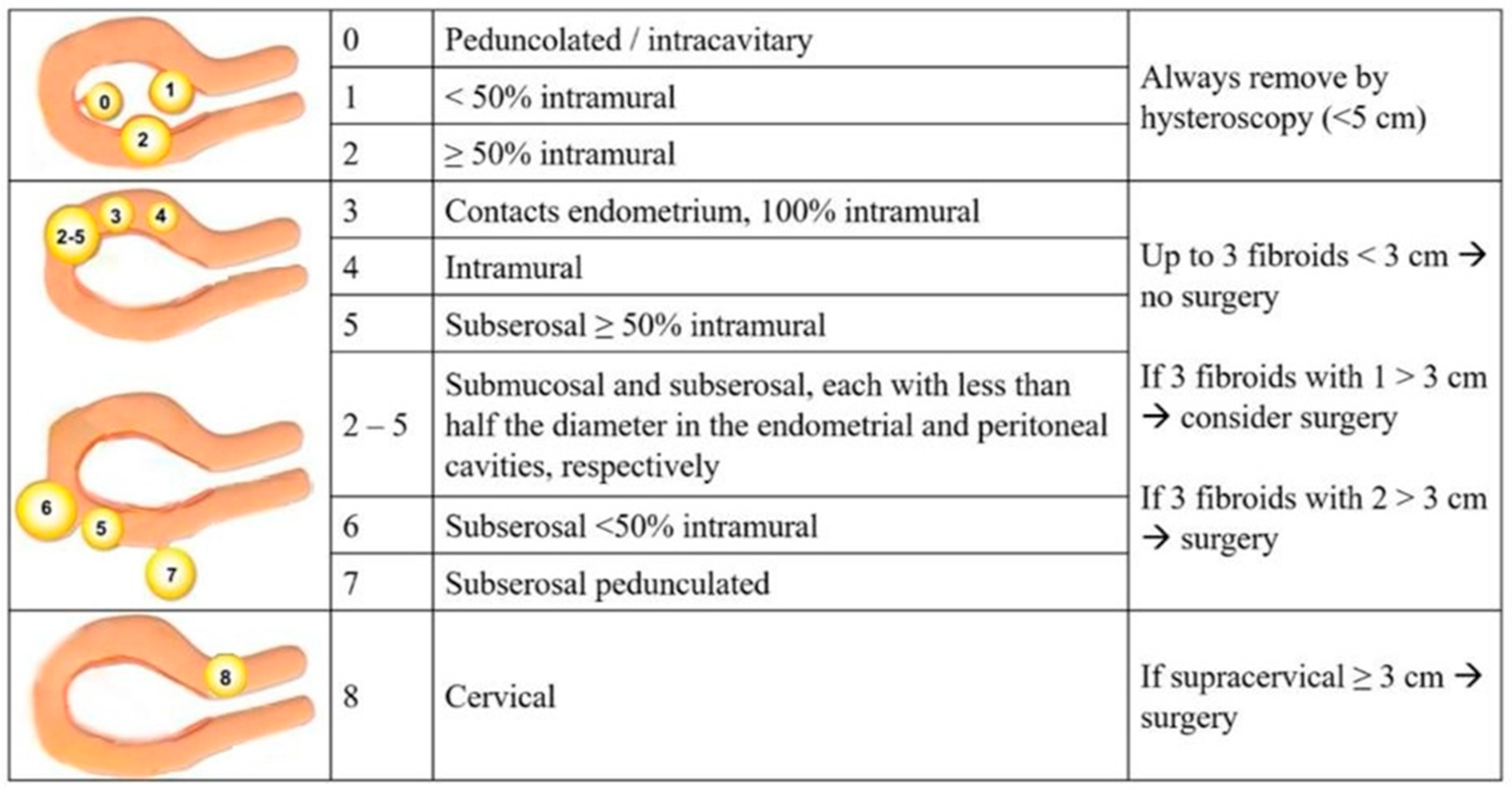
- Lakabi, R.; Harth, S.; Meinhold-Heerlein, I.; Olsthoorn, A.V.; Munro, M.G.; Murji, A. Diagnosis and classification of uterine fibroids. Int. J. Gynaecol. Obstet. 2025, 171, 566–573. [Google Scholar]
- Conoscenti, G.; Di Spiezio Sardo, A.; Exacoustos, C.; Maiorana, A.; Meir, Y.J.; Parazzini, F.; Schettini, S.; Vignali, M.; Vizza, E.; Zullo, F.; et al. Guidelines for the Diagnosis and Treatment of Fibromyomatosis (Draft); AGUI—Associazione Ginecologi Universitari Italiani: Roma, Italy, 2017. [Google Scholar]
- Tanos, V.; Toney, Z.A. Uterine scar rupture—Prediction, prevention, diagnosis, and management. Best. Pract. Res. Clin. Obstet. Gynaecol. 2019, 59, 115–131. [Google Scholar] [CrossRef]
- Ton, R.; Kilic, G.S.; Phelps, J.Y. A medical-legal review of power morcellation in the face of the recent FDA warning and litigation. J. Minim. Invasive Gynecol. 2015, 22, 564–572. [Google Scholar] [CrossRef]
- Galanti, F.; Riccio, S.; Giannini, A.; D’Oria, O.; Buzzaccarini, G.; Scudo, M.; Muzii, L.; Battaglia, F.A. Placentation and complications of ART pregnancy. An update on the different possible etiopathogenic mechanisms involved in the development of obstetric complications. J. Reprod. Immunol. 2024, 162, 104191. [Google Scholar] [CrossRef]
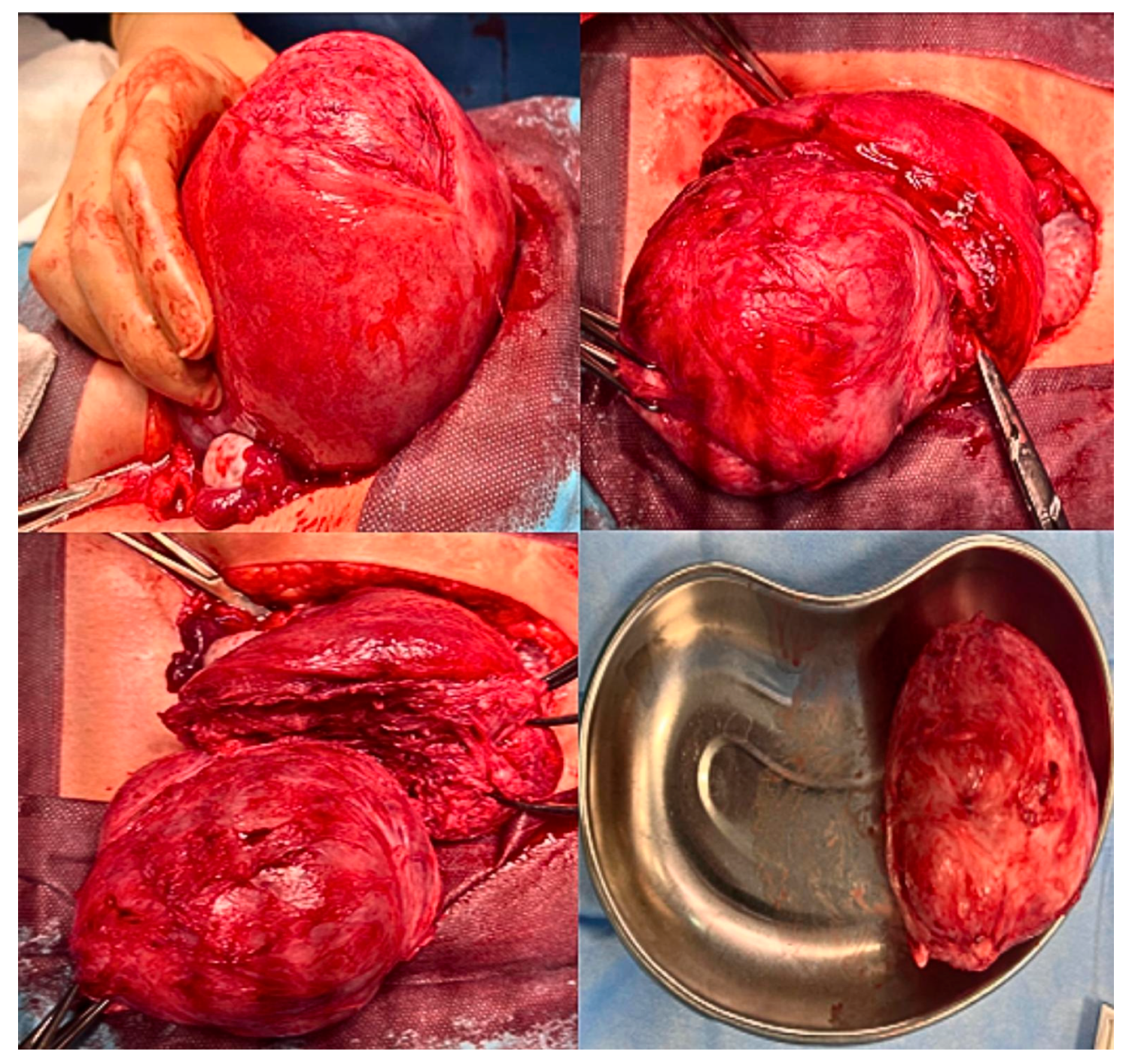
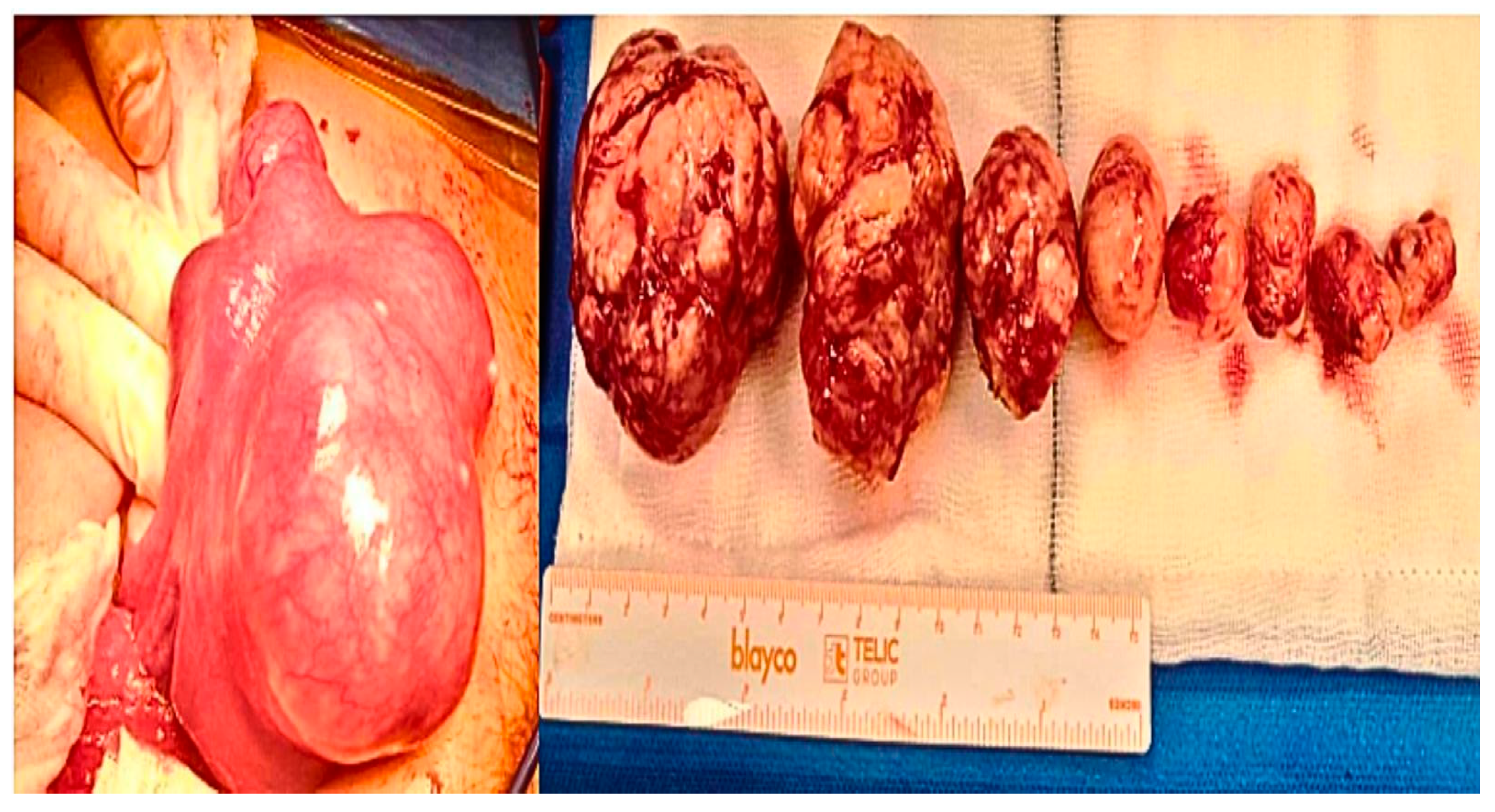
| Clinical Parameters | Mean (SD) |
|---|---|
| Age (years) | 36.53 ± 3.34 |
| BMI (kg/m2) | 25.18 ± 2.32 |
| Duration of infertility (years) | 3.2 ± 1.2 |
| Basal FSH (mIU/mL) | 6.19 ± 2.32 |
| AMH (ng/mL) | 1.26 ± 0.77 |
| Days of hormonal stimulation | 13.12 ± 2.06 |
| Total dose of gonadotropin (UI) | 2077.35 ± 999.15 |
| MII Oocytes retrieved | 4.29 ± 1.86 |
| Embryos obtained | 1.76 ± 1.15 |
| Clinical parameters and pregnancy outcomes | n° (%) |
| Tubal factor of infertility | 10 (58.8) |
| Idiopathic factor of infertility | 7 (41.2) |
| Implantation rate | 7 (41%) |
| Clinical pregnancy (CPR) | 6 (35.2%) |
| Live birth rate (LBR) | 6 (100%) |
| Miscarriage | 1 (5.8%) |
| Preterm delivery | 0 (0%) |
| Caesarean section (of total CPR) | 3 (50%) |
| Demographics and Clinical Parameters | Group B (n° 39) | Group C (n° 38) | p-Value |
|---|---|---|---|
| Age (years) Mean (SD) | 38.88 ± 3.38 | 38.84 ± 4.03 | Ns |
| BMI (kg/m2) Mean (SD) | 24.71 ± 5.23 | 23.2 ± 3.75 | 0.19 |
| Duration of infertility (years) Mean (SD) | 2.1 ± 1.2 | 2.2 ± 1.1 | Ns |
| Basal FSH (mIU/mL) Mean (SD) | 7.15 ± 2.20 | 6.55 ± 2.01 | 0.27 |
| AMH (ng/mL) Mean (SD) | 1.20 ± 0.79 | 1.10 ± 0.78 | Ns |
| Tubal factor of infertility n° (%) | 15 (38.5) | 16 (42) | Ns |
| Idiopathic factor of infertility n° (%) | 24 (61.5) | 22 (58) | Ns |
| MII Oocytes retrieved (n°) Mean (SD) | 3.66 ± 1.81 | 3.60 ± 1.85 | Ns |
| Embryos obtained (n°) Mean (SD) | 1.16 ± 0.99 | 1.13 ± 1.02 | Ns |
| Myomas Localization (According to FIGO 2011 Classification) | Group B (n° 39) | Group C (n° 38) | p-Value |
|---|---|---|---|
| Type 3 | 10 | 11 | Ns |
| Type 4 | 13 | 12 | Ns |
| Type 5 | 18 | 18 | Ns |
| Type 6 | 18 | 7 | Ns |
| Type 7 | 4 | 5 | Ns |
| Type 8 | 5 | 3 | Ns |
| Dimensions (cm) | |||
| Small (<3) | 10 | 5 | Ns |
| Medium (3–5) | 49 | 39 | Ns |
| Large (>5) | 6 | 3 | Ns |
| Min. dimension | 2 | 2 | Ns |
| 1st Quartile | 3 | 3 | Ns |
| Median | 4 | 4 | Ns |
| Mean | 4.1 | 3.7 | Ns |
| 3rd Quartile | 5 | 4.5 | Ns |
| Max. dimension | 12 | 8 | Ns |
| Typologies of myomas | |||
| Single | 23 | 28 | Ns |
| Multiple | 23 | 18 | Ns |
| Pregnancy Outcomes | Group B (n° 39) | Group C (n° 38) | p-Value |
|---|---|---|---|
| Pregnancy positive test n° (%) | 12 (31) | 5 (13) | 0.03 |
| Clinical pregnancy n° (%) | 11 (28.1) | 3 (7.8) | 0.02 |
| Miscarriage (<23 weeks) n° (%) | 1 (3.9) | 1 (3.9) | Ns |
| Preterm deliveries n° (%) | 0 (0) | 2 (5.2) | Ns |
| Spontaneous deliveries n° (%) | 3 (7.6) | 1 (3.8) | Ns |
| Caesarean section n° (%) | 8 (20.5) | 1 (3.8) | <0.01 |
| Live Birth n° (%) | 11 (100) | 1 (25) | <0.01 |
| Uterine rupture n° (%) | 0 (0) | 0 (0) | Ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Antonaci, D.; Galanti, F.; Dall’Alba, R.; Benedetti, E.; Rago, A.; Antonaci, L.; Miriello, D.; Rago, R. A Proposed Model of a Pragmatic Surgical Approach in Women Affected by Uterine Fibroids Undergoing IVF: A “Real Practice” Experience. J. Clin. Med. 2026, 15, 379. https://doi.org/10.3390/jcm15010379
Antonaci D, Galanti F, Dall’Alba R, Benedetti E, Rago A, Antonaci L, Miriello D, Rago R. A Proposed Model of a Pragmatic Surgical Approach in Women Affected by Uterine Fibroids Undergoing IVF: A “Real Practice” Experience. Journal of Clinical Medicine. 2026; 15(1):379. https://doi.org/10.3390/jcm15010379
Chicago/Turabian StyleAntonaci, Domenico, Francesco Galanti, Roberta Dall’Alba, Eleonora Benedetti, Andrea Rago, Laura Antonaci, Donatella Miriello, and Rocco Rago. 2026. "A Proposed Model of a Pragmatic Surgical Approach in Women Affected by Uterine Fibroids Undergoing IVF: A “Real Practice” Experience" Journal of Clinical Medicine 15, no. 1: 379. https://doi.org/10.3390/jcm15010379
APA StyleAntonaci, D., Galanti, F., Dall’Alba, R., Benedetti, E., Rago, A., Antonaci, L., Miriello, D., & Rago, R. (2026). A Proposed Model of a Pragmatic Surgical Approach in Women Affected by Uterine Fibroids Undergoing IVF: A “Real Practice” Experience. Journal of Clinical Medicine, 15(1), 379. https://doi.org/10.3390/jcm15010379









