Robot-Assisted Mirror Therapy for Upper Limb and Hand Recovery After Stroke: Clinical Efficacy and Insights into Neural Mechanisms
Abstract
1. Introduction
2. Materials and Methods
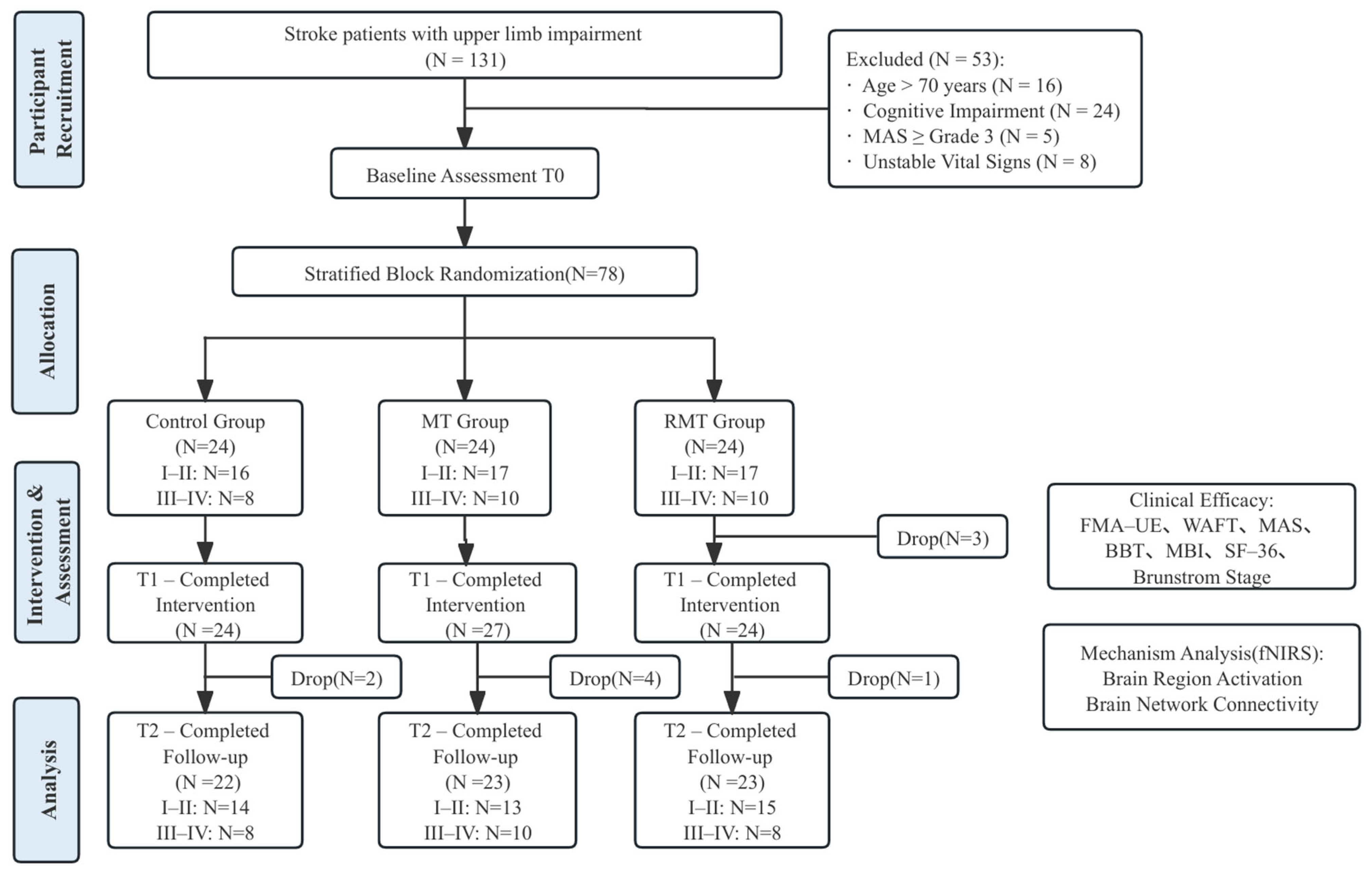
2.1. Study Design
2.2. Randomization and Blinding
2.3. Sample Size
2.4. Ethics
2.5. Participants
2.6. Interventions
2.7. Clinical Efficacy and Mechanistic Indicators
2.7.1. Clinical Efficacy Indicators
2.7.2. fNIRS Data Acquisition and Preprocessing
2.8. Statistical Analysis
3. Results
3.1. Basic Characteristics of Participants
3.2. Clinical Efficacy
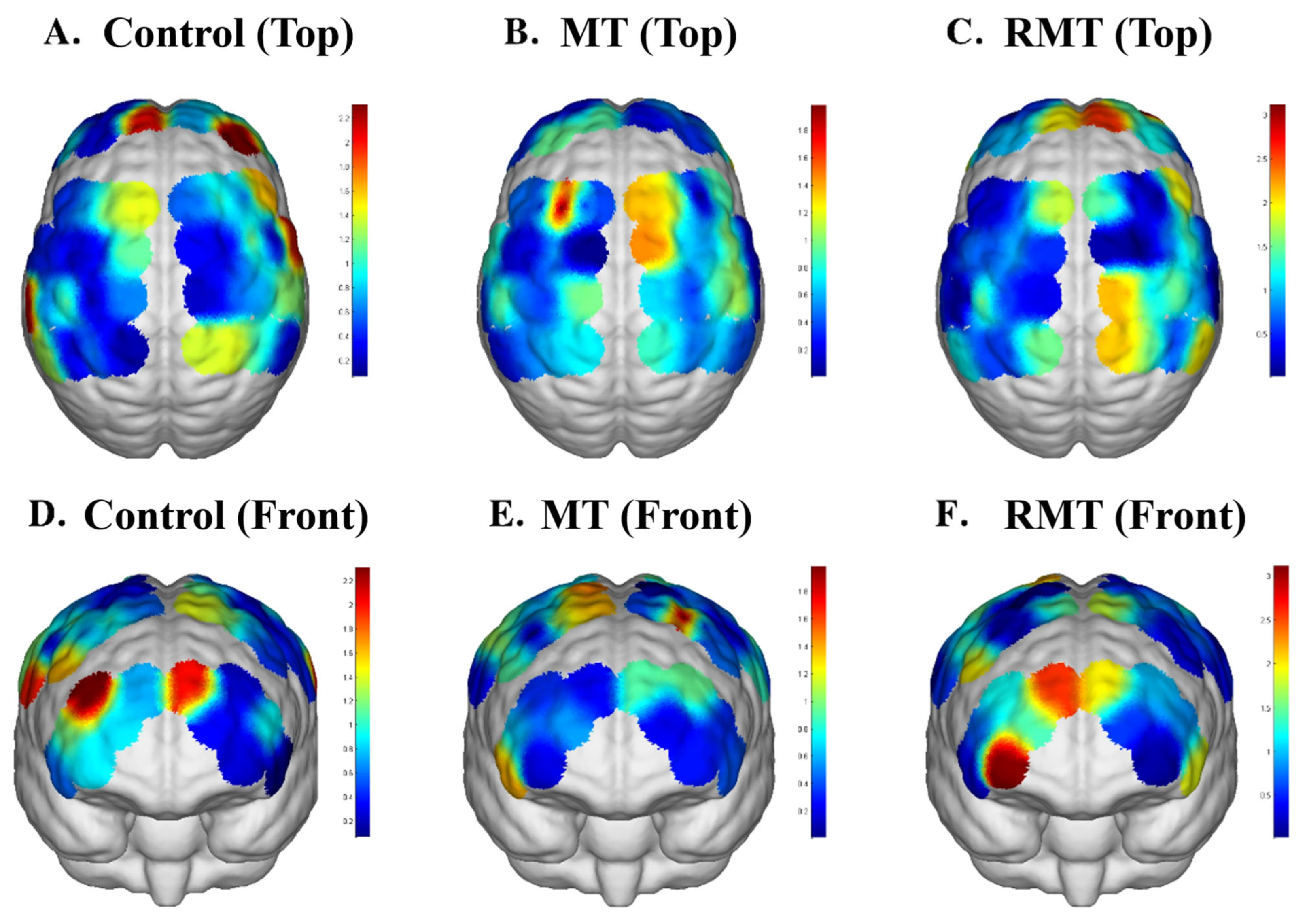
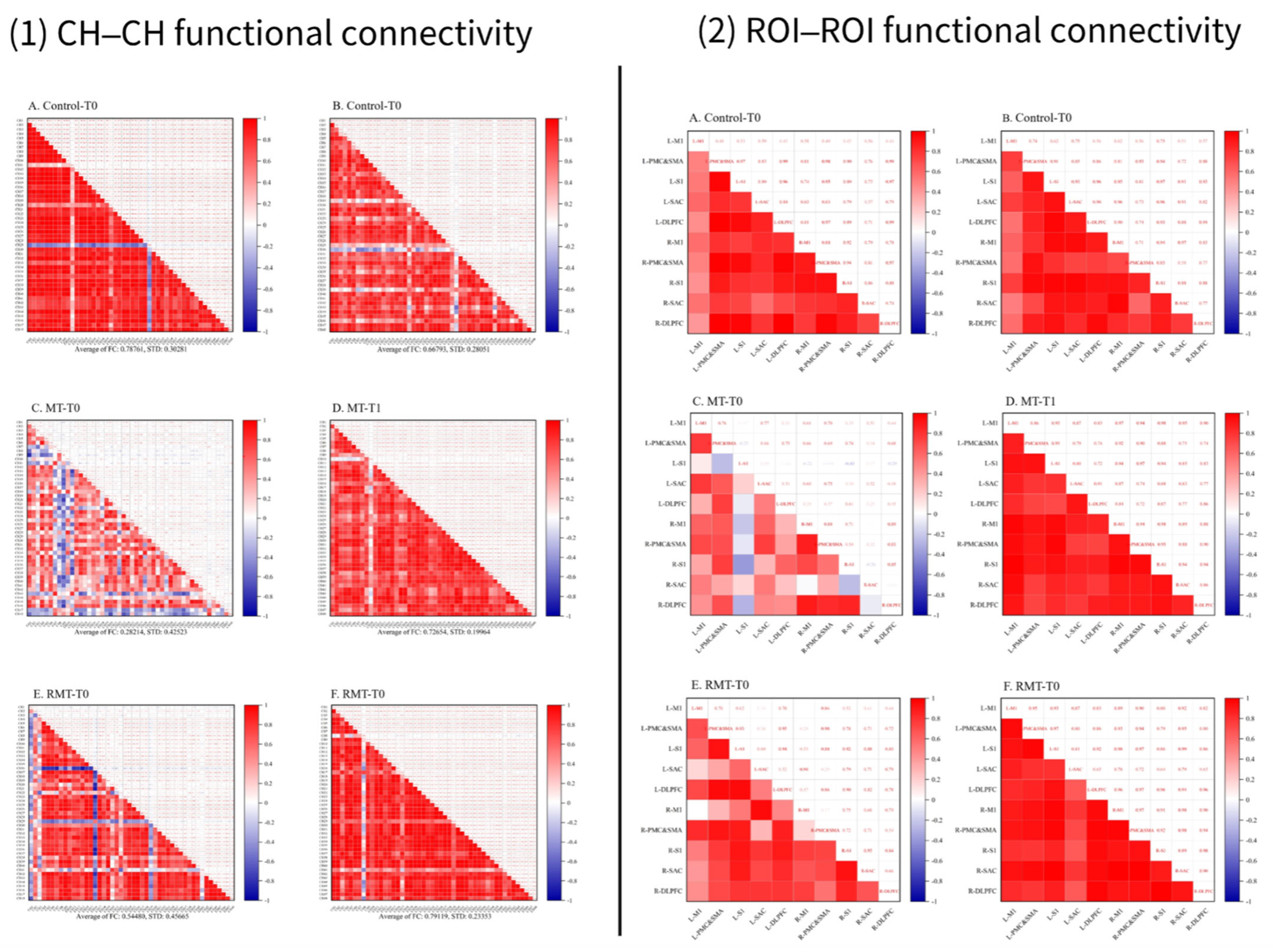
3.3. The Functional Connection of Brain Networks
4. Discussion
4.1. Clinical Significance of Functional Outcomes
4.2. Neural Mechanisms and Implications
4.3. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzik, A.; Bushnell, C. Stroke Epidemiology and Risk Factor Management. Continuum 2017, 23, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Yang, X.; Sun, M.; He, M.; Sa, R.; Zhang, K.; Zhu, B.; Li, T. Research trends and hotspots of post-stroke upper limb dysfunction: A bibliometric and visualization analysis. Front. Neurol. 2024, 15, 1449729. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.S.; Rogers-Ramachandran, D. Synaesthesia in phantom limbs induced with mirrors. Proc. Biol. Sci. 1996, 263, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Altschuler, E.L.; Wisdom, S.B.; Stone, L.; Foster, C.; Galasko, D.; Llewellyn, D.M.; Ramachandran, V.S. Rehabilitation of hemiparesis after stroke with a mirror. Lancet 1999, 353, 2035–2036. [Google Scholar] [CrossRef]
- Thieme, H.; Morkisch, N.; Mehrholz, J.; Pohl, M.; Behrens, J.; Borgetto, B.; Dohle, C. Mirror therapy for improving motor function after stroke. Cochrane Database Syst. Rev. 2018, 7, Cd008449. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Q.; Gou, W.; He, C. Effects of mirror therapy on walking ability, balance and lower limb motor recovery after stroke: A systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 2018, 32, 1007–1021. [Google Scholar] [CrossRef]
- Yavuzer, G.; Selles, R.; Sezer, N.; Sütbeyaz, S.; Bussmann, J.B.; Köseoğlu, F.; Atay, M.B.; Stam, H.J. Mirror therapy improves hand function in subacute stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2008, 89, 393–398. [Google Scholar] [CrossRef]
- Samuelkamaleshkumar, S.; Reethajanetsureka, S.; Pauljebaraj, P.; Benshamir, B.; Padankatti, S.M.; David, J.A. Mirror therapy enhances motor performance in the paretic upper limb after stroke: A pilot randomized controlled trial. Arch. Phys. Med. Rehabil. 2014, 95, 2000–2005. [Google Scholar] [CrossRef]
- Tyson, S.F.; Hanley, M.; Chillala, J.; Selley, A.B.; Tallis, R.C. Sensory loss in hospital-admitted people with stroke: Characteristics, associated factors, and relationship with function. Neurorehabil. Neural Repair 2008, 22, 166–172. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Altschuler, E.L. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain 2009, 132, 1693–1710. [Google Scholar] [CrossRef]
- Cattaneo, L.; Rizzolatti, G. The mirror neuron system. Arch. Neurol. 2009, 66, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Matthys, K.; Smits, M.; Van der Geest, J.N.; Van der Lugt, A.; Seurinck, R.; Stam, H.J.; Selles, R.W. Mirror-induced visual illusion of hand movements: A functional magnetic resonance imaging study. Arch. Phys. Med. Rehabil. 2009, 90, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Arya, K.N. Underlying neural mechanisms of mirror therapy: Implications for motor rehabilitation in stroke. Neurol. India 2016, 64, 38–44. [Google Scholar] [CrossRef]
- Nojima, I.; Oga, T.; Fukuyama, H.; Kawamata, T.; Mima, T. Mirror visual feedback can induce motor learning in patients with callosal disconnection. Exp. Brain Res. 2013, 227, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Ku, J.; Kim, H.J.; Park, H.K. Facilitation of corticospinal excitability according to motor imagery and mirror therapy in healthy subjects and stroke patients. Ann. Rehabil. Med. 2011, 35, 747–758. [Google Scholar] [CrossRef]
- Michielsen, M.E.; Selles, R.W.; van der Geest, J.N.; Eckhardt, M.; Yavuzer, G.; Stam, H.J.; Smits, M.; Ribbers, G.M.; Bussmann, J.B. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: A phase II randomized controlled trial. Neurorehabil. Neural Repair 2011, 25, 223–233. [Google Scholar] [CrossRef]
- Bhasin, A.; Padma Srivastava, M.V.; Kumaran, S.S.; Bhatia, R.; Mohanty, S. Neural interface of mirror therapy in chronic stroke patients: A functional magnetic resonance imaging study. Neurol. India 2012, 60, 570–576. [Google Scholar] [CrossRef]
- Hamzei, F.; Läppchen, C.H.; Glauche, V.; Mader, I.; Rijntjes, M.; Weiller, C. Functional plasticity induced by mirror training: The mirror as the element connecting both hands to one hemisphere. Neurorehabil. Neural Repair 2012, 26, 484–496. [Google Scholar] [CrossRef]
- Nogueira, N.; Parma, J.O.; Leão, S.; Sales, I.S.; Macedo, L.C.; Galvão, A.; de Oliveira, D.C.; Murça, T.M.; Fernandes, L.A.; Junqueira, C.; et al. Mirror therapy in upper limb motor recovery and activities of daily living, and its neural correlates in stroke individuals: A systematic review and meta-analysis. Brain Res. Bull. 2021, 177, 217–238. [Google Scholar] [CrossRef]
- Michielsen, M.E.; Smits, M.; Ribbers, G.M.; Stam, H.J.; van der Geest, J.N.; Bussmann, J.B.; Selles, R.W. The neuronal correlates of mirror therapy: An fMRI study on mirror induced visual illusions in patients with stroke. J. Neurol. Neurosurg. Psychiatry 2011, 82, 393–398. [Google Scholar] [CrossRef]
- Stecco, A.; Meneghini, A.; Stern, R.; Stecco, C.; Imamura, M. Ultrasonography in myofascial neck pain: Randomized clinical trial for diagnosis and follow-up. Surg. Radiol. Anat. 2014, 36, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Kuo, F.L.; Lin, Y.N.; Liou, T.H.; Lin, J.C.; Huang, S.W. Effects of Robot-Assisted Rehabilitation on Hand Function of People With Stroke: A Randomized, Crossover-Controlled, Assessor-Blinded Study. Am. J. Occup. Ther. 2021, 75, 7501205020p1–7501205020p11. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shi, X.; Xue, X.; Deng, Z. Efficacy of Robot-Assisted Training on Rehabilitation of Upper Limb Function in Patients With Stroke: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2023, 104, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Chien, W.T.; Chong, Y.Y.; Tse, M.K.; Chien, C.W.; Cheng, H.Y. Robot-assisted therapy for upper-limb rehabilitation in subacute stroke patients: A systematic review and meta-analysis. Brain Behav. 2020, 10, e01742. [Google Scholar] [CrossRef]
- Saita, K.; Morishita, T.; Hyakutake, K.; Ogata, T.; Fukuda, H.; Kamada, S.; Inoue, T. Feasibility of Robot-assisted Rehabilitation in Poststroke Recovery of Upper Limb Function Depending on the Severity. Neurol. Med. Chir. 2020, 60, 217–222. [Google Scholar] [CrossRef]
- Malik, A.N.; Tariq, H.; Afridi, A.; Rathore, F.A. Technological advancements in stroke rehabilitation. J. Pak. Med. Assoc. 2022, 72, 1672–1674. [Google Scholar] [CrossRef]
- Zhang, T.; Yao, Z.; Chen, F.; Wang, J.; Shi, W.; Zheng, J.; Zhang, Z.; Chen, Z. Enhancing Upper Limb Function and Motor Skills Post-Stroke Through an Upper Limb Rehabilitation Robot. J. Vis. Exp. 2024, 211, e66938. [Google Scholar] [CrossRef]
- Chen, Y.W.; Li, K.Y.; Lin, C.H.; Hung, P.H.; Lai, H.T.; Wu, C.Y. The effect of sequential combination of mirror therapy and robot-assisted therapy on motor function, daily function, and self-efficacy after stroke. Sci. Rep. 2023, 13, 16841. [Google Scholar] [CrossRef]
- Gunduz, M.E.; Pacheco-Barrios, K.; Bonin Pinto, C.; Duarte, D.; Vélez, F.G.S.; Gianlorenco, A.C.L.; Teixeira, P.E.P.; Giannoni-Luza, S.; Crandell, D.; Battistella, L.R.; et al. Effects of Combined and Alone Transcranial Motor Cortex Stimulation and Mirror Therapy in Phantom Limb Pain: A Randomized Factorial Trial. Neurorehabil. Neural Repair 2021, 35, 704–716. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Correction to: Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2017, 48, e78. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer Assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabilit. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.L.; Catlin, P.A.; Ellis, M.; Archer, A.L.; Morgan, B.; Piacentino, A. Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke 2001, 32, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Brunnstrom, S. Motor testing procedures in hemiplegia: Based on sequential recovery stages. Phys. Ther. 1966, 46, 357–375. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult norms for the Box and Block Test of manual dexterity. Am. J. Occup. Ther. 1985, 39, 386–391. [Google Scholar] [CrossRef]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Kwakkel, G.; Stinear, C.; Essers, B.; Munoz-Novoa, M.; Branscheidt, M.; Cabanas-Valdés, R.; Lakičević, S.; Lampropoulou, S.; Luft, A.L.; Verheyden, G.; et al. Motor rehabilitation after stroke: European Stroke Organisation (ESO) consensus-based definition and guiding framework. Eur. Stroke J. 2023, 8, 4. [Google Scholar] [CrossRef]
- Jin, H.; Xiong, W. Effect of rehabilitation robot glove combined with mirror therapy on hand function recovery after stroke. Yixue Lilun Yu Shijian 2024, 37, 1056–1058. [Google Scholar]
- He, Y.Z.; Huang, Z.M.; Deng, H.Y.; Huang, J.; Wu, J.H.; Wu, J.S. Feasibility, safety, and efficacy of task-oriented mirrored robotic training on upper-limb functions and activities of daily living in subacute poststroke patients: A pilot study. Eur. J. Phys. Rehabil. Med. 2023, 59, 660–668. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, G.; Kim, H.; Cho, D.Y.; Kwon, S. Combined effects and timing of robotic training and botulinum toxin on upper limb spasticity and motor function: A single-blinded randomized controlled pilot study. J. Neuroeng. Rehabil. 2025, 22, 50. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst. Rev. 2015, 2015, Cd006876. [Google Scholar] [CrossRef] [PubMed]
- Todorov, E.; Jordan, M.I. Optimal feedback control as a theory of motor coordination. Nat. Neurosci. 2002, 5, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Zhao, H.; Liang, Y.; Zhang, W.; Li, M.; Liu, H.; Hu, D.; Zhang, S.; Xing, E.; et al. The relationship between the prefrontal cortex and limb motor function in stroke: A study based on resting-state functional near-infrared spectroscopy. Brain Res. 2023, 1805, 148269. [Google Scholar] [CrossRef]
- D'Esposito, M.; Postle, B.R. The cognitive neuroscience of working memory. Annu. Rev. Psychol. 2015, 66, 115–142. [Google Scholar] [CrossRef]
- Abekawa, N.; Ito, S.; Gomi, H. Gaze-specific motor memories for hand-reaching. Curr. Biol. 2022, 32, 2747–2753.e6. [Google Scholar] [CrossRef]
- Spampinato, D.; Celnik, P. Multiple motor learning processes in humans: Defining their neurophysiological bases. Neurosci. 2021, 27, 246–267. [Google Scholar] [CrossRef]
- Popa, L.S.; Ebner, T.J. Cerebellum, predictions and errors. Front. Cell. Neurosci. 2019, 12, 524. [Google Scholar] [CrossRef]
- Markov, D.A.; Petrucco, L.; Kist, A.M.; Portugues, R. A cerebellar internal model calibrates a feedback controller involved in sensorimotor control. Nat. Commun. 2021, 12, 6694. [Google Scholar] [CrossRef]
- Antonioni, A.; Raho, E.M.; Straudi, S.; Granieri, E.; Koch, G.; Fadiga, L. The cerebellum and the mirror neuron system: A matter of inhibition? From neurophysiological evidence to neuromodulatory implications. A narrative review. Neurosci. Biobehav. Rev. 2024, 164, 105830. [Google Scholar] [CrossRef]
- Ishikawa, T.; Tomatsu, S.; Izawa, J.; Kakei, S. The cerebro-cerebellum: Could it be loci of forward models? Neurosci. Res. 2016, 104, 72–79. [Google Scholar] [CrossRef]
- Antonioni, A.; Galluccio, M.; Baroni, A.; Fregna, G.; Pozzo, T.; Koch, G.; Manfredini, F.; Fadiga, L.; Malerba, P.; Straudi, S. Event-related desynchronization during action observation is an early predictor of recovery in subcortical stroke: An EEG study. Ann. Phys. Rehabil. Med. 2024, 67, 101817. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Huang, S.; Huang, H.; Wu, W.; Wang, Y.; An, X.; Ming, D. Enhancing ERD activation and functional connectivity via the sixth-finger motor imagery in stroke patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 3902–3912. [Google Scholar] [CrossRef]
| Variable | Control (n = 22) | MT (n = 23) | RMT (n = 23) | p |
|---|---|---|---|---|
| Age(year), M [P25, P75] | 57.50 [49.50, 60.00] | 55.00 [42.00, 58.50] | 51.00 [44.00, 60.00] | 0.562 ➀ |
| Gender, n (%) | ||||
| Female (reference = Male) | 3 (13.6%) | 8 (34.8%) | 5 (21.7%) | 0.24 ➁ |
| Marital status, n (%) | ||||
| Married (reference = Others) | 20 (90.9%) | 19 (82.6%) | 22 (95.7%) | 0.338 ➂ |
| Degree of education, n (%) | 0.654 ➁ | |||
| Primary school & below | 1 (4.5%) | 4 (17.4%) | 5 (21.7%) | |
| Junior high school | 9 (40.9%) | 5 (21.7%) | 5 (21.7%) | |
| High school | 3 (13.6%) | 3 (13.0%) | 1 (4.3%) | |
| College | 8 (36.4%) | 10 (43.5%) | 11 (47.8%) | |
| Master & above | 1 (4.5%) | 1 (4.3%) | 1 (4.3%) | |
| Stroke types, n (%) | ||||
| Hemorrhagic (reference = Ischemic) | 13 (59.1%) | 8 (34.8%) | 11 (47.8%) | 0.262 ➂ |
| lesion location, n (%) | 0.056 ➁ | |||
| Cortex | 7 (31.8%) | 4 (17.4%) | 7 (30.4%) | |
| Subcortex | 15 (68.2%) | 13 (56.5%) | 9 (39.1%) | |
| Mix | 0 (0.0%) | 6 (26.1%) | 7 (30.4%) | |
| Hemiplegic side, n (%) | ||||
| Right (reference = Left) | 9 (40.9%) | 10 (43.5%) | 11 (47.8%) | 0.894 ➂ |
| Disease duration (months), M [P25, P75] | 1.20 [0.92, 2.98] | 1.30 [0.90, 2.50] | 1.00 [0.60, 2.20] | 0.6 ➀ |
| <3 months, n (%) | 16 (72.7%) | 18 (78.3%) | 20 (87.0%) | |
| ≥3 months, n (%) | 6 (27.3%) | 5 (21.7%) | 3 (13.0%) | 0.491 ➁ |
| Brunnstrom stage, n (%) | 0.812 ➂ | |||
| I–II | 14 (63.6%) | 13 (56.5%) | 15 (65.2%) | |
| III–IV | 8 (36.4%) | 10 (43.5%) | 8 (34.8%) | |
| Hypertension, n (%) | ||||
| Yes (reference = No) | 19 (86.4%) | 17 (73.9%) | 18 (78.3%) | 0.579 ➂ |
| Hyperlipidemia, n (%) | ||||
| Yes (reference = No) | 6 (27.3%) | 5 (21.7%) | 6 (26.1%) | 0.902 ➂ |
| Diabetes, n (%) | ||||
| Yes (reference = No) | 4 (18.2%) | 4 (17.4%) | 6 (26.1%) | 0.724 ➁ |
| Smoking, n (%) | ||||
| Yes (reference = No) | 10 (45.5%) | 3 (13.0%) | 3 (13.0%) | 0.013 ➁* |
| Drinking, n (%) | ||||
| Yes (reference = No) | 7 (31.8%) | 4 (17.4%) | 7 (30.4%) | 0.476 ➁ |
| Group | T0 | T1 | T2 | Time Effect | Intervention Effect | Interaction Effect | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |||||
| FMA-UE | Control | 16.23 ± 12.66 | 23.18 ± 13.03 | 27.95 ± 15.38 | 192.2 | <0.001 * | 0.2 | 0.854 | 1.6 | 0.175 |
| MT | 15.5 ± 11.01 | 24.74 ± 12.64 | 30.74 ± 13.89 | |||||||
| RMT | 15.30 ± 12.17 | 22.35 ± 13.22 | 27.00 ± 14.34 | |||||||
| WMFT | Control | 12.55 ± 12.66 | 19 ± 11.88 | 22.45 ± 12.98 | 185.3 | <0.001 * | 0.3 | 0.764 | 1.65 | 0.167 |
| MT | 13.96 ± 12.74 | 21.65 ± 13.78 | 27.17 ± 14.89 | |||||||
| RMT | 11.78 ± 13.62 | 20.7 ± 15.34 | 24.48 ± 16.75 | |||||||
| MAS (elbow fle) | Control | 1.77 ± 0.81 | 1.05 ± 0.9 | 1.23 ± 0.92 | 95.36 | <0.001 * | 0.43 | 0.65 | 0.54 | 0.703 |
| MT | 1.65 ± 0.88 | 0.78 ± 0.85 | 0.91 ± 0.9 | |||||||
| RMT | 1.74 ± 0.92 | 1 ± 1 | 1.09 ± 0.95 | |||||||
| MAS (elbow ext) | Control | 1.77 ± 0.87 | 1.09 ± 0.87 | 1.41 ± 0.8 | 59.17 | <0.001 * | 2.13 | 0.127 | 0.86 | 0.49 |
| MT | 1.43 ± 0.66 | 0.7 ± 0.82 | 1 ± 1 | |||||||
| RMT | 1.39 ± 0.78 | 0.7 ± 0.93 | 0.78 ± 1 | |||||||
| Brunnstrom (upper limb) | Control | 2.23 ± 1.02 | 2.82 ± 0.8 | 3.18 ± 0.73 | 182.08 | <0.001 * | 0.74 | 0.483 | 2.19 | 0.074 |
| MT | 2.35 ± 0.78 | 3.09 ± 0.73 | 3.65 ± 0.71 | |||||||
| RMT | 2.22 ± 1.09 | 3.13 ± 1.01 | 3.52 ± 0.9 | |||||||
| Brunnstrom (hand) | Control | 1.86 ± 0.83 | 2.41 ± 0.8 | 2.64 ± 0.79 | 87.75 | <0.001 * | 0.62 | 0.543 | 0.64 | 0.635 |
| MT | 2 ± 0.9 | 2.65 ± 0.78 | 3.04 ± 0.82 | |||||||
| RMT | 2 ± 1.09 | 2.61 ± 1.03 | 2.96 ± 1.07 | |||||||
| BBT | Control | 1.23 ± 3.21 | 3.77 ± 5.23 | 4.59 ± 6.11 | 33.59 | <0.001 * | 0.26 | 0.769 | 2.76 | 0.030 * |
| MT | 1.04 ± 2.53 | 4.52 ± 5.8 | 6.96 ± 7.75 | |||||||
| RMT | 2.09 ± 4.7 | 3.04 ± 6 | 4.48 ± 7.69 | |||||||
| MBI | Control | 57 ± 22.2 | 67.55 ± 20.35 | 72.95 ± 17.37 | 123.73 | <0.001 * | 0.36 | 0.7 | 1.46 | 0.22 |
| MT | 56 ± 24.06 | 70.87 ± 19.27 | 79.43 ± 14.21 | |||||||
| RMT | 51.87 ± 32.09 | 64.83 ± 28.79 | 73 ± 23.29 | |||||||
| SF-36 | Control | 63.5 ± 14 | 61 ± 14.19 | 57.64 ± 15.38 | 22.2 | <0.001 * | 0.79 | 0.458 | 0.83 | 0.439 |
| MT | 68 ± 18.50 | 66.35 ± 18 | 64.61 ± 19.15 | |||||||
| RMT | 66.56 ± 16.50 | 64.26 ± 16.06 | 60.87 ± 16.3 | |||||||
| Group | T0 | T1 | T2 | Time Effect | Intervention Effect | Interaction Effect | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |||||
| FMA-UE | ||||||||||
| I–II | Control | 11 ± 8.69 | 18 ± 11.1 | 21.86 ± 13.04 | 115 | <0.001 * | 0.59 | 0.56 | 1.29 | 0.28 |
| MT | 10.77 ± 8.04 | 19.38 ± 10.92 | 25.92 ± 12.76 | |||||||
| RMT | 8.93 ± 5.27 | 15.67 ± 6.32 | 20.4 ± 7.12 | |||||||
| III–IV | Control | 25.38 ± 13.79 | 32.25 ± 11.51 | 38.62 ± 13.77 | 70.51 | <0.001 * | 0.19 | 0.83 | 0.54 | 0.707 |
| MT | 21.7 ± 11.61 | 31.7 ± 11.68 | 37 ± 13.32 | |||||||
| RMT | 27.25 ± 12.65 | 34.88 ± 13.93 | 39.38 ± 16.64 | |||||||
| WMFT | ||||||||||
| I–II | Control | 4.71 ± 3.87 | 11.71 ± 6.53 | 15 ± 9.32 | 128.64 | <0.001 * | 0.64 | 0.533 | 0.78 | 0.542 |
| MT | 6.77 ± 6.14 | 13.46 ± 6.49 | 18.85 ± 6.41 | |||||||
| RMT | 4.13 ± 4.14 | 12.87 ± 7.54 | 16.2 ± 7.82 | |||||||
| III–IV | Control | 26.25 ± 10.74 | 31.75 ± 7.25 | 35.5 ± 6.21 | 56.7 | <0.001 * | 0.12 | 0.891 | 1.07 | 0.384 |
| MT | 23.3 ± 13.23 | 32.3 ± 13.61 | 38 ± 16.02 | |||||||
| RMT | 26.12 ± 13.72 | 35.38 ± 15.45 | 40 ± 18.36 | |||||||
| MAS (elbow flexion) | ||||||||||
| I–II | Control | 1.71 ± 0.83 | 0.93 ± 0.92 | 1.14 ± 1.03 | 61.87 | <0.001 * | 0.12 | 0.887 | 0.65 | 0.626 |
| MT | 1.69 ± 0.85 | 0.77 ± 0.83 | 0.85 ± 0.9 | |||||||
| RMT | 1.67 ± 0.9 | 0.93 ± 1.03 | 1.07 ± 0.96 | |||||||
| III–IV | Control | 1.88 ± 0.83 | 1.25 ± 0.89 | 1.38 ± 0.74 | 31.28 | <0.001 * | 0.41 | 0.668 | 0.38 | 0.822 |
| MT | 1.6 ± 0.97 | 0.8 ± 0.92 | 1 ± 0.94 | |||||||
| RMT | 1.88 ± 0.99 | 1.12 ± 0.99 | 1.12 ± 0.99 | |||||||
| MAS (elbow extension) | ||||||||||
| I–II | Control | 1.64 ± 0.84 | 0.93 ± 0.83 | 1.36 ± 0.84 | 25.75 | <0.001 * | 0.21 | 0.814 | 0.61 | 0.654 |
| MT | 1.46 ± 0.66 | 0.92 ± 0.86 | 1.15 ± 1.07 | |||||||
| RMT | 1.47 ± 0.83 | 0.87 ± 0.99 | 1 ± 1.07 | |||||||
| III–IV | Control | 2 ± 0.93 | 1.38 ± 0.92 | 1.5 ± 0.76 | 39.6 | <0.001 * | 3.79 | 0.038 * | 1.33 | 0.271 |
| MT | 1.4 ± 0.7 | 0.4 ± 0.7 | 0.8 ± 0.92 | |||||||
| RMT | 1.25 ± 0.71 | 0.38 ± 0.74 | 0.38 ± 0.74 | |||||||
| Brunnstrom (upper limb) | ||||||||||
| I–II | Control | 1.57 ± 0.51 | 2.36 ± 0.5 | 2.79 ± 0.58 | 181.04 | <0.001 * | 1.82 | 0.176 | 1.21 | 0.312 |
| MT | 1.77 ± 0.44 | 2.62 ± 0.51 | 3.31 ± 0.48 | |||||||
| RMT | 1.53 ± 0.52 | 2.53 ± 0.64 | 3 ± 0.53 | |||||||
| Control | 3.38 ± 0.52 | 3.62 ± 0.52 | 3.88 ± 0.35 | |||||||
| III–IV | MT | 3.1 ± 0.32 | 3.7 ± 0.48 | 4.1 ± 0.74 | 37.63 | <0.001 * | 3.17 | 0.061 | 1.77 | 0.151 |
| RMT | 3.5 ± 0.53 | 4.25 ± 0.46 | 4.5 ± 0.53 | |||||||
| Brunnstrom (hand) | ||||||||||
| Control | 1.43 ± 0.51 | 2.07 ± 0.73 | 2.43 ± 0.85 | |||||||
| I–II | MT | 1.54 ± 0.52 | 2.31 ± 0.48 | 2.77 ± 0.73 | 73.48 | <0.001 * | 0.93 | 0.4 | 0.29 | 0.883 |
| RMT | 1.33 ± 0.49 | 2.07 ± 0.8 | 2.4 ± 0.63 | |||||||
| Control | 2.62 ± 0.74 | 3 ± 0.53 | 3 ± 0.53 | |||||||
| III–IV | MT | 2.6 ± 0.97 | 3.1 ± 0.88 | 3.4 ± 0.84 | 19.59 | <0.001 * | 0.1 | 2.535 | 0.99 | 0.425 |
| RMT | 3.25 ± 0.71 | 3.62 ± 0.52 | 4 ± 0.93 | |||||||
| BBT | ||||||||||
| Control | 0 ± 0 | 2.07 ± 3.6 | 3.14 ± 5.5 | |||||||
| I–II | MT | 0.69 ± 1.97 | 2.46 ± 4.72 | 4.08 ± 6.58 | 10.71 | <0.001 * | 2.08 | 0.14 | 1.6 | 0.183 |
| RMT | 0 ± 0 | 0 ± 0 | 0.67 ± 1.8 | |||||||
| Control | 3.38 ± 4.75 | 6.75 ± 6.48 | 7.12 ± 6.64 | |||||||
| III–IV | MT | 1.5 ± 3.17 | 7.2 ± 6.2 | 10.7 ± 7.85 | 29 | <0.001 * | 0.53 | 0.6 | 2.2 | 0.08 |
| RMT | 6 ± 6.52 | 8.75 ± 7.48 | 11.62 ± 9.49 | |||||||
| MBI | ||||||||||
| Control | 49 ± 21.16 | 60 ± 19.12 | 66.93 ± 17.12 | |||||||
| I–II | MT | 46.08 ± 18.23 | 61.54 ± 15.4 | 72.92 ± 12.57 | 105.38 | <0.001 * | 0.76 | 0.477 | 1.53 | 0.2 |
| RMT | 37.67 ± 27.93 | 52.93 ± 27.6 | 63.87 ± 23.07 | |||||||
| Control | 71 ± 17.16 | 80.75 ± 15.81 | 83.5 ± 12.71 | |||||||
| III–IV | MT | 68.9 ± 25.39 | 83 ± 17.39 | 87.9 ± 11.9 | 29.84 | <0.001 * | 0.4 | 0.673 | 0.82 | 0.516 |
| RMT | 78.5 ± 20.82 | 87.12 ± 14.52 | 90.12 ± 11.26 | |||||||
| SF-36 | ||||||||||
| Control | 60 ± 12.50 | 57.71 ± 12.39 | 53.43 ± 12.74 | |||||||
| I–II | MT | 60.75 ± 17.22 | 63.77 ± 16.88 | 61 ± 18.33 | 30.93 | <0.001 * | 1.27 | 0.293 | 0.47 | 0.632 |
| RMT | 59.2 ± 11.80 | 56.6 ± 11.62 | 52.67 ± 11.37 | |||||||
| Control | 60.88 ± 16.50 | 66.75 ± 16.11 | 65 ± 17.64 | |||||||
| III–IV | MT | 71.36 ± 20.57 | 69.7 ± 19.75 | 69.3 ± 20.14 | 1.66 | 0.21 | 0.99 | 0.388 | 0.26 | 0.771 |
| RMT | 80.07 ± 13.51 | 78.62 ± 13.38 | 76.25 ± 12.74 | |||||||
| Group | T0 | T1 | Ptime | Pintervention | Pinteraction | |
|---|---|---|---|---|---|---|
| R-M1 | ||||||
| MeandiffHbO2 | Control | 0.057 ± 0.077 | 0.011 ± 0.039 | |||
| MT | 0.005 ± 0.053 | 0.036 ± 0.064 | 0.741 | 0.173 | 0.604 | |
| RMT | −0.003 ± 0.12 | 0.027 ± 0.047 | ||||
| MeandiffHbT | Control | 0.092 ± 0.186 | −0.014 ± 0.056 | |||
| MT | −0.018 ± 0.054 | 0.008 ± 0.121 | 0.686 | 0.168 | 0.373 | |
| RMT | −0.023 ± 0.152 | 0.003 ± 0.088 | ||||
| L-M1 | ||||||
| MeandiffHbO2 | Control | 0.012 ± 0.096 | 0.029 ± 0.022 | |||
| MT | −0.018 ± 0.085 | 0.045 ± 0.042 | 0.858 | 0.271 | 0.595 | |
| RMT | 0.019 ± 0.089 | 0.008 ± 0.051 | ||||
| MeandiffHbT | Control | 0.053 ± 0.091 | 0.019 ± 0.041 | |||
| MT | −0.037 ± 0.109 | 0.047 ± 0.087 | 0.962 | 0.066 | 0.495 | |
| RMT | 0.004 ± 0.099 | 0.008 ± 0.057 | ||||
| R-PMC&SMA | ||||||
| MeandiffHbO2 | Control | 0.039 ± 0.088 | 0.066 ± 0.099 | |||
| MT | 0.041 ± 0.156 | 0.055 ± 0.109 | 0.953 | 0.911 | 0.901 | |
| RMT | 0.063 ± 0.188 | 0.075 ± 0.096 | ||||
| MeandiffHbT | Control | 0.032 ± 0.189 | 0.021 ± 0.102 | |||
| MT | −0.004 ± 0.156 | 0.045 ± 0.153 | 0.064 | 0.987 | 0.803 | |
| RMT | 0.041 ± 0.213 | 0.069 ± 0.083 | ||||
| L-PMC&SMA | ||||||
| MeandiffHbO2 | Control | 0.047 ± 0.121 | 0.011 ± 0.058 | |||
| MT | 0.085 ± 0.183 | 0.082 ± 0.097 | 0.519 | 0.739 | 0.274 | |
| RMT | 0.056 ± 0.148 | 0.012 ± 0.107 | ||||
| MeandiffHbT | Control | 0.041 ± 0.168 | 0.03 ± 0.096 | |||
| MT | 0.058 ± 0.224 | 0.112 ± 0.127 | 0.681 | 0.589 | 0.392 | |
| RMT | 0.061 ± 0.212 | −0.018 ± 0.168 | ||||
| R-S1 | ||||||
| MeandiffHbO2 | Control | 0.014 ± 0.085 | 0.031 ± 0.059 | |||
| MT | 0.025 ± 0.093 | 0.071 ± 0.067 | 0.885 | 0.764 | 0.401 | |
| RMT | 0.02 ± 0.09 | 0.029 ± 0.088 | ||||
| MeandiffHbT | Control | 0.002 ± 0.135 | 0.022 ± 0.079 | |||
| MT | −0.006 ± 0.101 | 0.082 ± 0.109 | 0.145 | 0.700 | 0.433 | |
| RMT | 0.014 ± 0.116 | 0.039 ± 0.083 | ||||
| L-S1 | ||||||
| MeandiffHbO2 | Control | −0.009 ± 0.124 | 0.016 ± 0.057 | |||
| MT | 0.047 ± 0.105 | 0.068 ± 0.081 | 0.989 | 0.971 | 0.245 | |
| RMT | 0.048 ± 0.09 | 0.026 ± 0.073 | ||||
| MeandiffHbT | Control | 0.02 ± 0.073 | 0.002 ± 0.092 | |||
| MT | 0.033 ± 0.138 | 0.079 ± 0.103 | 0.979 | 0.701 | 0.434 | |
| RMT | 0.053 ± 0.104 | 0.016 ± 0.078 | ||||
| R-SAC | ||||||
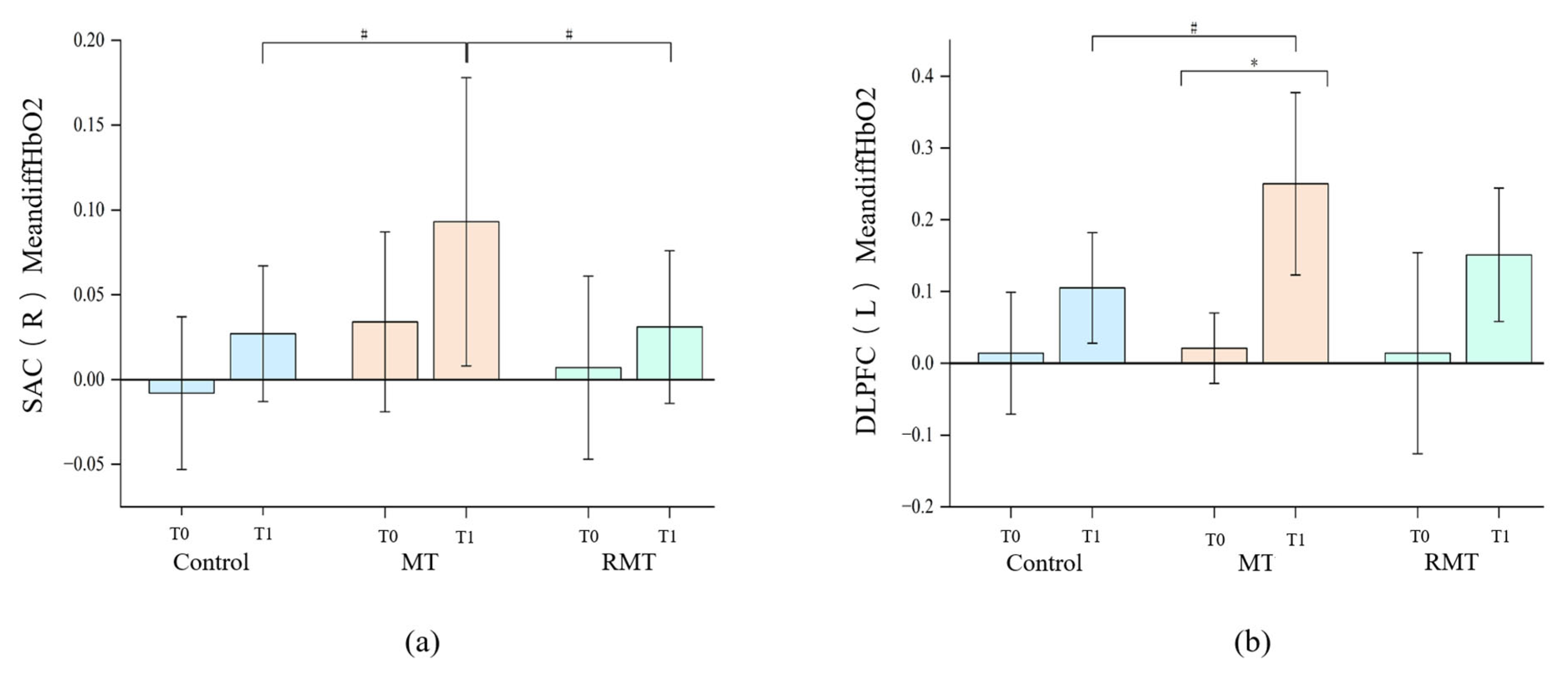
| MeandiffHbO2 | Control | −0.008 ± 0.045 | 0.027 ± 0.040 | |||
| MT | 0.034 ± 0.053 | 0.093 ± 0.085 | 0.529 | 0.008 * | 0.610 | |
| RMT | 0.007 ± 0.054 | 0.031 ± 0.045 | ||||
| MeandiffHbT | Control | −0.023 ± 0.089 | 0.05 ± 0.084 | |||
| MT | −0.019 ± 0.073 | 0.041 ± 0.072 | 0.582 | 0.491 | 0.100 | |
| RMT | 0.008 ± 0.095 | 0.018 ± 0.046 | ||||
| L-SAC | ||||||
| MeandiffHbO2 | Control | 0.021 ± 0.061 | 0.015 ± 0.045 | |||
| MT | 0.001 ± 0.057 | 0.051 ± 0.049 | 0.840 | 0.214 | 0.494 | |
| RMT | −0.001 ± 0.08 | 0.01 ± 0.064 | ||||
| MeandiffHbT | Control | 0.005 ± 0.05 | 0.018 ± 0.051 | |||
| MT | −0.017 ± 0.075 | 0.055 ± 0.057 | 0.823 | 0.102 | 0.210 | |
| RMT | −0.012 ± 0.09 | −0.022 ± 0.093 | ||||
| R-DLPFC | ||||||
| MeandiffHbO2 | Control | 0.033 ± 0.132 | 0.057 ± 0.087 | |||
| MT | 0.109 ± 0.172 | 0.046 ± 0.267 | 0.653 | 0.709 | 0.778 | |
| RMT | 0.057 ± 0.125 | 0.054 ± 0.104 | ||||
| MeandiffHbT | Control | 0.052 ± 0.171 | 0.028 ± 0.082 | |||
| MT | 0.074 ± 0.13 | 0.009 ± 0.37 | 0.569 | 0.628 | 0.854 | |
| RMT | 0.042 ± 0.158 | 0.098 ± 0.155 | ||||
| L-DLPFC | ||||||
| MeandiffHbO2 | Control | 0.014 ± 0.085 | 0.105 ± 0.077 | |||
| MT | 0.021 ± 0.049 | 0.250 ± 0.127 | 0.042 * | 0.059 | 0.101 | |
| RMT | 0.014 ± 0.140 | 0.151 ± 0.093 | ||||
| MeandiffHbT | Control | 0.022 ± 0.09 | 0.012 ± 0.07 | |||
| MT | 0.141 ± 0.221 | 0.106 ± 0.104 | 0.579 | 0.474 | 0.076 | |
| RMT | 0.1 ± 0.123 | 0.064 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Li, S.; Zhang, J.; Xu, Y.; Yang, Y. Robot-Assisted Mirror Therapy for Upper Limb and Hand Recovery After Stroke: Clinical Efficacy and Insights into Neural Mechanisms. J. Clin. Med. 2026, 15, 350. https://doi.org/10.3390/jcm15010350
Li S, Zhang J, Xu Y, Yang Y. Robot-Assisted Mirror Therapy for Upper Limb and Hand Recovery After Stroke: Clinical Efficacy and Insights into Neural Mechanisms. Journal of Clinical Medicine. 2026; 15(1):350. https://doi.org/10.3390/jcm15010350
Chicago/Turabian StyleLi, Shixin, Jiayi Zhang, Yang Xu, and Yonghong Yang. 2026. "Robot-Assisted Mirror Therapy for Upper Limb and Hand Recovery After Stroke: Clinical Efficacy and Insights into Neural Mechanisms" Journal of Clinical Medicine 15, no. 1: 350. https://doi.org/10.3390/jcm15010350
APA StyleLi, S., Zhang, J., Xu, Y., & Yang, Y. (2026). Robot-Assisted Mirror Therapy for Upper Limb and Hand Recovery After Stroke: Clinical Efficacy and Insights into Neural Mechanisms. Journal of Clinical Medicine, 15(1), 350. https://doi.org/10.3390/jcm15010350





