Lipoprotein(a) and Aortic Valve Stenosis: From Pathophysiology to Emerging Pharmacological Agents
Abstract
1. Introduction
2. Biochemical Structure and Metabolism of Lp(a)
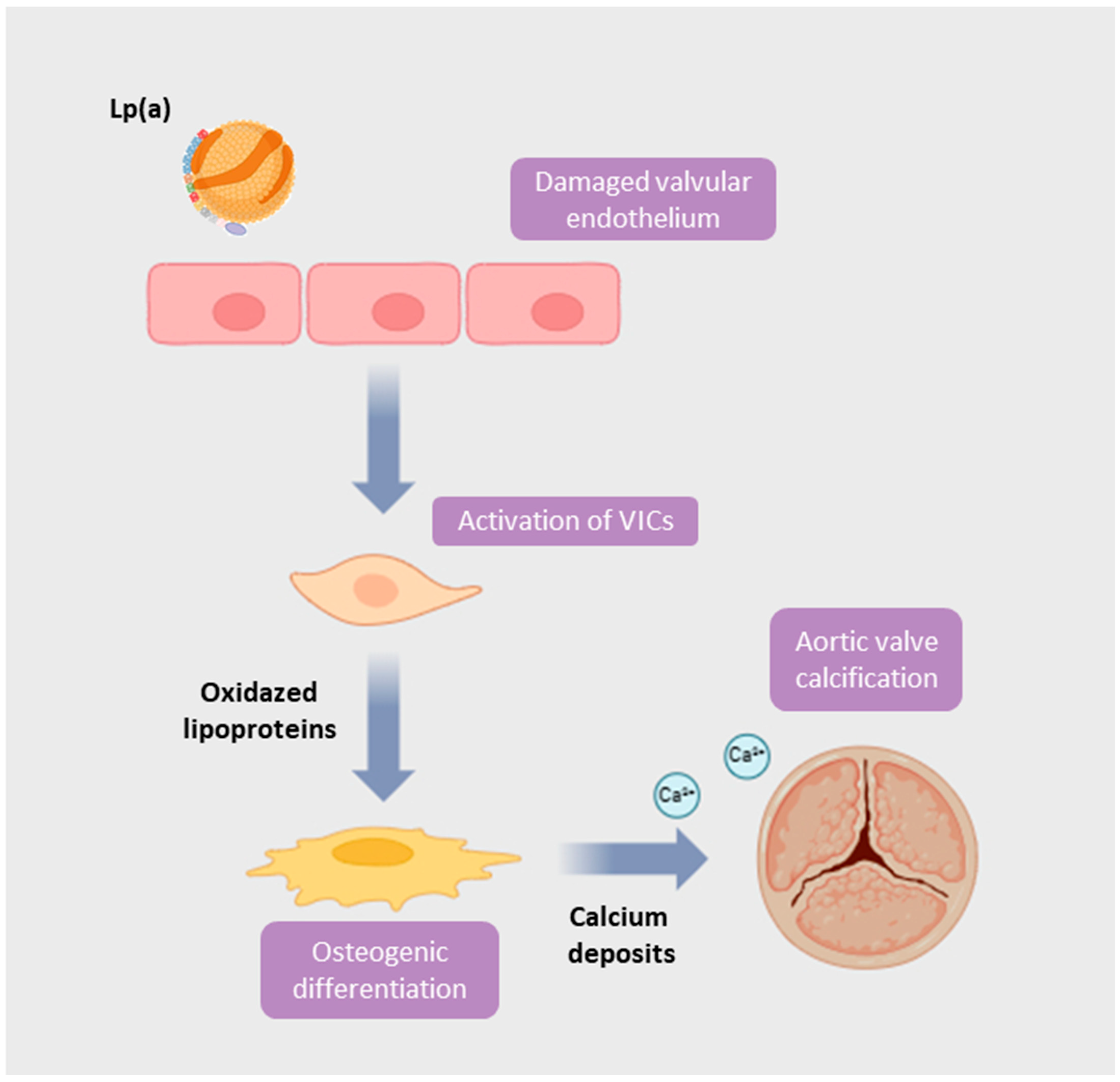
3. The Aortic Valve: From Endothelial Dysfunction to Calcific Stenosis
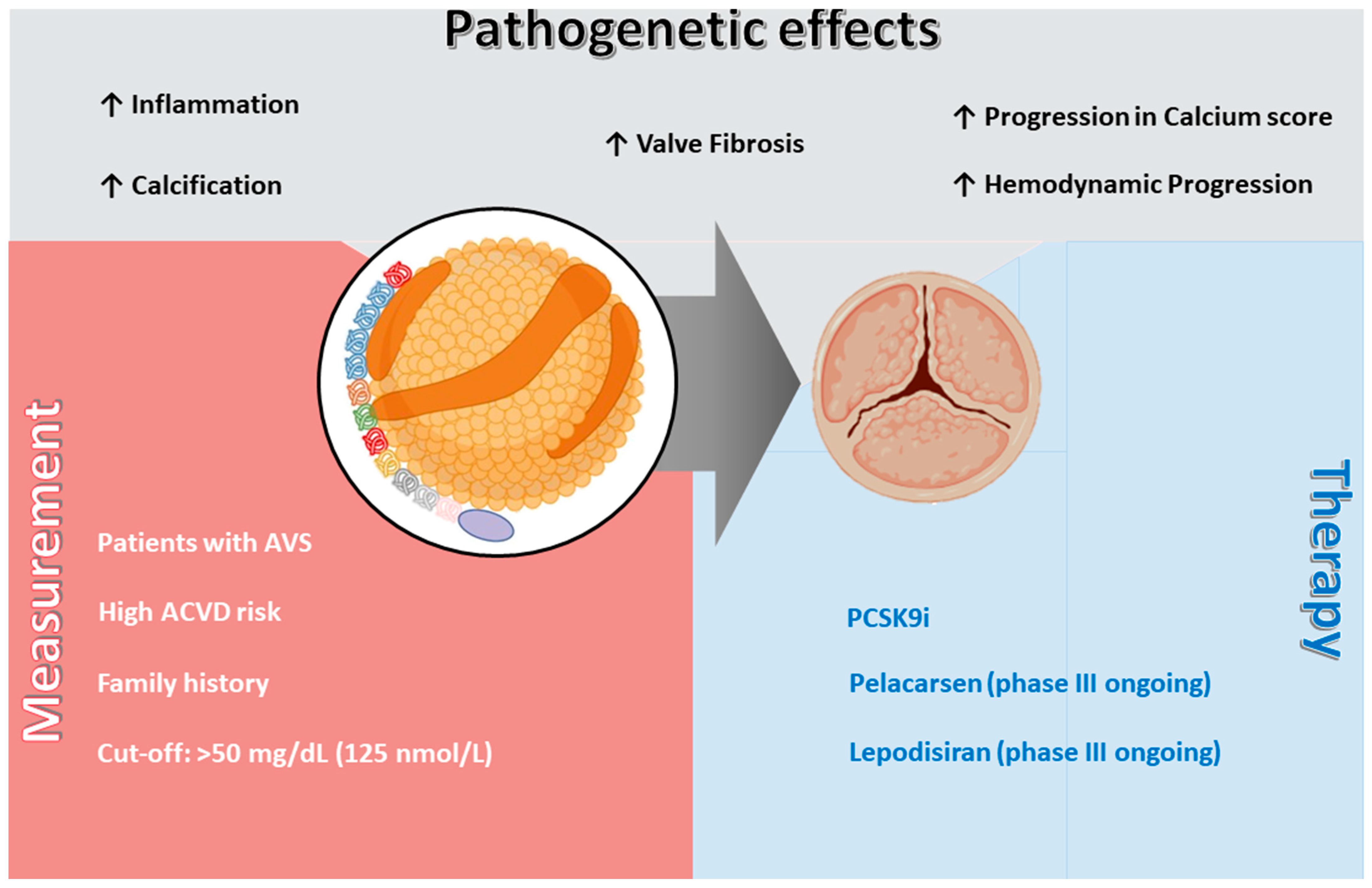
4. Measurement of Lp(a) and Clinical Guidelines
5. Non-Specific Pharmacological Approaches Impacting Lp(a)
5.1. Statins
5.2. Ezetimibe
5.3. PCSK9i
5.4. Bempedoic Acid
5.5. Omega-3 Fatty Acids
6. Specific Lp(a)-Lowering Therapies: Mechanisms and Trials
6.1. Antisense Oligonucleotides
6.2. siRNAs
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santangelo, G.; Bursi, F.; Faggiano, A.; Moscardelli, S.; Simeoli, P.S.; Guazzi, M.; Lorusso, R.; Carugo, S.; Faggiano, P. The Global Burden of Valvular Heart Disease: From Clinical Epidemiology to Management. J. Clin. Med. 2023, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Praz, F.; Borger, M.A.; Lanz, J.; Marin-Cuartas, M.; Abreu, A.; Adamo, M.; Marsan, N.A.; Barili, F.; Bonaros, N.; Cosyns, B.; et al. 2025 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2025, 46, 4635–4736. [Google Scholar] [CrossRef] [PubMed]
- Helske, S.; Kupari, M.; Lindstedt, K.A.; Kovanen, P.T. Aortic valve stenosis: An active atheroinflammatory process. Curr. Opin. Lipidol. 2007, 18, 483–491. [Google Scholar] [CrossRef]
- New, S.E.P.; Aikawa, E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ. Res. 2011, 108, 1381–1391. [Google Scholar] [CrossRef]
- Agnello, F.; Capodanno, D. Anti-inflammatory strategies for atherosclerotic artery disease. Expert Opin. Drug Saf. 2022, 21, 661–672. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Bottaro, G.; Zappulla, P.; Deste, W.; Famà, F.; Agnello, F.; Trovato, D.; Indelicato, A.; Barbanti, M.; Sgroi, C.; Monte, I.P.; et al. Severe aortic valve stenosis: Symptoms, biochemical markers, and global longitudinal strain. J. Cardiovasc. Echogr. 2020, 30, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Miksenas, H.; Januzzi, J.L.; Natarajan, P. Lipoprotein(a) and Cardiovascular Diseases. JAMA—J. Am. Med. Assoc. 2021, 326, 352–353. [Google Scholar] [CrossRef]
- Reyes-Soffer, G.; Ginsberg, H.N.; Berglund, L.; Duell, P.B.; Heffron, S.P.; Kamstrup, P.R.; Lloyd-Jones, D.M.; Marcovina, S.M.; Yeang, C.; Koschinsky, M.L.; et al. Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, E48–E60. [Google Scholar] [CrossRef]
- Spence, J.D.; Koschinsky, M. Mechanisms of lipoprotein(a) pathogenicity: Prothrombotic, proatherosclerotic, or both? Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1550–1551. [Google Scholar] [CrossRef]
- Capoulade, R.; Chan, K.L.; Yeang, C.; Mathieu, P.; Bossé, Y.; Dumesnil, J.G.; Tam, J.W.; Teo, K.K.; Mahmut, A.; Yang, X.; et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J. Am. Coll. Cardiol. 2015, 66, 1236–1246. [Google Scholar] [CrossRef]
- Capoulade, R.; Yeang, C.; Chan, K.L.; Pibarot, P.; Tsimikas, S. Association of Mild to Moderate Aortic Valve Stenosis Progression with Higher Lipoprotein(a) and Oxidized Phospholipid Levels: Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 1212–1217. [Google Scholar] [CrossRef]
- Zheng, K.H.; Tsimikas, S.; Pawade, T.; Kroon, J.; Jenkins, W.S.; Doris, M.K.; White, A.C.; Timmers, N.K.; Hjortnaes, J.; Rogers, M.A.; et al. Lipoprotein(a) and Oxidized Phospholipids Promote Valve Calcification in Patients with Aortic Stenosis. J. Am. Coll. Cardiol. 2019, 73, 2150–2162. [Google Scholar] [CrossRef]
- Nanda, N.C. Genetic associations with valvular calcification and aortic stenosis. Cardiol. Rev. 2013, 29, 503–512. [Google Scholar] [CrossRef]
- Kamstrup, P.R.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J. Am. Coll. Cardiol. 2014, 63, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Dufresne, L.; Burr, H.; Ambikkumar, A.; Yasui, N.; Luk, K.; Ranatunga, D.K.; Whitmer, R.A.; Lathrop, M.; Engert, J.C.; et al. Association of LPA variants with aortic stenosis a large-scale study using diagnostic and procedural codes from electronic health records. JAMA Cardiol. 2018, 3, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, B.J.; Boekholdt, S.M.; Dubé, M.-P.; Rhéaume, É.; Wareham, N.J.; Khaw, K.-T.; Sandhu, M.S.; Tardif, J.-C. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis a prospective mendelian randomization study and replication in a case-control cohort. Circ. Cardiovasc. Genet. 2014, 7, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Agnello, F.; Finocchiaro, S.; Laudani, C.; Legnazzi, M.; Mauro, M.S.; Rochira, C.; Scalia, L.; Capodanno, D. A review of polypills for the prevention of atherosclerotic cardiovascular disease. Am. Heart J. 2023, 266, 74–85. [Google Scholar] [CrossRef]
- Schmidt, K.; Noureen, A.; Kronenberg, F.; Utermann, G. Structure, function, and genetics of lipoprotein(a). J. Lipid Res. 2016, 57, 1339–1359. [Google Scholar] [CrossRef]
- Tsimikas, S. A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J. Am. Coll. Cardiol. 2017, 69, 692–711. [Google Scholar] [CrossRef]
- Kamstrup, P.R. Lipoprotein(a) and Cardiovascular Disease. Clin. Chem. 2021, 67, 154–166. [Google Scholar] [CrossRef]
- Borrelli, M.J.; Youssef, A.; Boffa, M.B.; Koschinsky, M.L. New Frontiers in Lp(a)-Targeted Therapies. Trends Pharmacol. Sci. 2019, 40, 212–225. [Google Scholar] [CrossRef]
- Hoover-Plow, J.; Huang, M. Lipoprotein(a) metabolism: Potential sites for therapeutic targets. Metabolism 2013, 62, 479–491. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P.A.; Schneider, W.J. Lipoprotein(a) catabolism: A case of multiple receptors. Pathology 2019, 51, 155–164. [Google Scholar] [CrossRef]
- Vuorio, A.; Watts, G.F.; Schneider, W.J.; Tsimikas, S.; Kovanen, P.T. Familial hypercholesterolemia and elevated lipoprotein(a): Double heritable risk and new therapeutic opportunities. J. Intern. Med. 2020, 287, 2–18. [Google Scholar] [CrossRef]
- Sharma, M.; Redpath, G.M.; Williams, M.J.A.; McCormick, S.P.A. Recycling of apolipoprotein(a) after PlgRKT-mediated endocytosis of lipoprotein(a). Circ. Res. 2017, 120, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, M.; Chen, H.Y.; Rong, J.; Dufresne, L.; Yao, J.; Guo, X.; Tsai, M.Y.; Tsimikas, S.; Post, W.S.; Vasan, R.S.; et al. Genome-Wide Association Study Highlights APOH as a Novel Locus for Lipoprotein(a) Levels-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 458–464. [Google Scholar] [CrossRef]
- Enkhmaa, B.; Anuurad, E.; Berglund, L. Lipoprotein(a): Impact by ethnicity and environmental and medical conditions. J. Lipid Res. 2016, 57, 1111–1125. [Google Scholar] [CrossRef]
- Marcovina, S.M.; Viney, N.J.; Hughes, S.G.; Xia, S.; Witztum, J.L.; Tsimikas, S. Temporal variability in lipoprotein(a) levels in patients enrolled in the placebo arms of IONIS-APO(a) Rx and IONIS-APO(a)-L Rx antisense oligonucleotide clinical trials. J. Clin. Lipidol. 2018, 12, 122–129.e2. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Basra, S.S.; Skolnick, A.H.; Wenger, N.K. Aortic valve disease in the older adult. J. Geriatr. Cardiol. 2016, 13, 941–944. [Google Scholar] [CrossRef]
- Arishiro, K.; Hoshiga, M.; Negoro, N.; Jin, D.; Takai, S.; Miyazaki, M.; Ishihara, T.; Hanafusa, T. Angiotensin Receptor-1 Blocker Inhibits Atherosclerotic Changes and Endothelial Disruption of the Aortic Valve in Hypercholesterolemic Rabbits. J. Am. Coll. Cardiol. 2007, 49, 1482–1489. [Google Scholar] [CrossRef]
- Small, A.M.; Yutzey, K.E.; Binstadt, B.A.; Key, K.V.; Bouatia-Naji, N.; Milan, D.; Aikawa, E.; Otto, C.M.; Hilaire, C.S. Unraveling the Mechanisms of Valvular Heart Disease to Identify Medical Therapy Targets: A Scientific Statement from the American Heart Association. Circulation 2024, 150, e109–e128. [Google Scholar] [CrossRef]
- Sengupta, P.P.; Kluin, J.; Lee, S.P.; Oh, J.K.; Smits, A.I.P.M. The future of valvular heart disease assessment and therapy. Lancet 2024, 403, 1590–1602. [Google Scholar] [CrossRef]
- Gotoh, T.; Kuroda, T.; Yamasawa, M.; Nishinaga, M.; Mitsuhashi, T.; Seino, Y.; Nagoh, N.; Kayaba, K.; Yamada, S.; Matsuo, H.; et al. Correlation between lipoprotein(a) and aortic valve sclerosis assessed by echocardiography (the JMS Cardiac Echo and Cohort Study). Am. J. Cardiol. 1995, 76, 928–932. [Google Scholar] [CrossRef]
- Kaiser, Y.; van der Toorn, J.E.; Singh, S.S.; Zheng, K.H.; Kavousi, M.; Sijbrands, E.J.G.; Stroes, E.S.G.; Vernooij, M.W.; de Rijke, Y.B.; Boekholdt, S.M.; et al. Lipoprotein(a) is associated with the onset but not the progression of aortic valve calcification. Eur. Heart J. 2022, 43, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
- Pantelidis, P.; Oikonomou, E.; Lampsas, S.; Zakynthinos, G.E.; Lysandrou, A.; Kalogeras, K.; Katsianos, E.; Theofilis, P.; Siasos, G.; Vavuranakis, M.A.; et al. Lipoprotein(a) and calcific aortic valve disease initiation and progression: A systematic review and meta-analysis. Cardiovasc. Res. 2023, 119, 1641–1655. [Google Scholar] [CrossRef]
- Botezatu, S.B.; Tzolos, E.; Kaiser, Y.; Cartlidge, T.R.G.; Kwiecinski, J.; Barton, A.K.; Yu, X.; Williams, M.C.; van Beek, E.J.R.; White, A.; et al. Serum lipoprotein(a) and bioprosthetic aortic valve degeneration. Eur. Heart J.-Cardiovasc. Imaging 2023, 24, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, B.J.; Loganath, K.; Girard, A.; Botezatu, S.; Zheng, K.H.; Tzolos, E.; Abdoun, K.; Tastet, L.; Capoulade, R.; Côté, N.; et al. Lipoprotein(a) and Calcific Aortic Valve Stenosis Progression: A Systematic Review and Meta-Analysis. JAMA Cardiol. 2024, 9, 835–842. [Google Scholar] [CrossRef]
- Owens, D.S.; Katz, R.; Takasu, J.; Kronmal, R.; Budoff, M.J.; O’Brien, K.D. Incidence and Progression of Aortic Valve Calcium in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Cardiol. 2010, 105, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F. Lipoprotein(a) measurement issues: Are we making a mountain out of a molehill? Atherosclerosis 2022, 349, 123–135. [Google Scholar] [CrossRef]
- Tsimikas, S.; Marcovina, S.M. Ancestry, Lipoprotein(a), and Cardiovascular Risk Thresholds: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 80, 934–946. [Google Scholar] [CrossRef]
- Yeang, C.; Witztum, J.L.; Tsimikas, S. Novel method for quantification of lipoprotein(a)-cholesterol: Implications for improving accuracy of LDL-C measurements. J. Lipid Res. 2021, 62, 100053. [Google Scholar] [CrossRef]
- Moriarty, P.M.; Yeang, C.; Varvel, S.A.; McConnell, J.P.; Tsimikas, S. Removing The Lipoprotein(A)-Cholesterol from the LDL-C Measurement Results in a 38% Reduction in Prevalence of Elevated LDL-C at Any Threshold: Implications for the Prevalence and Diagnosis of LDL-Mediated Risk. J. Am. Coll. Cardiol. 2018, 71, A1783. [Google Scholar] [CrossRef]
- Yeang, C.; Clopton, P.C.; Tsimikas, S. Lipoprotein(a)-cholesterol levels estimated by vertical auto profile correlate poorly with Lp(a) mass in hyperlipidemic subjects: Implications for clinical practice interpretation of Lp(a)-mediated risk. J. Clin. Lipidol. 2016, 10, 1389–1396. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Pearson, G.J.; Thanassoulis, G.; Anderson, T.J.; Barry, A.R.; Couture, P.; Dayan, N.; Francis, G.A.; Genest, J.; Gregoire, J.; Grover, S.A.; et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can. J. Cardiol. 2021, 37, 1129–1150. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef]
- Cegla, J.; Neely, R.G.; France, M.; Ferns, G.; Byrne, C.D.; Halcox, J.; Datta, D.; Capps, N.; Shoulders, C.; Qureshi, N.; et al. HEART UK consensus statement on Lipoprotein(a): A call to action. Atherosclerosis 2019, 291, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Senders, M.L.; Que, X.; Cho, Y.S.; Yeang, C.; Groenen, H.; Fay, F.; Calcagno, C.; Meerwaldt, A.E.; Green, S.; Miu, P.; et al. PET/MR Imaging of Malondialdehyde-Acetaldehyde Epitopes with a Human Antibody Detects Clinically Relevant Atherothrombosis. J. Am. Coll. Cardiol. 2018, 71, 321–335. [Google Scholar] [CrossRef]
- Kostner, G.M.; Gavish, D.; Leopold, B.; Bolzano, K.; Weintraub, M.S.; Breslow, J.L. HMG CoA reductase inhibitors lower LDL cholesterol without reducing Lp(a) levels. Circulation 1989, 80, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Yahya, R.; Berk, K.; Verhoeven, A.; Bos, S.; van der Zee, L.; Touw, J.; Erhart, G.; Kronenberg, F.; Timman, R.; Sijbrands, E.; et al. Statin treatment increases lipoprotein(a) levels in subjects with low molecular weight apolipoprotein(a) phenotype. Atherosclerosis 2019, 289, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Gordts, P.L.S.M.; Nora, C.; Yeang, C.; Witztum, J.L. Statin therapy increases lipoprotein(a) levels. Eur. Heart J. 2020, 41, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Tie, C.; Gao, K.; Zhang, N.; Zhang, S.; Shen, J.; Xie, X.; Wang, J.-A. Ezetimibe attenuates atherosclerosis associated with lipid reduction and inflammation inhibition. PLoS ONE 2015, 10, e0142430. [Google Scholar] [CrossRef]
- Qin, L.; Yang, Y.-B.; Yang, Y.-X.; Zhu, N.; Li, S.-X.; Liao, D.-F.; Zheng, X.-L. Anti-inflammatory activity of ezetimibe by regulating NF-κB/MAPK pathway in THP-1 macrophages. Pharmacology 2014, 93, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Dolezelova, E.; Stein, E.; Derosa, G.; Maffioli, P.; Nachtigal, P.; Sahebkar, A. Effect of ezetimibe on plasma adipokines: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2017, 83, 1380–1396. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Théroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Awad, K.; Mikhailidis, D.P.; Katsiki, N.; Muntner, P.; Banach, M. Effect of Ezetimibe Monotherapy on Plasma Lipoprotein(a) Concentrations in Patients with Primary Hypercholesterolemia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Drugs 2018, 78, 453–462. [Google Scholar] [CrossRef]
- Agnello, F.; Mauro, M.S.; Rochira, C.; Landolina, D.; Finocchiaro, S.; Greco, A.; Ammirabile, N.; Raffo, C.; Mazzone, P.M.; Spagnolo, M.; et al. PCSK9 inhibitors: Current status and emerging frontiers in lipid control. Expert Rev. Cardiovasc. Ther. 2023, 22, 41–58. [Google Scholar] [CrossRef]
- Agnello, F.; Ingala, S.; Laterra, G.; Scalia, L.; Barbanti, M. Novel and Emerging LDL-C Lowering Strategies: A New Era of Dyslipidemia Management. J. Clin. Med. 2024, 13, 1251. [Google Scholar] [CrossRef]
- Romagnuolo, R.; Scipione, C.A.; Boffa, M.B.; Marcovina, S.M.; Seidah, N.G.; Koschinsky, M.L. Lipoprotein(a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 through the low density lipoprotein receptor. J. Biol. Chem. 2015, 290, 11649–11662. [Google Scholar] [CrossRef]
- O’dOnoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.S.; Kanevsky, E.; Gouni-Berthold, I.; Im, K.; Pineda, A.L.; Wasserman, S.M.; Češka, R.; et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk insights from the FOURIER trial. Circulation 2019, 139, 1483–1492. [Google Scholar] [CrossRef]
- Raal, F.J.; Giugliano, R.P.; Sabatine, M.S.; Koren, M.J.; Blom, D.; Seidah, N.G.; Honarpour, N.; Lira, A.; Xue, A.; Chiruvolu, P.; et al. PCSK9 inhibition-mediated reduction in Lp(a) with evolocumab: An analysis of 10 clinical trials and the LDL receptor’s role. J. Lipid Res. 2016, 57, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.; Watts, G.F.; Robinson, J.G.; Minini, P.; Sasiela, W.J.; Edelberg, J.; Louie, M.J.; Raal, F.J. Effect of Alirocumab on Lipoprotein(a) Over ≥1.5 Years (from the Phase 3 ODYSSEY Program). Am. J. Cardiol. 2017, 119, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Landmesser, U.; Leiter, L.A.; Kallend, D.; Dufour, R.; Karakas, M.; Hall, T.; Troquay, R.P.; Turner, T.; Visseren, F.L.; et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N. Engl. J. Med. 2017, 376, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520–1530. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Nurmohamed, N.S.; Navar, A.M.; Kastelein, J.J.P. New and Emerging Therapies for Reduction of LDL-Cholesterol and Apolipoprotein B: JACC Focus Seminar ¼. J. Am. Coll. Cardiol. 2021, 77, 1564–1575. [Google Scholar] [CrossRef]
- Rubino, J.; MacDougall, D.E.; Sterling, L.R.; Kelly, S.E.; McKenney, J.M.; Lalwani, N.D. Lipid lowering with bempedoic acid added to a proprotein convertase subtilisin/kexin type 9 inhibitor therapy: A randomized, controlled trial. J. Clin. Lipidol. 2021, 15, 593–601. [Google Scholar] [CrossRef]
- Ward, N.C.; Ying, Q.; Chan, D.C.; Pang, J.; Mori, T.A.; Schultz, C.J.; Dwivedi, G.; Francis, R.J.; Watts, G.F. Improved arterial inflammation with high dose omega-3 fatty acids in patients with elevated lipoprotein(a): Selective effect of eicosapentaenoic acid? J. Clin. Lipidol. 2023, 17, 694–699. [Google Scholar] [CrossRef]
- Tsimikas, S.; Karwatowska-Prokopczuk, E.; Gouni-Berthold, I.; Tardif, J.-C.; Baum, S.J.; Steinhagen-Thiessen, E.; Shapiro, M.D.; Stroes, E.S.; Moriarty, P.M.; Nordestgaard, B.G.; et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N. Engl. J. Med. 2020, 382, 244–255. [Google Scholar] [CrossRef]
- Tsimikas, S.; Viney, N.J.; Hughes, S.G.; Singleton, W.; Graham, M.J.; Baker, B.F.; Burkey, J.L.; Yang, Q.; Marcovina, S.M.; Geary, R.S.; et al. Antisense therapy targeting apolipoprotein(a): A randomised, double-blind, placebo-controlled phase 1 study. Lancet 2015, 386, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Langsted, A.; Nordestgaard, B.G. Antisense Oligonucleotides Targeting Lipoprotein(a). Curr. Atheroscler. Rep. 2019, 21, 30. [Google Scholar] [CrossRef]
- Yeang, C.; Karwatowska-Prokopczuk, E.; Su, F.; Dinh, B.; Xia, S.; Witztum, J.L.; Tsimikas, S. Effect of Pelacarsen on Lipoprotein(a) Cholesterol and Corrected Low-Density Lipoprotein Cholesterol. J. Am. Coll. Cardiol. 2022, 79, 1035–1046. [Google Scholar] [CrossRef]
- Viney, N.J.; van Capelleveen, J.C.; Geary, R.S.; Xia, S.; Tami, J.A.; Yu, R.Z.; Marcovina, S.M.; Hughes, S.G.; Graham, M.J.; Crooke, R.M.; et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): Two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016, 388, 2239–2253. [Google Scholar] [CrossRef]
- Cho, L.; Nicholls, S.J.; Nordestgaard, B.G.; Landmesser, U.; Tsimikas, S.; Blaha, M.J.; Leitersdorf, E.; Lincoff, A.M.; Lesogor, A.; Manning, B.; et al. Design and Rationale of Lp(a)HORIZON Trial: Assessing the Effect of Lipoprotein(a) Lowering with Pelacarsen on Major Cardiovascular Events in Patients with CVD and Elevated Lp(a). Am. Heart J. 2025, 287, 1–9. [Google Scholar] [CrossRef]
- Biessen, E.A.L.; Van Berkel, T.J.C. N-Acetyl Galactosamine Targeting: Paving the Way for Clinical Application of Nucleotide Medicines in Cardiovascular Diseases. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2855–2865. [Google Scholar] [CrossRef]
- Landmesser, U.; Poller, W.; Tsimikas, S.; Most, P.; Paneni, F.; Luscher, T.F. From traditional pharmacological towards nucleic acid-based therapies for cardiovascular diseases. Eur. Heart J. 2020, 41, 3884–3899. [Google Scholar] [CrossRef]
- Koren, M.J.; Moriarty, P.M.; Baum, S.J.; Neutel, J.; Hernandez-Illas, M.; Weintraub, H.S.; Florio, M.; Kassahun, H.; Melquist, S.; Varrieur, T.; et al. Preclinical development and phase 1 trial of a novel siRNA targeting lipoprotein(a). Nat. Med. 2022, 28, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Linnebjerg, H.; Shen, X.; Wolski, K.; Ma, X.; Lim, S.; Michael, L.F.; Ruotolo, G.; Gribble, G.; Navar, A.M.; et al. Lepodisiran, an Extended-Duration Short Interfering RNA Targeting Lipoprotein(a): A Randomized Dose-Ascending Clinical Trial. JAMA 2023, 330, 2075–2083. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K.; Balog, C.; Swerdlow, D.I.; Scrimgeour, A.C.; Rambaran, C.; Wilson, R.J.; Boyce, M.; Ray, K.K.; Cho, L.; et al. Single Ascending Dose Study of a Short Interfering RNA Targeting Lipoprotein(a) Production in Individuals with Elevated Plasma Lipoprotein(a) Levels. JAMA—J. Am. Med. Assoc. 2022, 327, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, M.L.; Rosenson, R.S.; Gencer, B.; López, J.A.G.; Lepor, N.E.; Baum, S.J.; Stout, E.; Gaudet, D.; Knusel, B.; Kuder, J.F.; et al. Small Interfering RNA to Reduce Lipoprotein(a) in Cardiovascular Disease. N. Engl. J. Med. 2022, 387, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.; Winkle, P.; Neutel, J.; Wu, Y.; Jabari, F.; Terrio, C.; Varrieur, T.; Wang, J.; Hellawell, J. Pharmacokinetics, Pharmacodynamics, and Tolerability of Olpasiran in Healthy Japanese and Non-Japanese Participants: Results from a Phase I, Single-dose, Open-label Study. Clin. Ther. 2022, 44, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Ni, W.; Shen, X.; Wang, Q.; Navar, A.M.; Nicholls, S.J.; Wolski, K.; Michael, L.; Haupt, A.; Krege, J.H. Lepodisiran—A Long-Duration Small Interfering RNA Targeting Lipoprotein(a). N. Engl. J. Med. 2025, 392, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
| Drug | Mechanism of Action | Effect on Lp(a) |
|---|---|---|
| Non-Specific Lp(a)-lowering therapies | ||
| Statins | HMG-CoA reductase inhibition; increased LPA mRNA expression and apo(a) production | Increase ~12–24% (dose and potency dependent) |
| Ezetimibe | Inhibition of intestinal cholesterol absorption; possible LDLR upregulation and anti-inflammatory effects | Decrease ~7% |
| PCSK9-i | Increased LDLR activity and Lp(a) clearance | Decrease 14–30% |
| Bempedoic acid | ATP-citrate lyase inhibition | Minimal effect |
| Omega-3 fatty acids (EPA/DHA) | Reduced hepatic VLDL synthesis, increased clearance of TG-rich particles, anti-inflammatory effects | Decrease ~5% (preliminary data, small sample) |
| Specific Lp(a)-lowering therapies | ||
| Pelacarsen | Antisense oligonucleotide targeting hepatic LPA mRNA, inhibiting apo(a) synthesis | Decrease up to ~80% (dose-dependent, monthly SC dosing) |
| Olpasiran | GalNAc-conjugated siRNA inhibiting LPA mRNA translation in hepatocytes | Decrease up to ~95–98% (quarterly or biannual dosing) |
| Zerlasiran | GalNAc-conjugated siRNA targeting LPA mRNA | Decrease ~90–96% (every 6–12 months) |
| Lepodisiran | Long-acting GalNAc-siRNA targeting LPA mRNA | Decrease up to 98% sustained ≥48 weeks after a single dose |
| Trial | Lp(a)HORIZON | OCEAN(a)–Outcomes Trial | ACCLAIM Lp(a) |
|---|---|---|---|
| Sample | N = 8323 | N = 7297 | N = 12,500 |
| Population | Patients with established ASCVD and Lp(a) > 175 nmol/L (70 mg/dL) | Patients with Lp(a) > 200 nmol/L, a history of ASCVD (defined as either a previous type 1 MI or previous revascularization with PCI) and at least 1 prespecified risk-enhancing feature | Patients with Lp(a) > 175 nmol/L and at high risk of cardiovascular events or with established ASCVD |
| Investigational drug | Pelacarsen 80 mg, injected subcutaneously once per month | Olpasiran, injected subcutaneously every 12 weeks | Lepodisiran, injected subcutaneously |
| Primary outcome(s) | Time to first MACE (cardiovascular death, nonfatal MI, nonfatal stroke, or urgent coronary revascularization requiring hospitalization) | Time to first MACE (death from coronary artery disease, MI, or urgent coronary revascularization) | Time to first MACE (cardiovascular death, MI, stroke, or urgent coronary revascularization) |
| Expected completion date (month/year) | May 2025 | December 2026 | March 2029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Agnello, F.; Laterra, G.; Scalia, L.; Mauro, M.S.; Strazzieri, O.; Reddavid, C.; Ingala, S.; Guarino, S.; Barbera, C.; Russo, M.D.; et al. Lipoprotein(a) and Aortic Valve Stenosis: From Pathophysiology to Emerging Pharmacological Agents. J. Clin. Med. 2026, 15, 274. https://doi.org/10.3390/jcm15010274
Agnello F, Laterra G, Scalia L, Mauro MS, Strazzieri O, Reddavid C, Ingala S, Guarino S, Barbera C, Russo MD, et al. Lipoprotein(a) and Aortic Valve Stenosis: From Pathophysiology to Emerging Pharmacological Agents. Journal of Clinical Medicine. 2026; 15(1):274. https://doi.org/10.3390/jcm15010274
Chicago/Turabian StyleAgnello, Federica, Giulia Laterra, Lorenzo Scalia, Maria Sara Mauro, Orazio Strazzieri, Claudia Reddavid, Salvatore Ingala, Simona Guarino, Chiara Barbera, Maria Daniela Russo, and et al. 2026. "Lipoprotein(a) and Aortic Valve Stenosis: From Pathophysiology to Emerging Pharmacological Agents" Journal of Clinical Medicine 15, no. 1: 274. https://doi.org/10.3390/jcm15010274
APA StyleAgnello, F., Laterra, G., Scalia, L., Mauro, M. S., Strazzieri, O., Reddavid, C., Ingala, S., Guarino, S., Barbera, C., Russo, M. D., & Barbanti, M. (2026). Lipoprotein(a) and Aortic Valve Stenosis: From Pathophysiology to Emerging Pharmacological Agents. Journal of Clinical Medicine, 15(1), 274. https://doi.org/10.3390/jcm15010274






