Lanadelumab in Hereditary Angioedema: Real-World Outcomes and Implications for Access Practices in Europe
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Variables
2.3. Outcomes
2.4. Statistics
2.5. Review of Reimbursement Criteria in European Countries
3. Results
3.1. Patient Characteristics
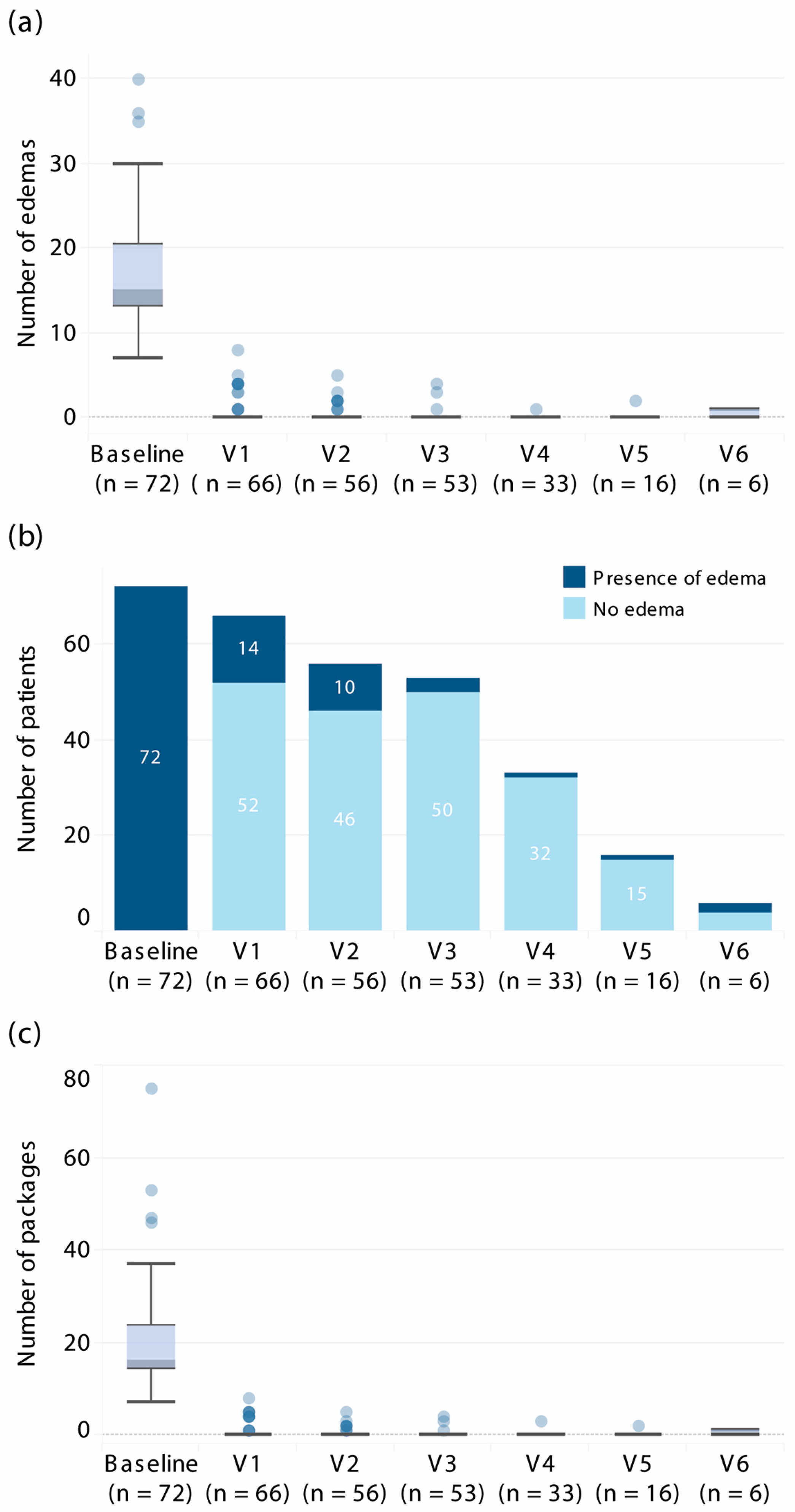
3.2. Efficacy Outcomes
3.3. Predictors of Response
3.4. Reimbursement Criteria for Lanadelumab in Selected European Countries
3.4.1. Availability and Reimbursement Process
3.4.2. Eligibility Criteria
3.4.3. Positioning Within Treatment Lines
3.4.4. Distribution Setting
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C1-INH | C1 esterase inhibitor |
| CI | Confidence interval |
| HAE | Hereditary angioedema |
| HELP | Hereditary Angioedema Long-term Prophylaxis study |
| IQR | Interquartile range |
| LTP | Long-term prophylaxis |
| SD | Standard deviation |
References
- O’Connor, M.; Busse, P.J.; Craig, T.J.; Radojicic, C.; Wedner, H.J.; Danese, S.; Ulloa, J.; Desai, V.; Andriotti, T.; Audhya, P.K.; et al. Impact of hereditary angioedema attacks on health-related quality of life and work productivity. World Allergy Organ. J. 2025, 18, 101083. [Google Scholar] [CrossRef]
- Banerji, A.; Sloane, D.E.; Sheffer, A.L. Hereditary angioedema: A current state-of-the-art review, V: Attenuated androgens for the treatment of hereditary angioedema. Ann. Allergy Asthma Immunol. 2008, 100, S19–S22. [Google Scholar] [CrossRef] [PubMed]
- Riedl, M.A.; Banerji, A.; Busse, P.J.; Johnston, D.T.; Davis-Lorton, M.A.; Patel, S.; Parr, H.; Chiao, J.; Watson, D.J.; Burrell, E.; et al. Patient satisfaction and experience with intravenously administered C1-inhibitor concentrates in the United States. Ann. Allergy Asthma Immunol. 2017, 119, 59–64. [Google Scholar] [CrossRef]
- Valerieva, A.; Nedeva, D.; Yordanova, V.; Petkova, E.; Staevska, M. Therapeutic management of hereditary angioedema: Past, present, and future. Balkan Med. J. 2021, 38, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Takhzyro (Lanadelumab). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/takhzyro (accessed on 6 August 2025).
- Banerji, A.; Bernstein, J.A.; Johnston, D.T.; Lumry, W.R.; Magerl, M.; Maurer, M.; Martinez-Saguer, I.; Zanichelli, A.; Hao, J.; Inhaber, N.; et al. Long-term prevention of hereditary angioedema attacks with lanadelumab: The HELP OLE Study. Allergy 2022, 77, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Magerl, M.; Betschel, S.; Aberer, W.; Ansotegui, I.J.; Aygören-Pürsün, E.; Banerji, A.; Bara, N.A.; Boccon-Gibod, I.; Bork, K.; et al. The international WAO/EAACI guideline for the management of hereditary angioedema-The 2021 revision and update. Allergy 2022, 77, 1961–1990. [Google Scholar] [CrossRef] [PubMed]
- Betschel, S.; Badiou, J.; Binkley, K.; Borici-Mazi, R.; Hébert, J.; Kanani, A.; Keith, P.; Lacuesta, G.; Waserman, S.; Yang, B.; et al. The International/Canadian Hereditary Angioedema Guideline. Allergy Asthma Clin. Immunol. 2019, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Obwieszczenia Ministra Zdrowia—Lista Leków Refundowanych. Available online: https://www.gov.pl/web/zdrowie/obwieszczenia-ministra-zdrowia-lista-lekow-refundowanych (accessed on 6 August 2025).
- Buttgereit, T.; Vera Ayala, C.; Aykanat, S.; Weller, K.; Gutsche, A.; Maurer, M.; Magerl, M. The real life experience goes on: Update after 4 years on the first cohort treated with lanadelumab at our center. Front. Immunol. 2024, 15, 1405317. [Google Scholar] [CrossRef] [PubMed]
- Nutzenbewertungsverfahren zum Wirkstoff Lanadelumab (Überschreitung 50 Mio. € Grenze: Hereditäres Angioödem, Prophylaxe, ≥ 12 Jahre). Available online: https://www.g-ba.de/bewertungsverfahren/nutzenbewertung/688/#nutzenbewertung (accessed on 6 August 2025).
- BIFIMED: Buscador de la Información Sobre la Situación de Financiación de los Medicamentos—Nomenclátor de AGOSTO. 2025. Available online: https://www.sanidad.gob.es/profesionales/medicamentos.do (accessed on 6 August 2025).
- Regime di Rimborsabilita’ e Prezzo del Medicinale per uso Umano «Takhzyro». (Determina n. DG/180/2021). Available online: https://www.medicoeleggi.com/argomenti000/italia2021/413111.htm (accessed on 6 August 2025).
- TAKHZYRO (Lanadélumab). Available online: https://www.has-sante.fr/jcms/pprd_2982720/fr/takhzyro-lanadelumab (accessed on 6 August 2025).
- Remboursement Takhzyro sol. inj. s.c. [ser. Préremplie] 1 × 300 mg/2 mL. Available online: https://h.cbip.be/fr/ampps/184606?cat=a (accessed on 6 August 2025).
- GVS Advice on Lanadelumab (Takhzyro®) for the Prevention of Recurrent Attacks of Hereditary Angioedema (HAE). Available online: https://english.zorginstituutnederland.nl/documents/2023/04/28/gvs-advice-on-lanadelumab-takhzyro (accessed on 6 August 2025).
- Informační Číselník. Svazu Zdravotních Pojišťoven České Republiky. Available online: https://szpcr.cz/wp-content/cis/hvlp/2024/InfoCis240501.pdf (accessed on 6 August 2025).
- Gutová, V. Hereditary angioedema with C1 inhibitor deficiency in children. Remedia 2023, 33, 87–91. [Google Scholar]
- Bernstein, J.A.; Betschel, S.D.; Busse, P.J.; Banerji, A.; Wedner, H.J.; Manning, M.; Zaragoza-Urdaz, R.H.; Anderson, J.; Gagnon, R.; Baptist, A.P.; et al. Sustained Effectiveness, Tolerability, and Safety of Long-Term Prophylaxis with Lanadelumab in Hereditary Angioedema: The Prospective, Phase 4, Noninterventional EMPOWER Real-World Study. Adv. Ther. 2025, 42, 3882–3901. [Google Scholar] [CrossRef] [PubMed]
- Magerl, M.; Bouillet, L.; Martinez-Saguer, I.; Gavini, F.; Bent-Ennakhil, N.; Sayegh, L.; Andresen, I. Real-World Effectiveness of Lanadelumab in Hereditary Angioedema: Multicountry INTEGRATED Observational Study. J. Allergy Clin. Immunol. Pract. 2025, 13, 378–387.e2. [Google Scholar] [CrossRef] [PubMed]
- Takhzyro, Lanadelumabum, Roztwór do Wstrzykiwań, 300 mg, 1 Fiol., Kod EAN: 05060147027884 w Ramach Programu Lekowego We Wskazaniu Wynikającym ze Złożonego Wniosku i Treści Uzgodnionego Programu. Available online: https://bip.aotm.gov.pl/zlecenia-mz-2020/939-materialy-2020/6562-24-2020-zlc?highlight=WyJsYW5hZGVsdW1hYnVtIl0= (accessed on 6 August 2025).
- Kucharczyk, A.; Porębski, G.; Rząd, M.; Grzela, K.; Juchacz, A.; Kurowski, M.; Kuziemski, K.; Łukaszyk, M.; Matuszewski, T.; Pawlukiewicz, M.; et al. Lanadelumab demonstrates high efficacy in reducing the frequency of angioedema attacks in patients with severe HAE in real-life settings. Pediatr. Med. Rodz. 2023, 19, 334–342. [Google Scholar] [CrossRef]
- Fain, O.; Du-Thanh, A.; Gobert, D.; Launay, D.; Inhaber, N.; Boudjemia, K.; Aubineau, M.; Sobel, A.; Boccon-Gibod, I.; Weiss, L.; et al. Long-term prophylaxis with lanadelumab for HAE: Authorization for temporary use in France. Allergy Asthma Clin. Immunol. 2022, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Machín, I.; González-Pérez, R.; Mederos-Luis, E.; García-Gil, S.; Poza-Guedes, P. Real-World Outcomes and Healthcare Utilization of Lanadelumab in Spain: Insights from First Cohort of Difficult-to-Treat Hereditary Angioedema Cases. Allergies 2025, 5, 3. [Google Scholar] [CrossRef]
- Nzeako, U.C.; Frigas, E.; Tremaine, W.J. Hereditary angioedema: A broad review for clinicians. Arch. Intern. Med. 2001, 161, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Zanichelli, A.; Wuillemin, W.A.; Aygören-Pürsün, E.; Banerji, A.; Busse, P.J.; Betschel, S.D.; Cancian, M.; Gagnon, R.; Goodyear, M.D.; Kinaciyan, T.; et al. Lanadelumab’s impact on hereditary angioedema control and quality of life across disease activity subgroups: Real-world evidence. Ann. Allergy Asthma Immunol. 2025, 135, 560–569.e2. [Google Scholar] [CrossRef] [PubMed]
- Craig, T.; Tachdjian, R.; Bernstein, J.A.; Anderson, J.; Nurse, C.; Watt, M.; Yu, M.; Juethner, S. Long-term prevention of hereditary angioedema attacks with lanadelumab in adolescents. Ann. Allergy Asthma Immunol. 2024, 133, 712–719.e1. [Google Scholar] [CrossRef] [PubMed]
- Karska, J. (Takeda, Warsaw, Poland). Presonal communication, 2025.
- GVS-Advies Lanadelumab (Takhzyro®) Voor Het Voorkomen van Terugkerende Aanvallen van Hereditair Angio-Oedeem (HAE). Available online: https://www.zorginstituutnederland.nl/documenten/2023/04/28/gvs-advies-lanadelumab-takhzyro (accessed on 6 August 2025).
| Characteristics | Value |
|---|---|
| Mean age (±SD), years | 38.0 (13.6) |
| Female sex, n (%) | 55 (76) |
| Median BMI (IQR), kg/m2 | 24.8 (22.8–28.4) |
| HAE type, n (%) | |
| I | 67 (93) |
| II | 5 (7) |
| Median C1-inhibitor level (IQR), g/L | 0.05 (0.04–0.07) |
| Mean C1-inhibitor activity (±SD), % | 16.3 (8.1) |
| Median C4 level (IQR), g/L | 0.04 (0.03–0.06) |
| First-degree relatives diagnosed with HAE, n (%) | 59 (82) |
| Median number of attacks six months before treatment (IQR) | |
| Overall | 15.0 (13.0–20.8) |
| Abdominal attacks | 13.0 (10.3–17.0) |
| Pharyngeal attacks | 0.0 (0.0–1.0) |
| Laryngeal attacks | 0.0 (0.0–2.0) |
| Median age at disease onset (IQR), years | 7.5 (5.0–12.0) |
| Mean age at diagnosis (±SD), years | 26.0 (13.7) |
| Long-term prophylaxis use, n (%) | 26 (36) |
| Median number of standard doses of on-demand treatment used in the 6 months before treatment (IQR) | |
| Overall | 16.0 (14.0–23.8) |
| pdC1-INH 1500 IU | 8.0 (2.0–17.0) |
| Icatibant | 8.0 (0.0–13.0) |
| Variable | B Coefficient | Standard Error | p-Value |
|---|---|---|---|
| Age | −0.008 | 0.760 | 0.992 |
| Sex | 1.754 | 1.413 | 0.214 |
| Age of symptom onset | −0.083 | 1.437 | 0.954 |
| Time from symptom onset to diagnosis | −0.121 | 1.445 | 0.933 |
| Time from symptom onset to treatment | −0.106 | 1.445 | 0.942 |
| Height | −0.211 | 0.530 | 0.691 |
| Body mass | 0.341 | 0.668 | 0.610 |
| Body mass index | −0.981 | 1.880 | 0.602 |
| C1-INH concentration | 0.046 | 6.948 | 0.995 |
| C1-INH activity | 0.050 | 0.047 | 0.285 |
| Baseline frequency of angioedema attacks | 0.004 | 0.114 | 0.970 |
| Number of treatment lines | 0.088 | 0.092 | 0.337 |
| Age at treatment with lanadelumab | 0.131 | 1.550 | 0.933 |
| HAE type | 0.208 | 1.965 | 0.916 |
| Country | Reimbursement Criteria |
|---|---|
| Poland | Reimbursed since: September 2021. |
| Eligibility | Patients ≥12 years of age with ≥12 severe abdominal/laryngeal/pharyngeal HAE attacks in 6 months with documented on-demand medication use (2021–2024); threshold relaxed to ≥6 attacks/6 months from 2025 [9]. |
| Patient cost | Free of charge. |
| Germany | Reimbursed since: 2019 [10]. |
| Eligibility | Per EMA indication. In practice, used for patients with frequent or severe attacks; no formal attack-count threshold; no prior prophylaxis required [11]. |
| Patient cost | Statutory co-payment of 10% of the retail price (min €5, max €10 per pack); annual cap 2% of household gross income (1% for chronically ill). Exempt for patients ≤ 18 years. |
| Spain | Reimbursed since: March 2021. |
| Eligibility | Per EMA indication [12]. Initiated in expert centers, typically for frequent (e.g., ≥1 attack/month) or severe attacks in line with WAO/EAACI guidelines [7]. No prior prophylaxis failure required. |
| Patient cost | Free of charge. |
| Italy | Reimbursed since: February 2021. |
| Eligibility | Patients ≥12 years with intolerance or contraindications for danazol-based LTP; requires an AIFA therapeutic plan issued by a specialist in an authorized center; plan valid for up to 12 months with reassessment thereafter. No formal numeric attack-frequency threshold [13]. |
| Patient cost | Free of charge. |
| France | Reimbursed since: 2020. |
| Eligibility | Patients ≥2 years who failed, did not tolerate, or had contraindications to first-line LTP (attenuated androgens or pdC1-INH); reserved for severe or life-threatening disease. No formal attack-count threshold [14]. |
| Patient cost | Free of charge. |
| Belgium | Reimbursed since: July 2022. |
| Eligibility | Patients ≥12 years with HAE type I/II who have: (a) >1 severe angioedema attack/month (>5 months/year affected), or (b) ≥1 life-threatening upper-airway swelling, or (c) inadequate control with repeated on-demand therapy. Initial approval is for 3 months; continuation requires ≥50% attack reduction at 3 months. Subsequent authorizations in 12-month cycles with annual specialist re-application and insurer review [15]. |
| Patient cost | Free of charge. |
| The Netherlands | Reimbursed since: 2023. |
| Eligibility | Covered under basic insurance per EU label; initiation at HAE specialist’s discretion, guided by consensus (prophylaxis typically if ≥1 severe attack/month or significant QoL impact). No step-through required [16]. |
| Patient cost | Covered under basic insurance; patients pay up to the statutory annual deductible (eigen risico, €385 in 2025). Beyond this amount, treatment is fully reimbursed. |
| Czech Republic | Reimbursed since: Regular reimbursement from 2024; previously case-by-case [17]. |
| Eligibility | Czech expert guidance recommends first-line LTP (lanadelumab or pdC1-INH) for (a) ≥18 attacks/year or (b) ≥1 life-threatening attack in the last year, or (c) extremely severe disease (e.g., frequent multifocal attacks, multiple acute doses), or (d) insufficient effect of or contraindication to other prophylaxis [18]. Final official criteria pending publication. |
| Patient cost | Free of charge. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Różyk, D.; Wrona, W.; Kucharczyk, B.; Tomaszewska, A.; Kucharczyk, A. Lanadelumab in Hereditary Angioedema: Real-World Outcomes and Implications for Access Practices in Europe. J. Clin. Med. 2026, 15, 189. https://doi.org/10.3390/jcm15010189
Różyk D, Wrona W, Kucharczyk B, Tomaszewska A, Kucharczyk A. Lanadelumab in Hereditary Angioedema: Real-World Outcomes and Implications for Access Practices in Europe. Journal of Clinical Medicine. 2026; 15(1):189. https://doi.org/10.3390/jcm15010189
Chicago/Turabian StyleRóżyk, Dagmara, Witold Wrona, Barbara Kucharczyk, Agata Tomaszewska, and Aleksandra Kucharczyk. 2026. "Lanadelumab in Hereditary Angioedema: Real-World Outcomes and Implications for Access Practices in Europe" Journal of Clinical Medicine 15, no. 1: 189. https://doi.org/10.3390/jcm15010189
APA StyleRóżyk, D., Wrona, W., Kucharczyk, B., Tomaszewska, A., & Kucharczyk, A. (2026). Lanadelumab in Hereditary Angioedema: Real-World Outcomes and Implications for Access Practices in Europe. Journal of Clinical Medicine, 15(1), 189. https://doi.org/10.3390/jcm15010189






