Impact of Superficial Keratectomy on Corneal Topography, Aberration, and Densitometry in Salzmann Nodular Degeneration
Abstract
1. Introduction
2. Materials and Methods
- a.
- Anterior layer with 120 µm of the outermost cornea.
- b.
- The mid-stromal layer is between the anterior and posterior layers.
- c.
- Posterior layer with a thickness of 60 µm.
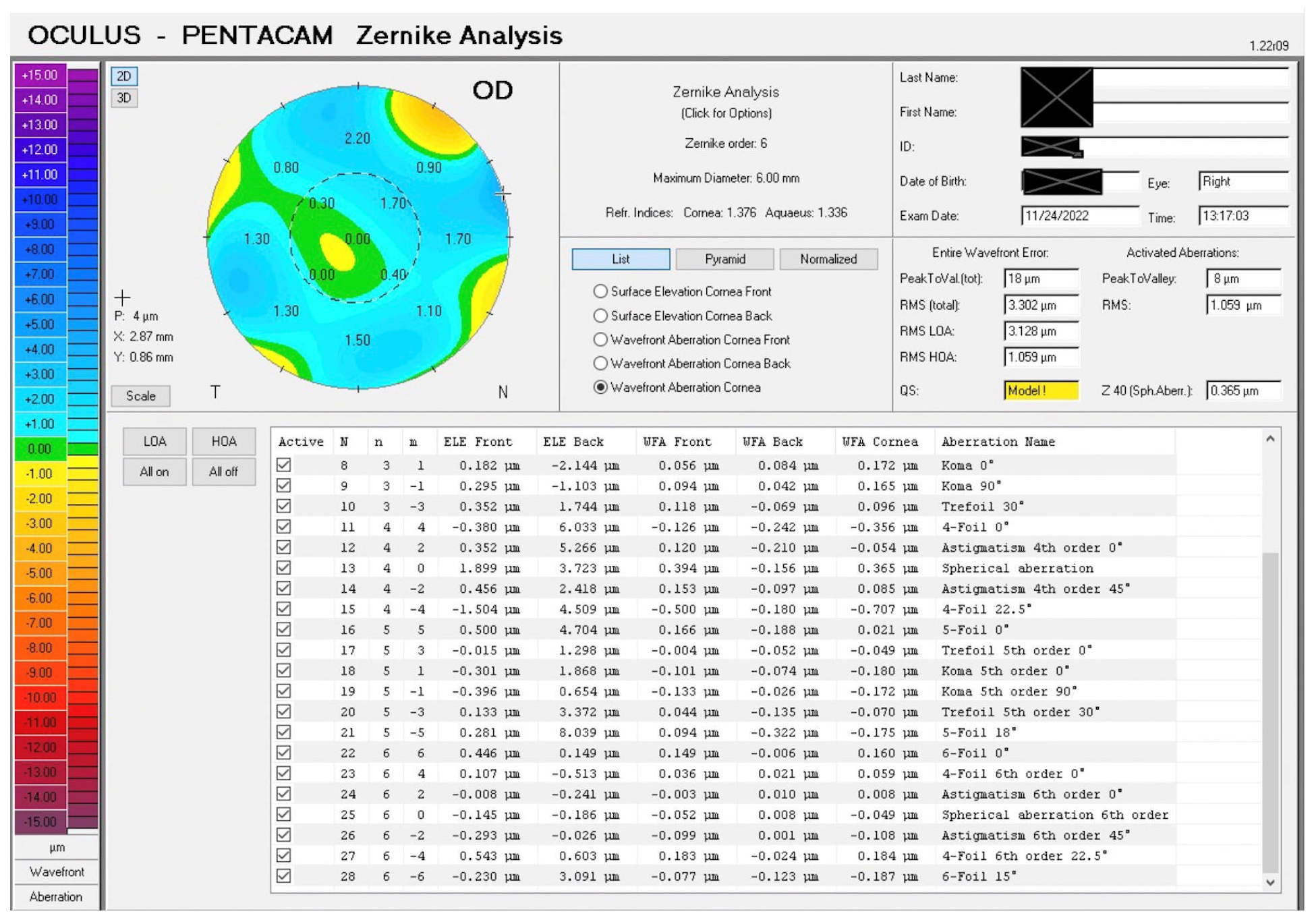
3. Results
3.1. Primary Outcome Measure
3.2. Secondary Outcomes
- (a)
- Visual Acuity, refractive cylinder, and spherical equivalent:
- (b)
- Keratometry:
- (c) Corneal Optical Densitometry (COD):
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maharana, P.K.; Sharma, N.; Das, S.; Titiyal, J.S.; Jain, R.; Vajpayee, R.B. Salzmann’s nodular degeneration. Ocul. Surf. 2016, 14, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Darrad, K.; McDonnell, P.J. Salzmann’s nodular corneal degeneration (SNCD): Clinical findings, risk factors, prognosis and the role of previous contact lens wear. Cont. Lens Anterior Eye 2011, 34, 173–178. [Google Scholar] [CrossRef]
- Wang, K.; See, C.W. Salzmann’s nodular degeneration. Exp. Eye Res. 2021, 202, 108351. [Google Scholar] [CrossRef]
- Bae, S.S.; Chan, C.C. Superficial keratectomy: Indications and outcomes. Can. J. Ophthalmol. 2018, 53, 553–559. [Google Scholar] [CrossRef]
- Khaireddin, R.; Katz, T.; Baile, R.B.; Richard, G.; Linke, S.J. Superficial keratectomy, PTK, and mitomycin C as a combined treatment option for Salzmann’s nodular degeneration: A follow-up of eight eyes. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1211–1215. [Google Scholar] [CrossRef]
- Shankar, H.; Taranath, D.; Santhirathelagan, C.T.; Pesudovs, K. Anterior segment biometry with the Pentacam: Comprehensive assessment of repeatability of automated measurements. J. Cataract Refract. Surg. 2008, 34, 103–113. [Google Scholar] [CrossRef]
- Nanavaty, M.A.; Favor, M.P.; Lake, D.B. Comparison of equivalent keratometric indices on Scheimpflug tomography with Placido-based topography system at different optical zones. Br. J. Ophthalmol. 2013, 97, 350–356. [Google Scholar] [CrossRef]
- Li, M.; Kong, X. Comparing Generalized Estimating Equation and Linear Mixed Effects Model for Estimating Marginal Association with Bivariate Continuous Outcomes. Ophthalmic Epidemiol. 2023, 30, 307–316. [Google Scholar] [CrossRef]
- Roszkowska, A.M.; Colosi, P.; De Grazia, L.; Mirabelli, E.; Romeo, G. One-year outcome of manual alcohol-assisted removal of Salzmann’s nodular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 1431–1434. [Google Scholar] [CrossRef]
- Helm, S.; Wiedemann, J.; Reinking, N.; Rosswinkel, B.; Bachmann, B.; Cursiefen, C.; Schlereth, S. Visual, topographic and aberrometric outcomes after phototherapeutic keratectomy (PTK) for Salzmann nodular degeneration. Med. Sci. 2025, 13, 197. [Google Scholar] [CrossRef]
- Chen, S.; Chu, X.; Zhang, C.; Jia, Z.; Yang, L.; Yang, R.; Huang, Y.; Zhao, S. Safety and efficacy of the phototherapeutic keratectomy for treatment of recurrent corneal erosions: A systematic review and meta-analysis. Ophthalmic Res. 2023, 66, 1114–1127. [Google Scholar] [CrossRef] [PubMed]
- Kligman, B.E.; Baartman, B.J.; Dupps, W.J., Jr. Errors in treatment of lower-order aberrations and induction of higher-order aberrations in laser refractive surgery. Int. Ophthalmol. Clin. 2016, 56, 19–45. [Google Scholar] [CrossRef]
- Messina, M.; Giannaccare, G.; Cagini, C.; Fogagnolo, P.; Poddi, M.; Bonifazi, T.; Mirabella, G.; Coco, G.; Della Lena, F. Enhancing visual quality: The impact of alcohol-assisted delamination on corneal aberrations in patients with central epithelial basement membrane dystrophy. J. Clin. Med. 2025, 14, 2342. [Google Scholar] [CrossRef]
- Salari, F.; Beikmarzehei, A.; Liu, G.; Zarei-Ghanavati, M.; Liu, C. Superficial keratectomy: A review of literature. Front. Med. 2022, 9, 915284. [Google Scholar] [CrossRef]
- Raber, I.M.; Eagle, R.C., Jr. Peripheral hypertrophic subepithelial corneal degeneration. Cornea 2022, 41, 183–191. [Google Scholar] [CrossRef]
- Goerlitz-Jessen, M.F.; Gupta, P.K.; Kim, T. Impact of epithelial basement membrane dystrophy and Salzmann nodular degeneration on biometry measurements. J. Cataract Refract. Surg. 2019, 45, 1119–1123. [Google Scholar] [CrossRef]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Scorsetti, M.M.; Eguiza, V.S.; Duran, J.A. Manual superficial keratectomy is the first choice treatment for Salzmann nodular degeneration. Cornea 2024, 43, 716–719. [Google Scholar] [CrossRef]
- Messina, M.; Poddi, M.; De Santi, N.; Bianchi, E.; Cagini, C. Combined superficial keratectomy, alcohol delamination and amniotic membrane patch with fibrin glue in Salzmann nodular degeneration. Eur. J. Ophthalmol. 2024, 34, 1281–1285. [Google Scholar] [CrossRef]
- He, X.; Donaldson, K.E. Superficial keratectomy for Salzmann nodular degeneration following laser in situ keratomileusis. Can. J. Ophthalmol. 2019, 54, e149–e151. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ma, B.S.; Zeng, J.H.; Ma, D.J. Corneal optical density: Structural basis, measurements, influencing factors, and roles in refractive surgery. Front. Bioeng. Biotechnol. 2023, 11, 1144455. [Google Scholar] [CrossRef]
- Roszkowska, A.M.; Aragona, P.; Spinella, R.; Pisani, A.; Puzzolo, D.; Micali, A. Morphologic and confocal investigation on Salzmann nodular degeneration of the cornea. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5910–5919. [Google Scholar] [CrossRef] [PubMed]
- Otri, A.M.; Fares, U.; Al-Aqaba, M.A.; Dua, H.S. Corneal densitometry as an indicator of corneal health. Ophthalmology 2012, 119, 501–508. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Y.; Zhang, J.; Lei, Y.; Ma, Z.; Zhang, Y.; Zheng, X. Corneal densitometry after allogeneic small-incision intrastromal lenticule implantation for hyperopia correction. BMC Ophthalmol. 2022, 22, 286. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.; Ramos, I.; Ambrosio, R., Jr. Corneal densitometry in keratoconus. Cornea 2014, 33, 1282–1286. [Google Scholar] [CrossRef]
- Mounir, A.; Awny, I.; Yousef, H.S.; Mostafa, E.M. Distribution of corneal densitometry in different grades of keratoconus. Indian J. Ophthalmol. 2023, 71, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Jian, W.; Sun, L.; Li, M.; Han, T.; Son, J.; Zhou, X. One-year follow-up of changes in corneal densitometry after accelerated (45 mW/cm2) transepithelial corneal collagen cross-linking for keratoconus: A retrospective study. Cornea 2016, 35, 1434–1440. [Google Scholar] [CrossRef]
| Age/Sex | Presenting Symptom or Indication for Surgery | Location of Nodule(s) | Pre-op LogMAR BCVA | Post-op LogMAR BCVA |
|---|---|---|---|---|
| 75/M | Blurred vision | Paracentral | 0.48 | 0.30 |
| 75/M | Blurred vision | Paracentral | 1.20 | 0.40 |
| 58/F | Foreign body sensation | Mid-peripheral | 0.00 | 0.00 |
| 65/F | Blurred vision | Paracentral | 0.30 | 0.00 |
| 66/F | Blurred vision | Mid-peripheral | 0.10 | 0.00 |
| 48/M | Foreign body sensation | Mid-peripheral | 0.18 | 0.10 |
| 63/M | Blurred vision | Mid-peripheral | 0.18 | 0.18 |
| 63/M | Blurred vision | Paracentral | 0.18 | 0.18 |
| 67/M | Soreness, epiphora | Paracentral | 0.18 | 0.10 |
| 48/F | Foreign body sensation | Paracentral and mid-peripheral | 0.18 | 0.00 |
| 77/M | Foreign body sensation | Paracentral and mid-peripheral | 0.30 | 0.15 |
| 69/F | Blurred vision, astigmatism | Paracentral | 0.48 | 0.30 |
| 52/M | Blurred vision | Paracentral and mid-peripheral | 0.48 | 0.00 |
| 47/F | Foreign body sensation | Mid-peripheral | 0.00 | 0.00 |
| 47/F | Foreign body sensation | Mid-peripheral | 0.15 | 0.10 |
| 66/M | Asymptomatic | Paracentral | 0.15 | 0.15 |
| 93/F | Foreign body sensation | Mid-peripheral and peripheral | 0.50 | 0.30 |
| 93/F | Foreign body sensation | Mid-peripheral and peripheral | 0.50 | 0.40 |
| 67/F | Blurred vision, astigmatism | Paracentral and mid-peripheral | 0.15 | 0.00 |
| 53/F | Recurrent corneal erosions | Paracentral | 0.00 | 0.00 |
| 68/F | Soreness, discomfort | Mid-peripheral | 0.00 | 0.00 |
| Parameter | Location (WFA) | Pre-op (μm) Mean (±SD) | Post-op (μm) Mean (±SD) | p-Value |
|---|---|---|---|---|
| RMS Total | - | 7.439 (±3.648) | 4.283 (±2.076) | 0.001 |
| RMS Lower-Order Aberrations (LOAs) | - | 6.858 (±3.524) | 3.965 (±1.830) | 0.001 |
| RMS Higher-Order Aberrations (HOAs) | - | 2.792 (±1.194) | 1.515 (±1.146) | 0.000 |
| Z11 Horizontal Tilt (Tilt in X) | Front | −1.622 (±3.978) | 0.409 (±1.627) | 0.038 |
| Total | −1.596 (±4.431) | 0.451 (±1.734) | 0.047 | |
| Z2−2 Primary Oblique Astigmatism | Total | −0.634 (±2.853) | 0.190 (±1.664) | 0.032 |
| Z31 Horizontal Coma | Front | −0.381 (±1.173) | 0.191 (±0.575) | 0.048 |
| Total | −0.373 (±1.296) | 0.245 (±0.663) | 0.048 |
| Keratometry from the Holladay EKR Report | |||
|---|---|---|---|
| Zone (mm)/Keratometry Meridian | Pre-op Mean (±SD) (D) | Post-op Mean (±SD) (D) | p-Value |
| 3 mm/K2 (Steep K) | 43.08 (±2.72) | 42.79 (±2.32) | 0.034 |
| 4.5 mm/K1 (Flat K) | 40.17 (±2.52) | 41.07 (±3.03) | 0.018 |
| 5 mm/K1 (Flat K) | 40.23 (±2.48) | 41.20 (±3.05) | 0.003 |
| 6 mm/K1 (Flat K) | 40.56 (± 2.30) | 41.59 (±3.06) | <0.001 |
| 7 mm/K1 (Flat K) | 40.89 (±2.23) | 41.83 (±3.13) | <0.001 |
| 7 mm/K2 (Steep K) | 44.60 (±2.59) | 43.97 (±1.91) | 0.037 |
| Corneal Optical Densitometry (COD) | |||
| Zone/Depth | Pre-op Mean (±SD) (µm) | Post-op Mean (±SD) (µm) | p-Value |
| 2–6 mm/Total | 18.76 (±6.46) | 15.73 (±4.27) | <0.001 |
| 6–10 mm/Total | 30.79 (±10.90) | 26.25 (±8.17) | 0.026 |
| 10–12 mm/Total | 33.47 (±9.66) | 28.46 (±5.83) | 0.002 |
| Overall cornea/Total depth or all zones | 24.85 (±7.23) | 20.94 (±4.90) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Qi, Z.; Mukhija, R.; Quiney, G.; Nanavaty, M.A. Impact of Superficial Keratectomy on Corneal Topography, Aberration, and Densitometry in Salzmann Nodular Degeneration. J. Clin. Med. 2026, 15, 178. https://doi.org/10.3390/jcm15010178
Qi Z, Mukhija R, Quiney G, Nanavaty MA. Impact of Superficial Keratectomy on Corneal Topography, Aberration, and Densitometry in Salzmann Nodular Degeneration. Journal of Clinical Medicine. 2026; 15(1):178. https://doi.org/10.3390/jcm15010178
Chicago/Turabian StyleQi, Ziqiao, Ritika Mukhija, Gabriella Quiney, and Mayank A. Nanavaty. 2026. "Impact of Superficial Keratectomy on Corneal Topography, Aberration, and Densitometry in Salzmann Nodular Degeneration" Journal of Clinical Medicine 15, no. 1: 178. https://doi.org/10.3390/jcm15010178
APA StyleQi, Z., Mukhija, R., Quiney, G., & Nanavaty, M. A. (2026). Impact of Superficial Keratectomy on Corneal Topography, Aberration, and Densitometry in Salzmann Nodular Degeneration. Journal of Clinical Medicine, 15(1), 178. https://doi.org/10.3390/jcm15010178







