A Novel Prophylactic Device Against Head Deformity to Prevent Severe Positional Plagiocephaly
Abstract
1. Introduction
2. Subjects and Methods
2.1. Control Cases
2.2. Novel Prophylactic Device Against Head Deformity
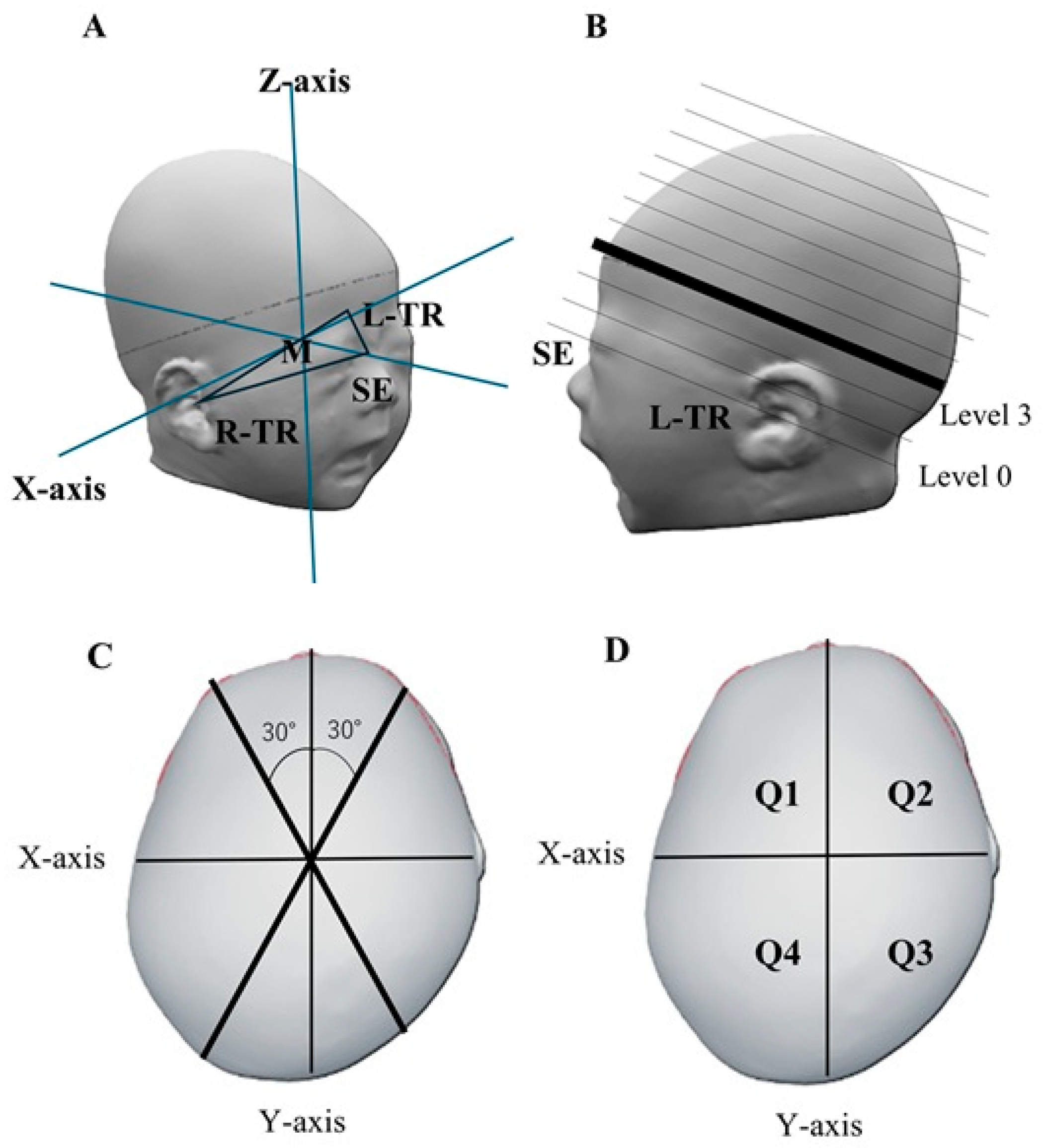
2.3. Cranial Shape Measurement
2.4. Head Deformity Parameters
2.5. Items for Consideration
2.6. Statistical Analysis
3. Results
3.1. Patient Background
3.2. Comparative Assessment of Cranial Shape at 3 Months of Age
3.3. Timing of Device Initiation
3.4. Device Safety
4. Discussion
4.1. Safety of the Prophylactic Device Against Head Deformity
4.2. Methods of Measuring Cranial Geometry
4.3. Prevention of Head Deformity
4.4. Prevention of Severe PP
4.5. Benefits of Preventing Head Deformity
4.6. Future Lines of Research
4.7. Limitations
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASR | Anterior symmetry rate |
| CA | Cranial asymmetry |
| CI | Cephalic index |
| CrL | Cranial length |
| CrW | Cranial width |
| CVAI | Cranial vault asymmetry index |
| PP | Positional plagiocephaly |
| PSR | Posterior symmetry rate |
| SIDS | Sudden infant death syndrome |
References
- Christensen, L.; Østergaard, J.R.; Nørholt, S.E. Positional plagiocephaly. Ugeskr. Laeger. 2002, 165, 46–50. (In Danish) [Google Scholar] [PubMed]
- Beuriat, P.A.; Szathmari, A.; Di Rocco, F.; Mottolese, C. Deformational plagiocephaly: State of the art and review of the literature. Neurochirurgie 2019, 65, 322–329. [Google Scholar] [CrossRef]
- Rogers, G.F. Deformational plagiocephaly, brachycephaly, and scaphocephaly. Part I: Terminology, diagnosis, and etiopathogenesis. J. Craniofac. Surg. 2011, 22, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Rekate, H.L. Occipital plagiocephaly: A critical review of the literature. J. Neurosurg. 1998, 89, 24–30. [Google Scholar] [CrossRef]
- Dec, W.; Warren, S.M. Current concepts in deformational plagiocephaly. J. Craniofac. Surg. 2011, 22, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Miyabayashi, H.; Nagano, N.; Kato, R.; Noto, T.; Hashimoto, S.; Saito, K.; Morioka, I. Cranial shape in infants aged one month can predict the severity of deformational plagiocephaly at the age of six months. J. Clin. Med. 2022, 11, 1797. [Google Scholar] [CrossRef]
- Miyabayashi, H.; Nagano, N.; Morioka, I. Cranial shape changes over time and prevention of deformities in healthy infants. J. Nihon Univ. Med. Assoc. 2024, 83, 45–49. (In Japanese) [Google Scholar] [CrossRef]
- Di Chiara, A.; La Rosa, E.; Ramieri, V.; Vellone, V.; Cascone, P. Treatment of deformational plagiocephaly with physiotherapy. J. Craniofac. Surg. 2019, 30, 2008–2013. [Google Scholar] [CrossRef]
- Vles, J.S.; Colla, C.; Weber, J.W.; Beuls, E.; Wilmink, J.; Kingma, H. Helmet versus nonhelmet treatment in nonsynostotic positional posterior plagiocephaly. J. Craniofac. Surg. 2000, 11, 572–574. [Google Scholar] [CrossRef]
- van Wijk, R.M.; van Vlimmeren, L.A.; Groothuis-Oudshoorn, C.G.; Van der Ploeg, C.P.; Ijzerman, M.J.; Boere-Boonekamp, M.M. Helmet therapy in infants with positional skull deformation: Randomised controlled trial. Br. Med. J. 2014, 348, g2741. [Google Scholar] [CrossRef]
- Martiniuk, A.L.; Vujovich-Dunn, C.; Park, M.; Yu, W.; Lucas, B.R. Plagiocephaly and developmental delay: A systematic review. J. Dev. Behav. Pediatr. 2017, 38, 67–78. [Google Scholar] [CrossRef]
- Infant Head-Shaping Pillows Can Cause Suffocation, FDA Warns. Available online: https://publications.aap.org/aapnews/news/22583/Infant-head-shaping-pillows-can-cause-suffocation (accessed on 21 March 2025).
- Holowka, M.A.; Reisner, A.; Giavedoni, B.; Lombardo, J.R.; Coulter, C. Plagiocephaly severity scale to aid in clinical treatment recommendations. J. Craniofac. Surg. 2017, 28, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Plank, L.H.; Giavedoni, B.; Lombardo, J.R.; Geil, M.D.; Reisner, A. Comparison of infant head shape changes in deformational plagiocephaly following treatment with a cranial remolding orthosis using a noninvasive laser shape digitizer. J. Craniofac. Surg. 2006, 17, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Ifflaender, S.; Rüdiger, M.; Koch, A.; Burkhardt, W. Three-dimensional digital capture of head size in neonates—A method evaluation. PLoS ONE 2013, 8, e61274. [Google Scholar] [CrossRef] [PubMed]
- Ifflaender, S.; Rüdiger, M.; Konstantelos, D.; Wahls, K.; Burkhardt, W. Prevalence of head deformities in preterm infants at term equivalent age. Early Hum. Dev. 2013, 89, 1041–1047. [Google Scholar] [CrossRef]
- Koizumi, T.; Komuro, Y.; Hashizume, K.; Yanai, A. Cephalic index of Japanese children with normal brain development. J. Craniofac. Surg. 2010, 21, 1434–1437. [Google Scholar] [CrossRef]
- Loveday, B.P.; de Chalain, T.B. Active counterpositioning or orthotic device to treat positional plagiocephaly? J. Craniofac. Surg. 2001, 12, 308–313. [Google Scholar] [CrossRef]
- Dörhage, K. Ursache und Diagnostik der lagebedingten Plagiozephalie. Man. Med. 2010, 48, 125–134. [Google Scholar] [CrossRef]
- Aihara, Y.; Komatsu, K.; Dairoku, H.; Kubo, O.; Hori, T.; Okada, Y. Cranial molding helmet therapy and establishment of practical criteria for management in Asian infant positional head deformity. Childs Nerv. Syst. 2014, 30, 1499–1509. [Google Scholar] [CrossRef]
- Miyabayashi, H.; Saito, K.; Kato, R.; Noto, T.; Nagano, N.; Morioka, I. Denominator of cranial vault asymmetry index: Choosing between longer and shorter diagonal lengths. J. Craniofac. Surg. 2023, 34, e369–e372. [Google Scholar] [CrossRef]
- Takamatsu, A.; Hikosaka, M.; Kaneko, T.; Mikami, M.; Kaneko, A. Evaluation of the molding helmet therapy for Japanese infants with deformational plagiocephaly. Jpn. Med. Assoc. J. 2021, 4, 50–60. [Google Scholar]
- Nelson, W.E.; Kliegman, R.N. Deformational Plagiocephaly. In Nelson Textbook of Pediatrics, 21st ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Miyabayashi, H.; Nagano, N.; Saito, K.; Kato, R.; Noto, T.; Morioka, I. Creating reference values for cranial shape in early healthy Japanese infants using a three-dimensional scanner. J. Jpn. Soc. Perinat. Neonatal Med. 2023, 59, 166–173. (In Japanese) [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.Y.; Carlin, R.F.; Hand, I.; TASK FORCE ON SUDDEN INFANT DEATH SYNDROME and THE COMMITTEE ON FETUS AND NEWBORN. Evidence Base for 2022 Updated Recommendations for a Safe Infant Sleeping Environment to Reduce the Risk of Sleep-Related Infant Deaths. Pediatrics 2022, 150, e2022057991. [Google Scholar] [CrossRef] [PubMed]
- Ganau, M.; Magdum, S.A.; Calisto, A. Pre-operative imaging and post-operative appearance of standard paediatric neurosurgical approaches: A training guide for neuroradiologists. Transl. Pediatr. 2021, 10, 1231–1243. [Google Scholar] [CrossRef]
- Ganau, M.; Syrmos, N.C.; Magdum, S.A. Imaging in Craniofacial Disorders With Special Emphasis on Gradient Echo Black-Bone and Zero Time Echo MRI Sequences. J. Pediatr. Neurosci. 2022, 17, S14–S20. [Google Scholar] [CrossRef]

| Total (n = 42) | At Birth (n = 32) | At 1 Month of Age (n = 10) | p-Value | |
|---|---|---|---|---|
| Male:Female | 15:27 | 10:22 | 5:5 | 0.280 |
| Number of deliveries (primiparous, multiparous) | 18:14 | 22:10 | 6:4 | 0.608 |
| Delivery method (vaginal, cesarean) | 27:15 | 18:14 | 9:1 | 0.052 |
| Gestational weeks | 39 (±1.3) | 39 (±1.3) | 39 (±1.2) | 0.604 |
| Birth weight (g) | 2947 (±377) | 2947 (±392) | 2946 (±323) | 0.998 |
| Head circumference (cm) at birth | 33.2 (±1.1) | 33.2 (±1.1) | 33.2 (±1.4) | 0.791 |
| (n = 42) | Before Device Use | At 3 Months of Age |
|---|---|---|
| CI (%) | 84.4 [82.3–86.9] | 88.9 [84.4–93.5] |
| CA (mm) | 2.8 [1.2–5.1] | 5.5 [2.3–9.1] |
| CVAI (%) | 2.3 [1.1–4.3] | 4.3 [1.8–7.2] |
| ASR (%) | 95.4 [91.3–98.6] | 95.7 [91.7–97.7] |
| PSR (%) | 93.8 [87.7–96.6] | 92.4 [88.8–96.5] |
| Number of infants with positional plagiocephaly (%) | 9 (21) | 17 (41) |
| Control Group (n = 110) | Prophylactic Device Group (n = 42) | p-Value | |
|---|---|---|---|
| Male:Female | 57:53 | 15:27 | 0.075 |
| Age in days | 100 ± 10.4 | 96 ± 6.3 | 0.019 |
| CI (%) | 88.7 [84.6–92.9] | 88.9 [84.4–93.5] | 0.670 |
| CA (mm) | 8.0 [4.4–11.3] | 5.5 [2.3–9.1] | 0.007 |
| CVAI (%) | 5.8 [3.2–8.1] | 4.3 [1.8–7.2] | 0.048 |
| ASR (%) | 94.7 [90.9–97.1] | 95.7 [91.7–97.7] | 0.210 |
| PSR (%) | 89.7 [83.9–94.4] | 92.4 [88.8–96.5] | 0.035 |
| Number of infants with positional plagiocephaly (%) | 63 (57) | 17 (41) | 0.094 |
| Number of infants with severe positional plagiocephaly (%) | 15 (14) | 0 (0) | 0.012 |
| Started at Birth (n = 32) | Started at 1 Month of Age (n = 10) | p-Value | |
|---|---|---|---|
| Male:Female | 10:22 | 5:5 | 0.280 |
| Age in days | 95 ± 6.3 | 97 ± 6.5 | 0.441 |
| CI (%) | 89.4 [84.6–94.2] | 88.3 [84.4–89.0] | 0.170 |
| CA (mm) | 5.9 [3.7–9.2] | 2.7 [2.0–6.2] | 0.226 |
| CVAI (%) | 4.7 [2.8–7.2] | 2.0 [1.6–4.8] | 0.165 |
| ASR (%) | 95.5 [91.4–97.7] | 95.9 [92.7–96.8] | 0.859 |
| PSR (%) | 91.4 [86.8–96.1] | 94.1 [89.7–99.1] | 0.092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, Y.; Miyabayashi, H.; Noto, T.; Kato, R.; Nagano, N.; Morioka, I. A Novel Prophylactic Device Against Head Deformity to Prevent Severe Positional Plagiocephaly. J. Clin. Med. 2025, 14, 3261. https://doi.org/10.3390/jcm14093261
Tanaka Y, Miyabayashi H, Noto T, Kato R, Nagano N, Morioka I. A Novel Prophylactic Device Against Head Deformity to Prevent Severe Positional Plagiocephaly. Journal of Clinical Medicine. 2025; 14(9):3261. https://doi.org/10.3390/jcm14093261
Chicago/Turabian StyleTanaka, Yukari, Hiroshi Miyabayashi, Takanori Noto, Risa Kato, Nobuhiko Nagano, and Ichiro Morioka. 2025. "A Novel Prophylactic Device Against Head Deformity to Prevent Severe Positional Plagiocephaly" Journal of Clinical Medicine 14, no. 9: 3261. https://doi.org/10.3390/jcm14093261
APA StyleTanaka, Y., Miyabayashi, H., Noto, T., Kato, R., Nagano, N., & Morioka, I. (2025). A Novel Prophylactic Device Against Head Deformity to Prevent Severe Positional Plagiocephaly. Journal of Clinical Medicine, 14(9), 3261. https://doi.org/10.3390/jcm14093261






