Tranilast Reduces Intestinal Ischemia Reperfusion Injury in Rats Through the Upregulation of Heme-Oxygenase (HO)-1
Abstract
1. Introduction
2. Methods
2.1. Animal Model
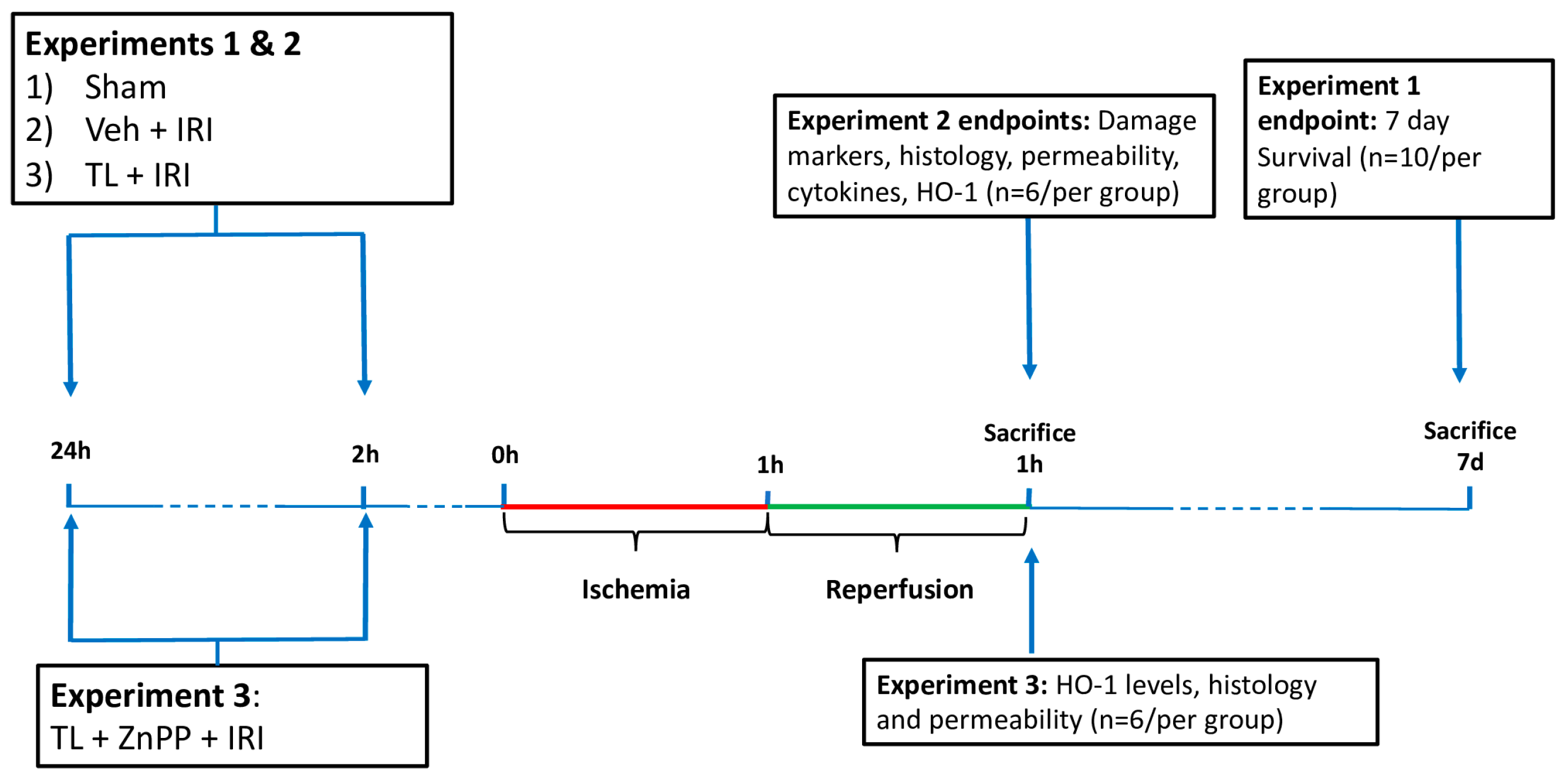
2.2. Experimental Design
2.3. Sample Collection
2.4. Damage Biomarkers
2.5. Histology
2.6. Epithelial Barrier Assessment
2.7. Bacterial Translocation
2.8. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
Western Blot
2.9. Statistical Analysis
3. Results
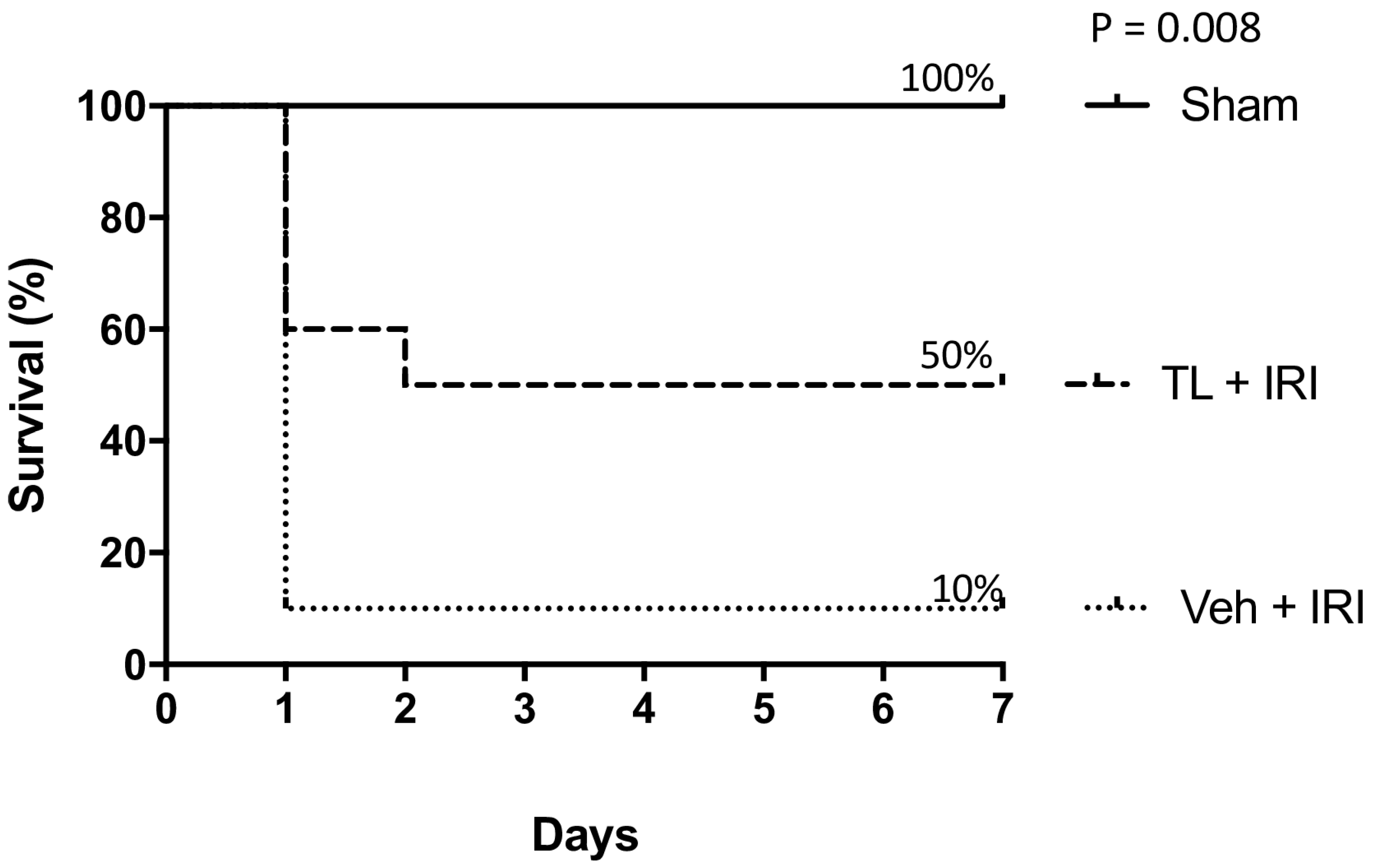
3.1. Experiment 1: Effect of Tranilast Pretreatment on Survival
TL Pretreatment Improved Seven-Day Survival After IRI
3.2. Experiment 2: Effect of Tranilast Pretreatment on Morphology, Barrier Function and Immune Activation
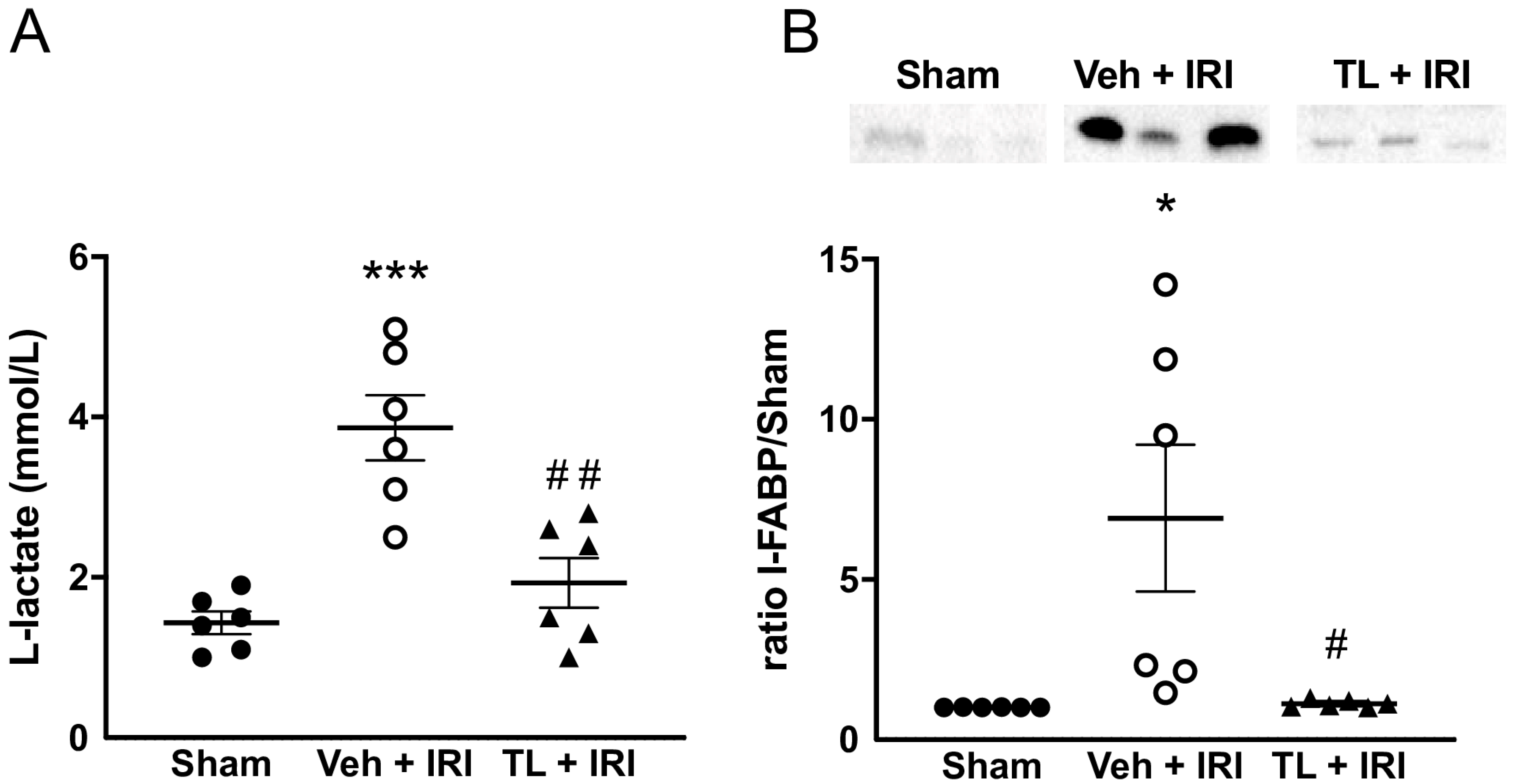
3.2.1. TL Pretreatment Reduced IRI Induced Epithelial Damage
3.2.2. TL Pretreatment Reduced IRI Induced Intestinal Wall Damage
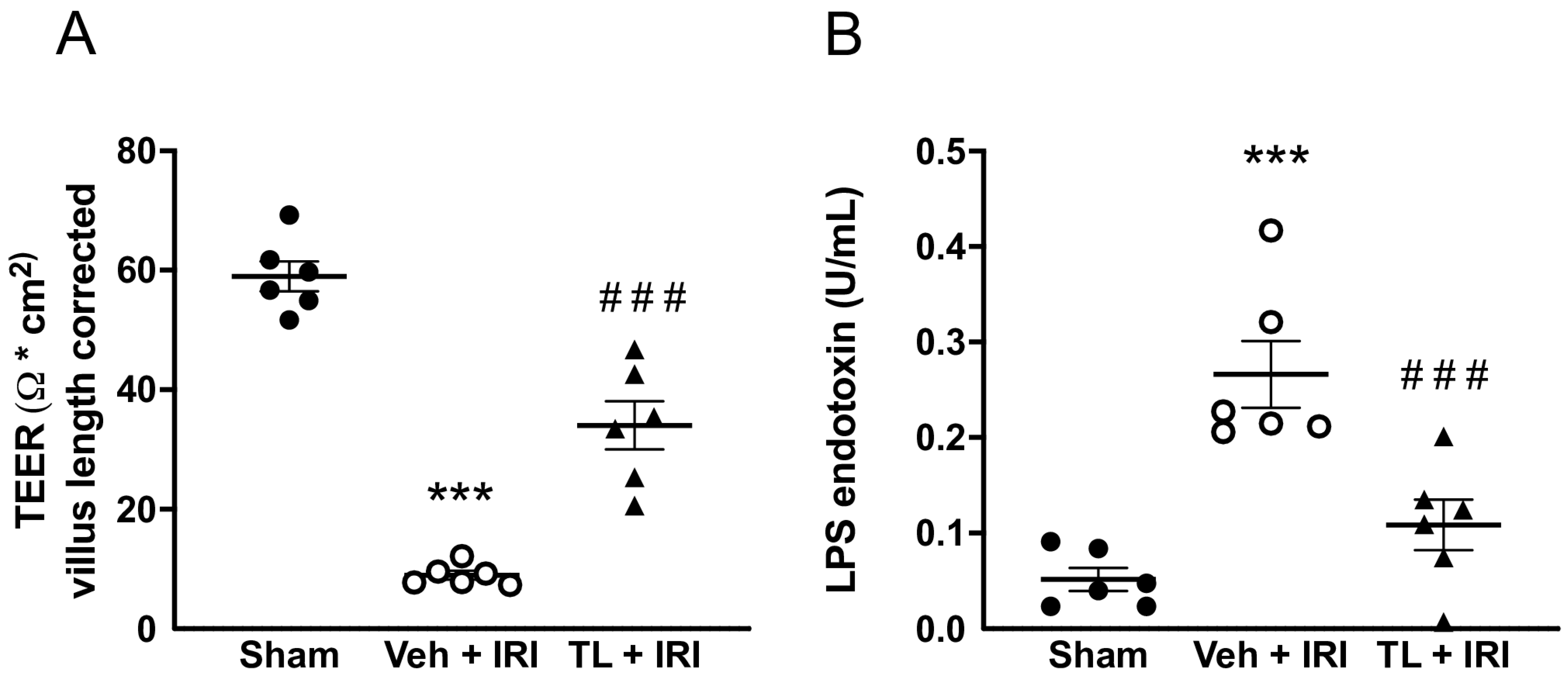
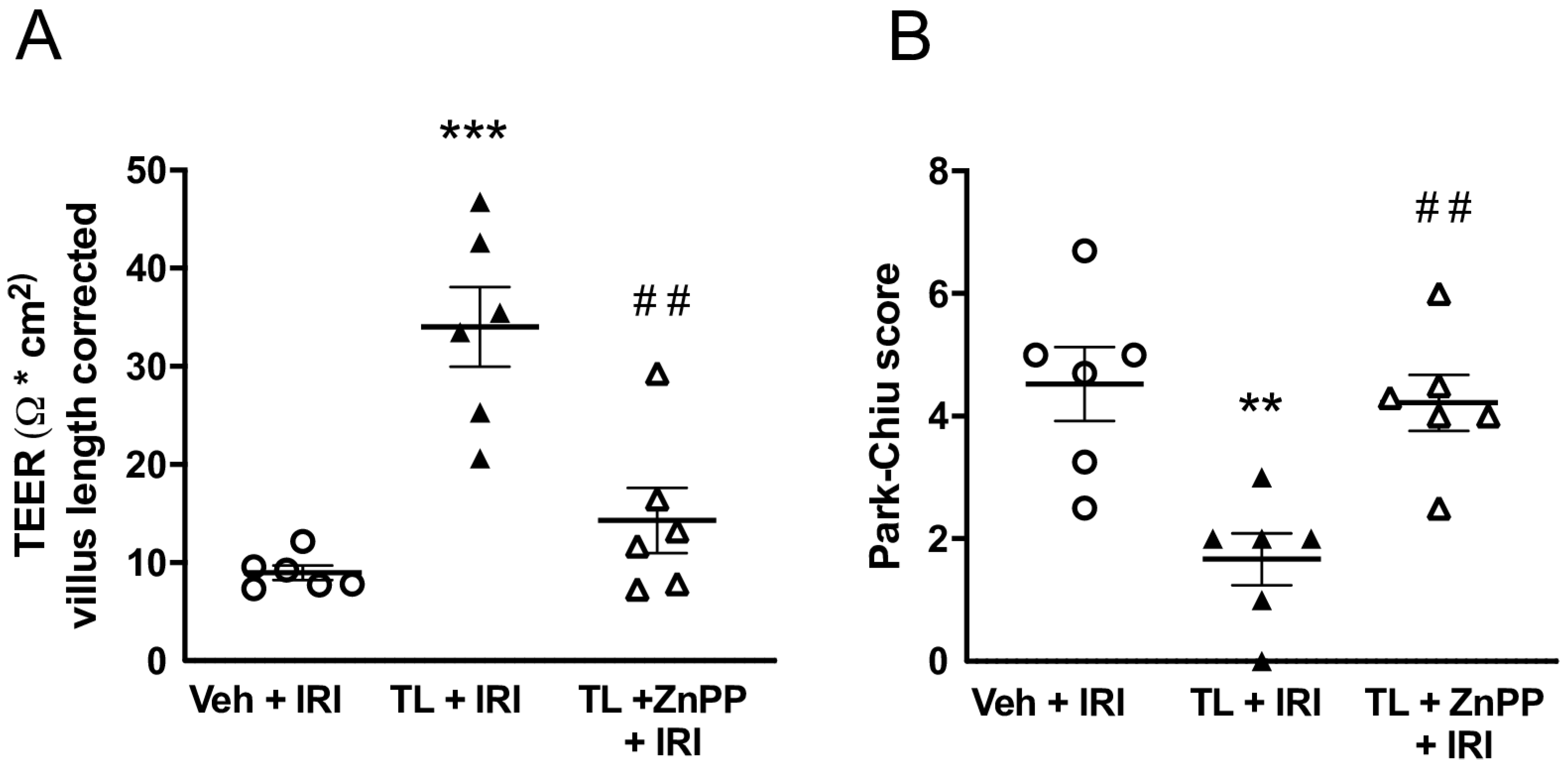
3.2.3. TL Pretreatment Reduced Epithelial Permeability of the Intestine
3.2.4. TL Pretreatment Reduced Pro-Inflammatory and Increased Anti-Inflammatory Cytokines
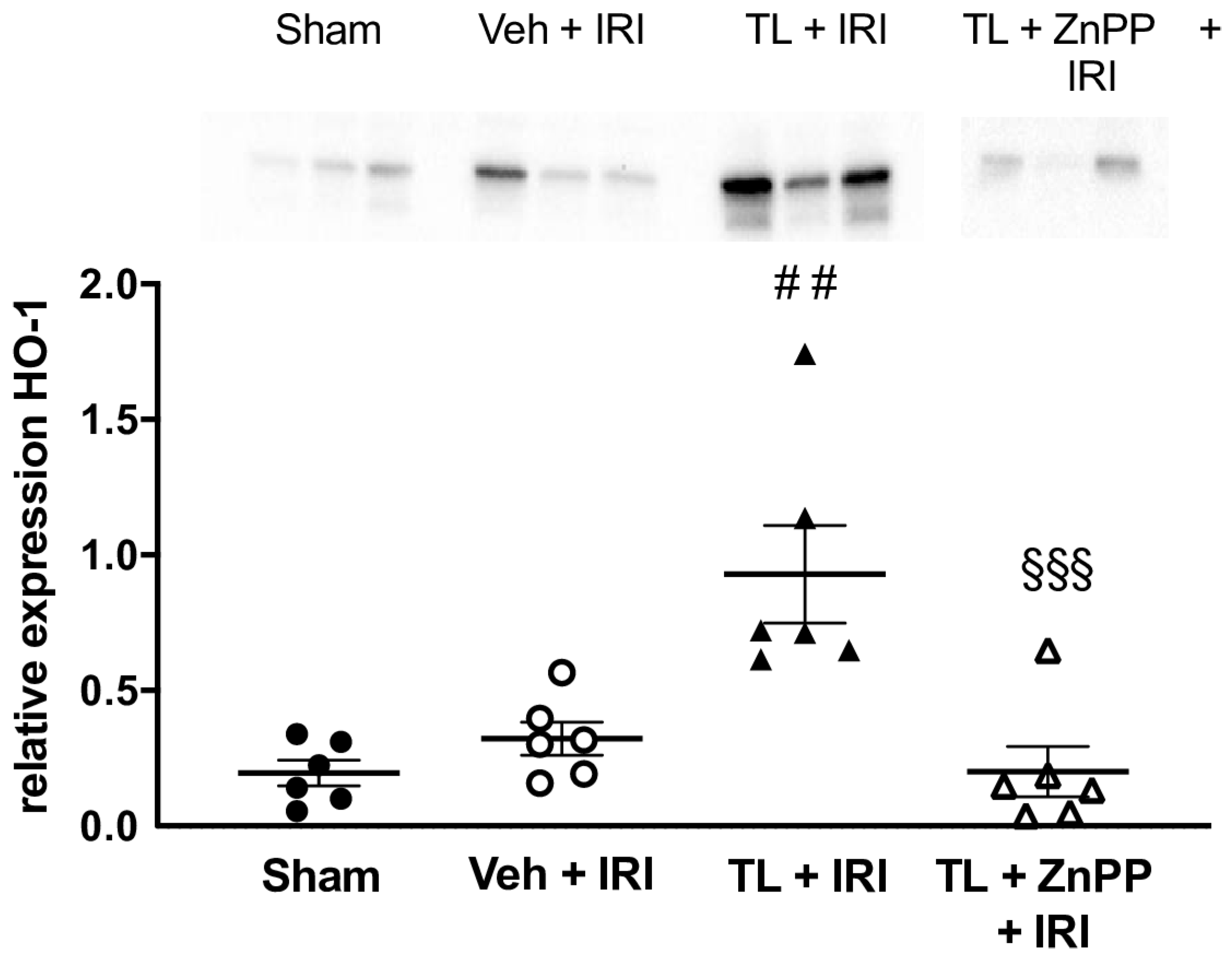
3.2.5. TL Led to Upregulation of HO-1 in the Ileal Tissue
3.3. Experiment 3: Effect of HO-1 Inhibition
ZnPP Abolishes the TL-Induced Upregulation of HO-1
3.4. Inhibiting HO-1 Upregulation aBolished the Protective Effect of TL Pretreatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CO | carbon monoxide |
| HO-1 | heme oxygenase-1 |
| I-FABP | intestinal fatty acid-binding protein |
| IFN-γ | interferon-γ |
| IL | interleukin |
| IRI | ischemia reperfusion injury |
| ITx | intestinal transplantation |
| LPS | lipopolysaccharide |
| qRT-PCR | quantitative reverse-transcription polymerase chain reaction |
| ROS | reactive oxygen species |
| T-regs | regulatory T cells |
| TEER | trans-epithelial electrical resistance |
| TL | tranilast |
| TNF-α | tumor necrosis factor-α |
| WB | western blot |
| ZnPP | zinc protoporphyrin |
References
- Duggan, C.; Gannon, J.; Walker, W.A. Protective nutrients and functional foods for the gastrointestinal tract. Am. J. Clin. Nutr. 2002, 75, 789–808. [Google Scholar] [CrossRef]
- Arrieta, M.C. Alterations in intestinal permeability. Gut 2006, 55, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Mallick, I.H.; Yang, W.; Winslet, M.C.; Seifalian, A.M. Ischemia–Reperfusion Injury of the Intestine and Protective Strategies Against Injury. Dig. Dis. Sci. 2004, 49, 1359–1377. [Google Scholar] [CrossRef]
- Acosta, S.; Ögren, M.; Sternby, N.H.; Bergqvist, D.; Björck, M. Clinical implications for the management of acute thromboembolic occlusion of the superior mesenteric artery: Autopsy findings in 213 patients. Ann. Surg. 2005, 241, 516–522. [Google Scholar] [CrossRef]
- Acosta, S. Epidemiology of mesenteric vascular disease: Clinical implications. Semin. Vasc. Surg. 2010, 23, 4–8. [Google Scholar] [CrossRef]
- Howard, T.J.; Plaskon, L.A.; Wiebke, E.A.; Wilcox, M.G.; Madura, J.A. Nonocclusive mesenteric ischemia remains a diagnostic dilemma. Am. J. Surg. 1996, 171, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.; Ögren, M.; Sternby, N.H.; Bergqvist, D.; Björck, M. Fatal nonocclusive mesenteric ischaemia: Population-based incidence and risk factors. J. Intern. Med. 2006, 259, 305–313. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Schoots, I.G.; Koffeman, G.I.; Legemate, D.A.; Levi, M.; Van Gulik, T.M. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br. J. Surg. 2004, 91, 17–27. [Google Scholar] [CrossRef]
- Björck, M.; Koelemay, M.; Acosta, S.; Goncalves, F.B.; Kölbel, T.; Kolkman, J.; Lees, T.; Lefevre, J.; Menyhei, G.; Oderich, G.; et al. Editor’s Choice—Management of the Diseases of Mesenteric Arteries and Veins. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 460–510. [Google Scholar] [CrossRef]
- Roussel, A.; Castier, Y.; Nuzzo, A.; Pellenc, Q.; Sibert, A.; Panis, Y.; Bouhnik, Y.; Corcos, O. Revascularization of acute mesenteric ischemia after creation of a dedicated multidisciplinary center. J. Vasc. Surg. 2015, 62, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.E.; Blennerhassett, L.R.; Heel, K.A.; McCauley, R.D.; Hall, J.C. Ischaemia-reperfusion injury to the intestine. Aust. N. Z. J. Surg. 1998, 68, 554–561. [Google Scholar] [CrossRef]
- Terada, Y.; Ogura, J.; Tsujimoto, T.; Kuwayama, K.; Koizumi, T.; Sasaki, S.; Maruyama, H.; Kobayashi, M.; Yamaguchi, H.; Iseki, K. Intestinal P-glycoprotein Expression is Multimodally Regulated by Intestinal Ischemia-Reperfusion. J. Pharm. Pharm. Sci. 2014, 17, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Andrés Juan, C.; Manuel Pérez de la Lastra, J.; Plou, F.J.; Pérez-Lebeña, E.; Reinbothe, S. Molecular Sciences The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Ulusoy Tangul, S.; Onat, T.; Aydoğan Kirmizi, D.; Doganyigit, Z.; Kaymak, E.; Oflamaz, A.; Şenayli, A.; Somuncu, S. Effect of bromelain on ischemia-reperfusion injury in the torsion model created in polycystic and normal ovarian tissues. Front. Pharmacol. 2024, 15, 1451592. [Google Scholar] [CrossRef]
- Meštrović, J.; Drmić-Hofman, I.; Pogorelić, Z.; Vilović, K.; Šupe-Domić, D.; Šešelja-Perišin, A.; Čapkun, V. Beneficial effect of nifedipine on testicular torsion-detorsion injury in rats. Urology 2014, 84, 1194–1198. [Google Scholar] [CrossRef]
- Grant, D.; Abu-Elmagd, K.; Mazariegos, G.; Vianna, R.; Langnas, A.; Mangus, R.; Farmer, D.G.; Lacaille, F.; Iyer, K.; Fishbein, T. Intestinal Transplant Registry Report: Global Activity and Trends. Am. J. Transplant. 2015, 15, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Grootjans, J.; Lenaerts, K.; Derikx, J.P.; Matthijsen, R.A.; de Bruïne, A.P.; van Bijnen, A.A.; van Dam, R.M.; Dejong, C.H.; Buurman, W.A. Human intestinal ischemia-reperfusion-induced inflammation characterized: Experiences from a new translational model. Am. J. Pathol. 2010, 176, 2283–2291. [Google Scholar] [CrossRef]
- Lenaerts, K.; Ceulemans, L.J.; Hundscheid, I.H.R.; Grootjans, J.; Dejong, C.H.C.; Olde Damink, S.W.M. New insights in intestinal ischemia–reperfusion injury. Curr. Opin. Organ. Transplant. 2013, 18, 298–303. [Google Scholar] [CrossRef]
- Darakhshan, S.; Pour, A.B. Tranilast: A review of its therapeutic applications. Pharmacol. Res. 2015, 91, 15–28. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef]
- Pae, H.O.; Jeong, S.O.; Koo, B.S.; Ha, H.Y.; Lee, K.M.; Chung, H.T. Tranilast, an orally active anti-allergic drug, up-regulates the anti-inflammatory heme oxygenase-1 expression but down-regulates the pro-inflammatory cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW264.7 macrophages. Biochem. Biophys. Res. Commun. 2008, 371, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.; Zhang, Y.; Cox, A.J.; Kelly, D.J.; Qi, W. Tranilast attenuates the up-regulation of thioredoxin-interacting protein and oxidative stress in an experimental model of diabetic nephropathy. Nephrol. Dial. Transplant. 2011, 26, 100–110. [Google Scholar] [CrossRef]
- Sener, G.; Akgün, U.; Satiroğlu, H.; Topaloğlu, U.; Keyer-Uysal, M. The effect of pentoxifylline on intestinal ischemia/reperfusion injury. Fundam. Clin. Pharmacol. 2001, 15, 19–22. [Google Scholar] [CrossRef]
- Pot, C.; Apetoh, L.; Kuchroo, V.K. Type 1 regulatory T cells (Tr1) in autoimmunity. Semin. Immunol. 2011, 23, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Shao, K.; Wang, X.H. Effects of Tranilast on renal structure and function in mice with ischemic reperfusion injury. J. Shanghai Jiaotong Univ. (Med. Sci.) 2012, 32, 446–451. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhuo, J. Tranilast Treatment Attenuates Cerebral Ischemia-Reperfusion Injury in Rats Through the Inhibition of Inflammatory Responses Mediated by NF-κB and PPARs. Clin. Transl. Sci. 2019, 12, 196–202. [Google Scholar] [CrossRef]
- Ceulemans, L.J.; Verbeke, L.; Decuypere, J.-P.; Farré, R.; De Hertogh, G.; Lenaerts, K.; Jochmans, I.; Monbaliu, D.; Nevens, F.; Tack, J.; et al. Farnesoid x receptor activation attenuates intestinal ischemia reperfusion injury in rats. PLoS ONE 2017, 12, e0169331. [Google Scholar] [CrossRef]
- Clarysse, M.; Accarie, A.; Panisello-Roselló, A.; Farré, R.; Canovai, E.; Monbaliu, D.; De Hertogh, G.; Vanuytsel, T.; Pirenne, J.; Ceulemans, L.J. Intravenous Polyethylene Glycol Alleviates Intestinal Ischemia-Reperfusion Injury in a Rodent Model. Int. J. Mol. Sci. 2023, 24, 10775. [Google Scholar] [CrossRef]
- Ménoret, S.; Bézie, S.; Li, X.L.; Usal, C.; Caron, L.; Anegon, I. Tranilast, an analogue of tryptophan catabolites, induces allograft tolerance by CD161+ cells. J. Transl. Med. 2011, 9 (Suppl. S2), P27. [Google Scholar] [CrossRef]
- Xia, Z.Y.; Gao, J.; Ancharaz, A.K. Protective effect of ischemic postconditioning on lung ischemia-reperfusion injury in rats and the role of heme oxygenase-1. Chin. J. Traumatol.—Engl. Ed. 2009, 12, 162–166. [Google Scholar] [CrossRef]
- Clarke, L.L. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1151–G1166. [Google Scholar] [CrossRef]
- Thomson, A.; Smart, K.; Somerville, M.S.; Lauder, S.N.; Appanna, G.; Horwood, J.; Raj, L.S.; Srivastava, B.; Durai, D.; Scurr, M.J.; et al. The Ussing chamber system for measuring intestinal permeability in health and disease. BMC Gastroenterol. 2019, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Thuijls, G.; van Wijck, K.; Grootjans, J.; Derikx, J.P.; van Bijnen, A.A.; Heineman, E.; Dejong, C.H.; Buurman, W.A.; Poeze, M. Early Diagnosis of Intestinal Ischemia Using Urinary and Plasma Fatty Acid Binding Proteins. Ann. Surg. 2011, 253, 303–308. [Google Scholar] [CrossRef]
- Park, P.O.; Haglund, U.; Bulkley, G.B.; Fält, K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery 1990, 107, 574–580. [Google Scholar] [PubMed]
- Farré, R.; Vicario, M. Abnormal barrier function in gastrointestinal disorders. Handb. Exp. Pharmacol. 2017, 239, 193–217. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef]
- Kärkkäinen, J.M.; Lehtimäki, T.T.; Manninen, H.; Paajanen, H. Acute Mesenteric Ischemia Is a More Common Cause than Expected of Acute Abdomen in the Elderly. J. Gastrointest. Surg. 2015, 19, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Nadatani, Y.; Watanabe, T.; Shimada, S.; Otani, K.; Tanigawa, T.; Fujiwara, Y. Microbiome and intestinal ischemia/reperfusion injury. J. Clin. Biochem. Nutr. 2018, 63, 26–32. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Fan, Z. Oxidative Stress in Intestinal Ischemia-Reperfusion. Front. Med. 2022, 8, 750731. [Google Scholar] [CrossRef]
- Koda, A.; Nagai, H.; Watanabe, S.; Yanagihara, Y.; Sakamoto, K. Inhibition of hypersensitivity reactions by a new drug, N(3′,4′-dimethoxycinnamoyl) anthranilic acid (N-5′). J. Allergy Clin. Immunol. 1976, 57, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.M.; Jiga, L.P.; Chuang, J.J.J.; Randazzo, M.; Opelz, G.; Terness, P. Studying the immunosuppressive role of indoleamine 2,3-dioxygenase: Tryptophan metabolites suppress rat allogeneic T-cell responses in vitro and in vivo. Transpl. Int. 2005, 18, 95–100. [Google Scholar] [CrossRef]
- Azuma, H.; Banno, K.; Yoshimura, T. Pharmacological properties of N-(3′,4′-dimethoxycinnamoyl) anthranilic acid (N-5′), a new anti-atopic agent. Br. J. Pharmacol. 1976, 58, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Shiota, N.; Kovanen, P.; Eklund, K.; Shibata, N.; Shimoura, K.; Niibayashi, T.; Shimbori, C.; Okunishi, H. The anti-allergic compound tranilast attenuates inflammation and inhibits bone destruction in collagen-induced arthritis in mice. Br. J. Pharmacol. 2010, 159, 626–635. [Google Scholar] [CrossRef]
- Oshitani, N.; Yamagami, H.; Watanabe, K.; Higuchi, K.; Arakawa, T. Long-term prospective pilot study with tranilast for the prevention of stricture progression in patients with Crohn’s disease. Gut 2007, 56, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Cadetg, P.; Ruf, R.; Mazzucchelli, L.; Ferrari, P.; Redaelli, C.A. Heme oxygenase-1 attenuates ischemia/reperfusion-induced apoptosis and improves survival in rat renal allografts. Kidney Int. 2003, 63, 1564–1573. [Google Scholar] [CrossRef]
- Liu, X.; Wei, J.; Peng, D.H.; Layne, M.D.; Yet, S.F. Absence of heme oxygenase-1 exacerbates myocardial ischemia/reperfusion injury in diabetic mice. Diabetes 2005, 54, 778–784. [Google Scholar] [CrossRef]
- Ryter, S.W.; Alam, J.; Choi, A.M.K. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Lu, H.T.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef]
- Cheng, Y.; Rong, J. Therapeutic Potential of Heme Oxygenase-1/carbon Monoxide System Against Ischemia-Reperfusion Injury. Curr. Pharm. Des. 2017, 23, 3884–3898. [Google Scholar] [CrossRef]
- Chung, S.W.; Liu, X.; Macias, A.A.; Baron, R.M.; Perrella, M.A. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J. Clin. Investig. 2008, 118, 239–247. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.-J.; Coronata, A.A.; Fredenburgh, L.E.; Chung, S.W.; Perrella, M.A.; Nakahira, K.; Ryter, S.W.; Choi, A.M. Carbon Monoxide Confers Protection in Sepsis by Enhancing Beclin 1-Dependent Autophagy and Phagocytosis. Antioxid. Redox Signal. 2014, 20, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; May, A.; Chin, B.Y. Carbon monoxide increases macrophage bacterial clearance through Toll-like receptor (TLR)4 expression. Cell. Mol. Biol. 2005, 51, 433–440. [Google Scholar] [PubMed]
- Andoh, A.; Kimura, T.; Fukuda, M.; Araki, Y.; Fujiyama, Y.; Bamba, T. Rapid intestinal ischaemia-reperfusion injury is suppressed in genetically mast cell-deficient Ws/Ws rats. Clin. Exp. Immunol. 1999, 116, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, R.; Murakami, M.; Kajimura, M.; Goda, N.; Makino, N.; Takamiya, Y.; Yamaguchi, T.; Ishimura, Y.; Hozumi, N.; Suematsu, M. Stabilization of mast cells by heme oxygenase-1: An anti-inflammatory role. Am. J. Physiol. Circ. Physiol. 2002, 283, H861–H870. [Google Scholar] [CrossRef]
- Jeong, S.; Kang, C.; Park, S.; Ju, S.; Yoo, J.-W.; Yoon, I.-S.; Yun, H.; Jung, Y. Eletrophilic chemistry of tranilast is involved in its anti-colitic activity via nrf2-ho-1 pathway activation. Pharmaceuticals 2021, 14, 1092. [Google Scholar] [CrossRef]
- Attuwaybi, B.; Kozar, R.; Moore-Olufemi, S.; Sato, N.; Hassoun, H.; Weisbrodt, N.; Moore, F. Heme oxygenase-1 induction by hemin protects against gut ischemia/reperfusion injury. J. Surg. Res. 2004, 118, 53–57. [Google Scholar] [CrossRef]
- Wasserberg, N.; Pileggi, A.; Salgar, S.K.; Ruiz, P.; Ricordi, C.; Inverardi, L.; Tzakis, A.G. Heme oxygenase-1 upregulation protects against intestinal ischemia/reperfusion injury: A laboratory based study. Int. J. Surg. 2007, 5, 216–224. [Google Scholar] [CrossRef]
- Kusama, H.; Kikuchi, S.; Tazawa, S.; Katsuno, K.; Baba, Y.; Zhai, Y.-L.; Nikaido, T.; Fujii, S. Tranilast inhibits the proliferation of human coronary smooth muscle cell through the activation of p21waf1. Atherosclerosis 1999, 143, 307–313. [Google Scholar] [CrossRef]
- Kawabata, Y.; Yamamoto, K.; Debari, K.; Onoue, S.; Yamada, S. Novel crystalline solid dispersion of tranilast with high photostability and improved oral bioavailability. Eur. J. Pharm. Sci. 2010, 39, 256–262. [Google Scholar] [CrossRef]
- Corcos, O.; Castier, Y.; Sibert, A.; Gaujoux, S.; Ronot, M.; Joly, F.; Paugam, C.; Bretagnol, F.; Abdel–Rehim, M.; Francis, F.; et al. Effects of a Multimodal Management Strategy for Acute Mesenteric Ischemia on Survival and Intestinal Failure. Clin. Gastroenterol. Hepatol. 2013, 11, 158–165.e2. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.M.; Tryphonopoulos, P.; DeFaria, W.; Ruiz, P.; Miller, J.; Barrett, T.A.; Tzakis, A.G.; Kato, T. Role of innate and acquired immune mechanisms in clinical intestinal transplant rejection. Transplantation 2015, 99, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, L.J.; Braza, F.; Monbaliu, D.; Jochmans, I.; De Hertogh, G.; Du Plessis, J.; Emonds, M.; Kitade, H.; Kawai, M.; Li, Y.; et al. The Leuven Immunomodulatory Protocol Promotes T-Regulatory Cells and Substantially Prolongs Survival After First Intestinal Transplantation. Am. J. Transplant. 2016, 16, 2973–2985. [Google Scholar] [CrossRef]
- Zaher, S.S.; Coe, D.; Chai, J.G.; Larkin, D.F.P.; George, A.J.T. Suppression of the allogeneic response by the anti-allergy drug N-(3,4-dimethoxycinnamonyl) anthranilic acid results from T-cell cycle arrest. Immunology 2013, 138, 157–164. [Google Scholar] [CrossRef]
- Prud’Homme, G.J.; Glinka, Y.; Toulina, A.; Ace, O.; Subramaniam, V.; Jothy, S. Breast cancer stem-like cells are inhibited by a non-toxic aryl hydrocarbon receptor agonist. PLoS ONE 2010, 5, e13831. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Qiu, J.; Bostick, J.W.; Ueda, A.; Schjerven, H.; Li, S.; Jobin, C.; Chen, Z.-M.E.; Zhou, L. The Aryl Hydrocarbon Receptor Preferentially Marks and Promotes Gut Regulatory T Cells. Cell Rep. 2017, 21, 2277–2290. [Google Scholar] [CrossRef]
- Sun, Q.-F.; Ding, J.-G.; Sheng, J.-F.; Zhu, M.-H.; Li, J.-J.; Sheng, Z.-K.; Tang, X.-F. Novel action of 3,4-DAA ameliorating acute liver allograft injury. Cell Biochem. Funct. 2011, 29, 673–678. [Google Scholar] [CrossRef]
- Quaedackers, J.; Beuk, R.; Bennet, L.; Charlton, A.; Egbrink, M.O.; Gunn, A.; Heineman, E. An Evaluation of Methods for Grading Histologic Injury Following Ischemia/Reperfusion of the Small Bowel. Transplant. Proc. 2000, 32, 1307–1310. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canovai, E.; Farré, R.; De Hertogh, G.; Dubois, A.; Vanuytsel, T.; Pirenne, J.; Ceulemans, L.J. Tranilast Reduces Intestinal Ischemia Reperfusion Injury in Rats Through the Upregulation of Heme-Oxygenase (HO)-1. J. Clin. Med. 2025, 14, 3254. https://doi.org/10.3390/jcm14093254
Canovai E, Farré R, De Hertogh G, Dubois A, Vanuytsel T, Pirenne J, Ceulemans LJ. Tranilast Reduces Intestinal Ischemia Reperfusion Injury in Rats Through the Upregulation of Heme-Oxygenase (HO)-1. Journal of Clinical Medicine. 2025; 14(9):3254. https://doi.org/10.3390/jcm14093254
Chicago/Turabian StyleCanovai, Emilio, Ricard Farré, Gert De Hertogh, Antoine Dubois, Tim Vanuytsel, Jacques Pirenne, and Laurens J. Ceulemans. 2025. "Tranilast Reduces Intestinal Ischemia Reperfusion Injury in Rats Through the Upregulation of Heme-Oxygenase (HO)-1" Journal of Clinical Medicine 14, no. 9: 3254. https://doi.org/10.3390/jcm14093254
APA StyleCanovai, E., Farré, R., De Hertogh, G., Dubois, A., Vanuytsel, T., Pirenne, J., & Ceulemans, L. J. (2025). Tranilast Reduces Intestinal Ischemia Reperfusion Injury in Rats Through the Upregulation of Heme-Oxygenase (HO)-1. Journal of Clinical Medicine, 14(9), 3254. https://doi.org/10.3390/jcm14093254





