Prevalence and Risk Factors of Metabolic-Associated Fatty Liver Disease in Children with Down Syndrome at a Tertiary Care Center
Abstract
1. Introduction
2. Methods
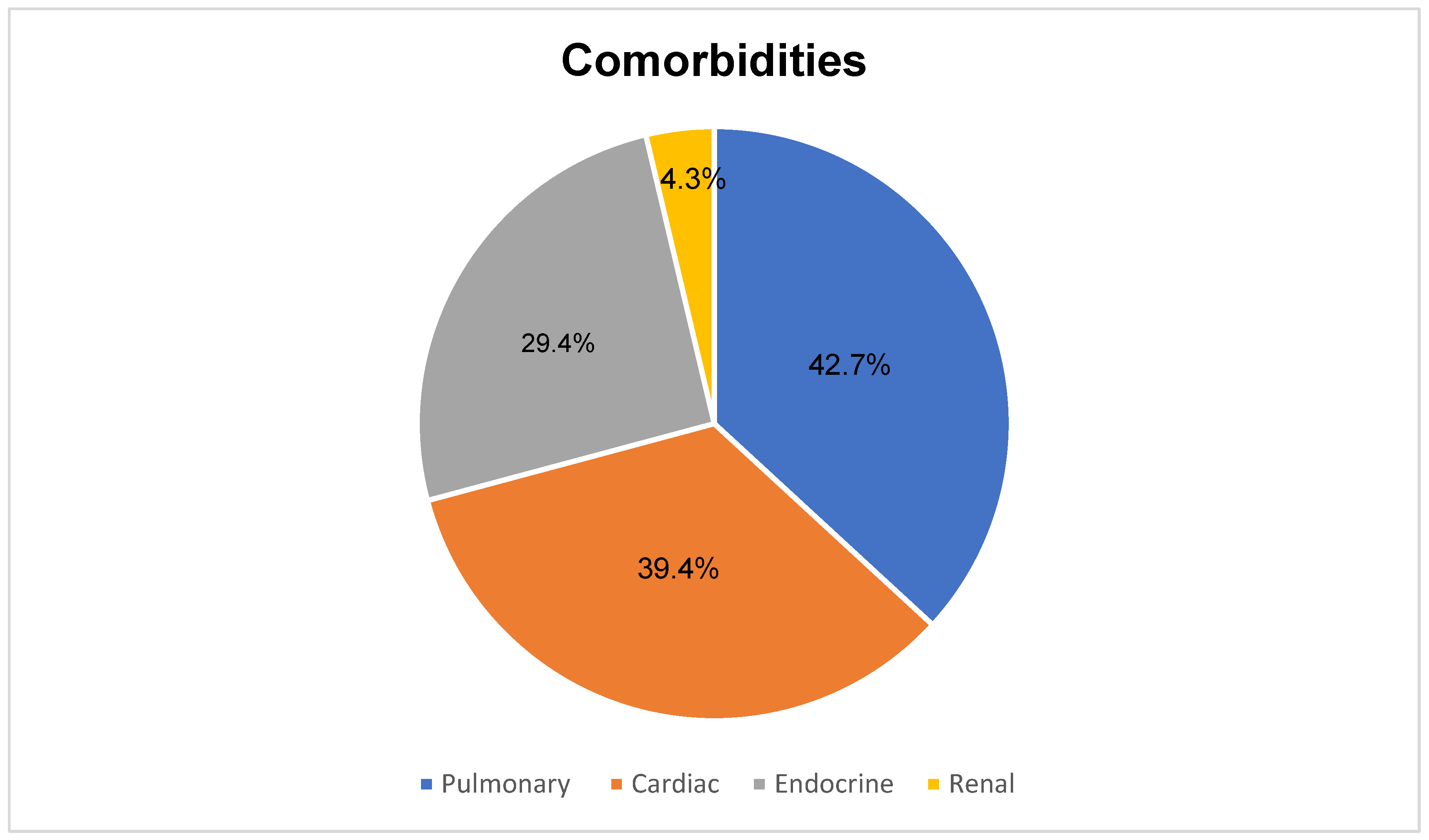
3. Results
3.1. Laboratory Findings
3.2. Comparative Analysis and Predictors of MAFLD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
References
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 319–334. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Eslam, M.; Alkhouri, N.; Vajro, P.; Baumann, U.; Weiss, R.; Socha, P.; Marcus, C.; Lee, W.S.; Kelly, D.; Porta, G.; et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: An international expert consensus statement. Lancet Gastroenterol. Hepatol. 2021, 6, 864–873. [Google Scholar] [CrossRef]
- Zhang, L.; El-Shabrawi, M.; Baur, L.A.; Byrne, C.D.; Targher, G.; Kehar, M.; Porta, G.; Lee, W.S.; Lefere, S.; Turan, S.; et al. An international multidisciplinary consensus on pediatric metabolic dysfunction-associated fatty liver disease. Med 2024, 5, 797–815. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Pierce, M.; Ramsey, K.; Pinter, J. Trends in Obesity and Overweight in Oregon Children With Down Syndrome. Glob. Pediatr. Health 2019, 6, 2333794X19835640. [Google Scholar] [CrossRef]
- Hawli, Y.; Nasrallah, M.; El-Hajj Fuleihan, G. Endocrine and musculoskeletal abnormalities in patients with Down syndrome. Nat. Rev. Endocrinol. 2009, 5, 327–334. [Google Scholar] [CrossRef]
- Foerste, T.; Sabin, M.; Reid, S.; Reddihough, D. Understanding the causes of obesity in children with trisomy 21: Hyperphagia vs physical inactivity. J. Intellect. Disabil. Res. 2016, 60, 856–864. [Google Scholar] [CrossRef]
- Seeff, L.B.; Levitsky, J.; Tillman, P.W.; Perou, M.L.; Zimmerman, H.J. Histopathology of the liver in Down’s syndrome. Am. J. Dig. Dis. 1967, 12, 1102–1113. [Google Scholar] [CrossRef]
- Valentini, D.; Alisi, A.; di Camillo, C.; Sartorelli, M.R.; Crudele, A.; Bartuli, A.; Nobili, V.; Villani, A. Nonalcoholic Fatty Liver Disease in Italian Children with Down Syndrome: Prevalence and Correlation with Obesity-Related Features. J. Pediatr. 2017, 189, 92–97.e1. [Google Scholar] [CrossRef]
- Valentini, D.; Mosca, A.; Di Camillo, C.; Crudele, A.; Sartorelli, M.R.; Scoppola, V.; Tarani, L.; Villani, A.; Raponi, M.; Novelli, A.; et al. PNPLA3 gene polymorphism is associated with liver steatosis in children with Down syndrome. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1564–1572. [Google Scholar] [CrossRef]
- Nobili, V.; Alisi, A.; Valenti, L.; Miele, L.; Feldstein, A.E.; Alkhouri, N. NAFLD in children: New genes, new diagnostic modalities and new drugs. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 517–530. [Google Scholar] [CrossRef]
- Toms, R.; Bonney, A.; Mayne, D.J.; Feng, X.; Walsan, R. Geographic and area-level socioeconomic variation in cardiometabolic risk factor distribution: A systematic review of the literature. Int. J. Health Geogr. 2019, 18, 1. [Google Scholar] [CrossRef]
- Khoury, M.; Bigras, J.L.; Cummings, E.A.; Harris, K.C.; Hegele, R.A.; Henderson, M.; Morrison, K.M.; St-Pierre, J.; Wong, P.D.; McCrindle, B.W. The Detection, Evaluation, and Management of Dyslipidemia in Children and Adolescents: A Canadian Cardiovascular Society/Canadian Pediatric Cardiology Association Clinical Practice Update. Can. J. Cardiol. 2022, 38, 1168–1179. [Google Scholar] [CrossRef]
- Garcia Cuartero, B.; Garcia Lacalle, C.; Jimenez Lobo, C.; Gonzalez Vergaz, A.; Calvo Rey, C.; Alcazar Villar, M.J.; Diaz Martinez, E. The HOMA and QUICKI indexes, and insulin and C-peptide levels in healthy children. Cut off points to identify metabolic syndrome in healthy children. An. Pediatr. 2007, 66, 481–490. [Google Scholar] [CrossRef]
- Keskin, M.; Kurtoglu, S.; Kendirci, M.; Atabek, M.E.; Yazici, C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 2005, 115, e500–e503. [Google Scholar] [CrossRef]
- Corsi, M.M.; Dogliotti, G.; Pedroni, F.; Galliera, E.; Malavazos, A.E.; Villa, R.; Chiappelli, M.; Licastro, F. Adipocytokines in Down’s syndrome, an atheroma-free model: Role of adiponectin. Arch. Gerontol. Geriatr. 2009, 48, 106–109. [Google Scholar] [CrossRef]
- Muchova, J.; Zitnanova, I.; Durackova, Z. Oxidative stress and Down syndrome. Do antioxidants play a role in therapy? Physiol. Res. 2014, 63, 535–542. [Google Scholar] [CrossRef]
- Hong, T.; Chen, Y.; Li, X.; Lu, Y. The Role and Mechanism of Oxidative Stress and Nuclear Receptors in the Development of NAFLD. Oxid. Med. Cell Longev. 2021, 2021, 6889533. [Google Scholar] [CrossRef]
- Anderson, E.L.; Howe, L.D.; Jones, H.E.; Higgins, J.P.; Lawlor, D.A.; Fraser, A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0140908. [Google Scholar] [CrossRef]
- Pecoraro, L.; Ferron, E.; Solfa, M.; Mirandola, M.; Lauriola, S.; Piacentini, G.; Pietrobelli, A. Body composition and laboratory parameters in children with down syndrome: The DONUT study. Clin. Nutr. ESPEN 2023, 57, 253–257. [Google Scholar] [CrossRef]
- Oreskovic, N.M.; Baumer, N.T.; Di Camillo, C.; Cornachia, M.; Franklin, C.; Hart, S.J.; Kishnani, P.S.; McCormick, A.; Milliken, A.L.; Patsiogiannis, V.; et al. Cardiometabolic profiles in children and adults with overweight and obesity and down syndrome. Am. J. Med. Genet. A 2023, 191, 813–822. [Google Scholar] [CrossRef]
- Startin, C.M.; D’Souza, H.; Ball, G.; Hamburg, S.; Hithersay, R.; Hughes, K.M.O.; Massand, E.; Karmiloff-Smith, A.; Thomas, M.S.C.; LonDown, S.C.; et al. Health comorbidities and cognitive abilities across the lifespan in Down syndrome. J. Neurodev. Disord. 2020, 12, 4. [Google Scholar] [CrossRef]
- Diefendorf, A.O.; Bull, M.J.; Casey-Harvey, D.; Miyamoto, R.T.; Pope, M.L.; Renshaw, J.J.; Schreiner, R.L.; Wagner-Escobar, M. Down syndrome: A multidisciplinary perspective. J. Am. Acad. Audiol. 1995, 6, 39–46. [Google Scholar]
- Hetman, M.; Mielko, K.; Placzkowska, S.; Bodetko, A.; Mlynarz, P.; Barg, E. Predisposition to atherosclerosis in children and adults with trisomy 21: Biochemical and metabolomic studies. Pediatr. Endocrinol. Diabetes Metab. 2023, 29, 143–155. [Google Scholar] [CrossRef]
- Adelekan, T.; Magge, S.; Shults, J.; Stallings, V.; Stettler, N. Lipid profiles of children with Down syndrome compared with their siblings. Pediatrics 2012, 129, e1382–e1387. [Google Scholar] [CrossRef]
- Nur Zati Iwani, A.K.; Jalaludin, M.Y.; Wan Mohd Zin, R.M.; Fuziah, M.Z.; Hong, J.Y.H.; Abqariyah, Y.; Mokhtar, A.H.; Wan Mohamud, W.N. TG: HDL-C Ratio Is a Good Marker to Identify Children Affected by Obesity with Increased Cardiometabolic Risk and Insulin Resistance. Int. J. Endocrinol. 2019, 2019, 8586167. [Google Scholar] [CrossRef]
- Zhu, J.L.; Hasle, H.; Correa, A.; Schendel, D.; Friedman, J.M.; Olsen, J.; Rasmussen, S.A. Survival among people with Down syndrome: A nationwide population-based study in Denmark. Genet. Med. 2013, 15, 64–69. [Google Scholar] [CrossRef]
- Konyn, P.; Ahmed, A.; Kim, D. Causes and risk profiles of mortality among individuals with nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S43–S57. [Google Scholar] [CrossRef]
- Muramatsu, M.; Osawa, T.; Miyamura, Y.; Nakagawa, S.; Tanaka, T.; Kodama, T.; Aburatani, H.; Sakai, J.; Ryeom, S.; Minami, T. Loss of Down syndrome critical region-1 leads to cholesterol metabolic dysfunction that exaggerates hypercholesterolemia in ApoE-null background. J. Biol. Chem. 2021, 296, 100697. [Google Scholar] [CrossRef]
- Giallongo, S.; Ferrigno, J.; Caltabiano, R.; Broggi, G.; Alanazi, A.M.; Distefano, A.; Tropea, E.; Tramutola, A.; Perluigi, M.; Volti, G.L.; et al. Aging exacerbates oxidative stress and liver fibrosis in an animal model of Down Syndrome. Aging 2024, 16, 10203–10215. [Google Scholar] [CrossRef] [PubMed]
- Mircher, C.; Briceno, L.G.; Toulas, J.; Conte, M.; Tanguy, M.L.; Cieuta-Walti, C.; Rethore, M.O.; Ravel, A. Growth curves for French people with Down syndrome from birth to 20 years of age. Am. J. Med. Genet. A 2018, 176, 2685–2694. [Google Scholar] [CrossRef]
- Xanthopoulos, M.S.; Walega, R.; Xiao, R.; Pipan, M.E.; Cochrane, C.I.; Zemel, B.S.; Kelly, A.; Magge, S.N. Physical Activity in Youth with Down Syndrome and Its Relationship with Adiposity. J. Dev. Behav. Pediatr. 2023, 44, e436–e443. [Google Scholar] [CrossRef] [PubMed]
- Souto, D.O.; de Sousa, M.O.; Ferreira, R.G.; Brandao, A.C.; Carrera, P.B.; Leite, H.R. What are the barriers and facilitators to participation of people with Down syndrome? A scoping review. Dev. Med. Child. Neurol. 2024, 66, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Schultz, E.E.; Sergi, K.; Twietmeyer, G.; Oreskovic, N.M.; Agiovlasitis, S. Factors That Influence Physical Activity in Individuals With Down Syndrome: Perspectives of Guardians and Health Professionals. Adapt. Phys. Activ Q. 2023, 40, 587–606. [Google Scholar] [CrossRef]
- Morkem, R.; Theal, R.; Barber, D.; Flemming, J.; Queenan, J.; Kehar, M. Screening Patterns of Nonalcoholic Fatty Liver Disease in Children with Obesity in Canadian Primary Care: A Cross-Sectional Study. Can. J. Gastroenterol. Hepatol. 2022, 2022, 8435581. [Google Scholar] [CrossRef]
- Lee-Kim, V.; Morkem, R.; Barber, D.; Flemming, J.A.; Kehar, M. Awareness, management, and practice patterns of pediatric NAFLD by primary care physicians. Paediatr. Child. Health 2021, 27, 93–98. [Google Scholar] [CrossRef]
| Variable | Value |
|---|---|
| Number of participants | 503 |
| Sex (Female/Male) full cohort | 231/271 |
| Median age, months | 172 |
| Median weight, kg | 44 |
| Median BMI | 21.75 |
| Children with MAFLD (%) | 54 (10.7%) |
| Sex (Female/Male) MAFLD cohort | 29/25 |
| Median age of MAFLD group, months | 205 |
| Median weight of MAFLD group, kg | 66 |
| Median BMI of MAFLD group | 31.39 |
| Median systolic BP, mmHg | 113 |
| Median diastolic BP, mmHg | 71 |
| MAFLD diagnosis based on imaging | 48/54 (88.9%) |
| MAFLD diagnosis due to adiposity | 49/54 (90.7%) |
| Laboratory Marker | Median | IQR |
| Total bilirubin | 5 µmol/L | 3–10 µmol/L |
| ALT | 35 U/L | 26–49 U/L |
| AST | 26 U/L | 22–38 U/L |
| GGT | 30 U/L | 23–50 U/L |
| ALP | 315 U/L | 107–231 U/L |
| Albumin | 41 g/L | 39–43 g/L |
| INR | 1.04 | 1–1.9 |
| Ferritin | 82 µg/L | 57–134 µg/L |
| Creatinine | 64 µmol/L | 52–80 µmol/L |
| Triglyceride | 4.4 mmol/L | 0.9–1.91 mmol/L |
| Total cholesterol | 1.32 mmol/L | 3.75–5.19 mmol/L |
| HDL cholesterol | 1.1 mmol/L | 1.01–1.25 mmol/L |
| LDL cholesterol | 2.59 mmol/L | 1.97–3.18 mmol/L |
| Fasting glucose | 5.15 mmol/L | 4.9–5.7 mmol/L |
| Fasting insulin | 182 pmol/L | 134–410 pmol/L |
| HbA1c | 5.3 | 5.1–5.5 |
| Liver stiffness (FibroScan, N-13) | 5.3 kPa | 4.7–6.6 kPa |
| CAP score | 262 dB/ms | 220–315 dB/ms |
| Variable | Univariate Analysis | Multivariate Analysis | ||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age (months) | 1.008 | 1.003–1.012 | <0.0001 | 0.997 | 0.99–1.003 | 0.4 |
| Sex | 0.707 | 0.40–1.20 | 0.23 | – | – | – |
| (Female = Ref) | ||||||
| BMI | 1.19 | 1.14–1.25 | <0.0001 | 1.2 | 1.10–1.20 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaceper, M.D.; Villegas, M.-J.; Sadh, S.; Kawesa, S.; Strain, J.; Nair, A.; Dupuis, A.; Pothos, M.; Zheng, M.-H.; Kehar, M. Prevalence and Risk Factors of Metabolic-Associated Fatty Liver Disease in Children with Down Syndrome at a Tertiary Care Center. J. Clin. Med. 2025, 14, 3239. https://doi.org/10.3390/jcm14093239
Karaceper MD, Villegas M-J, Sadh S, Kawesa S, Strain J, Nair A, Dupuis A, Pothos M, Zheng M-H, Kehar M. Prevalence and Risk Factors of Metabolic-Associated Fatty Liver Disease in Children with Down Syndrome at a Tertiary Care Center. Journal of Clinical Medicine. 2025; 14(9):3239. https://doi.org/10.3390/jcm14093239
Chicago/Turabian StyleKaraceper, Maria D., Maria-Jose Villegas, Sanathan Sadh, Sierra Kawesa, Jamie Strain, Asha Nair, Alissa Dupuis, Mary Pothos, Ming-Hua Zheng, and Mohit Kehar. 2025. "Prevalence and Risk Factors of Metabolic-Associated Fatty Liver Disease in Children with Down Syndrome at a Tertiary Care Center" Journal of Clinical Medicine 14, no. 9: 3239. https://doi.org/10.3390/jcm14093239
APA StyleKaraceper, M. D., Villegas, M.-J., Sadh, S., Kawesa, S., Strain, J., Nair, A., Dupuis, A., Pothos, M., Zheng, M.-H., & Kehar, M. (2025). Prevalence and Risk Factors of Metabolic-Associated Fatty Liver Disease in Children with Down Syndrome at a Tertiary Care Center. Journal of Clinical Medicine, 14(9), 3239. https://doi.org/10.3390/jcm14093239







