Reducing Infections and Improving Healing in Complex Wounds: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Information Sources
2.4. Search Strategy
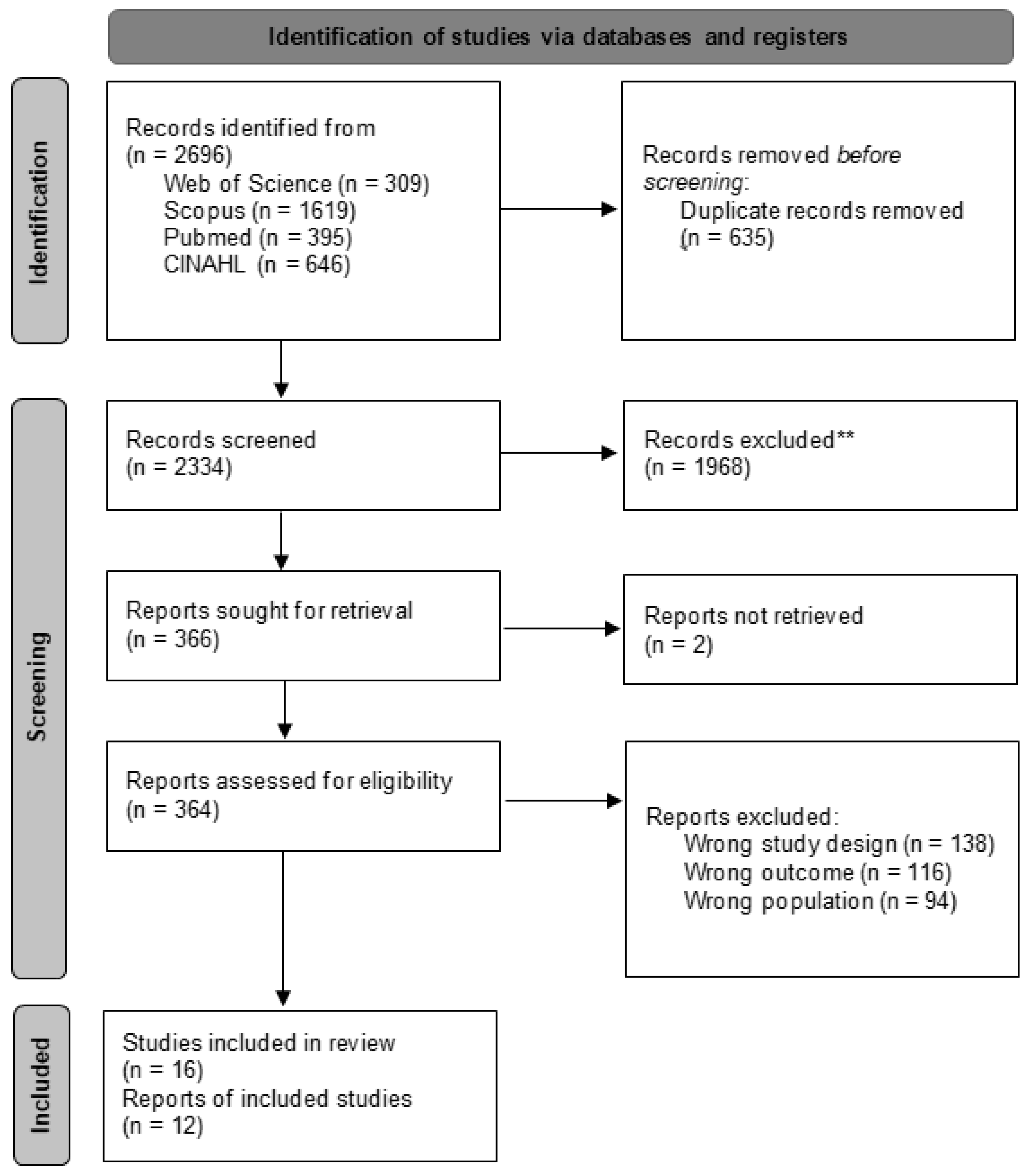
2.5. Study Selection Process
2.6. Data Extraction
2.7. Risk of Bias
2.8. Decisions for the Meta-Analysis
3. Results
3.1. Risk of Bias
3.2. Study Characteristics
3.3. Study Characteristics
Intervention
3.4. Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hall, C.; Regner, J.; Abernathy, S.; Isbell, C.; Isbell, T.; Kurek, S.; Smith, R.; Frazee, R. Surgical Site Infection after Primary Closure of High-Risk Surgical Wounds in Emergency General Surgery Laparotomy and Closed Negative-Pressure Wound Therapy. J. Am. Coll. Surg. 2019, 228, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Elhessy, A.H.; Chaudhry, A.R.; Hammouda, A.I.; Giacobbe, S.D.; Gesheff, M.G.; Conway, J.D. Experience with negative-pressure wound therapy with instillation in complex infected orthopaedic wounds. Int. Wound J. 2021, 18, 902–908. [Google Scholar] [CrossRef]
- Hsu, K.-F.; Chiu, Y.-L.; Chiao, H.Y.; Chen, C.-Y.; Chang, C.-K.; Wu, C.-J.; Peng, Y.-J.; Wang, C.-H.; Dai, N.-T.; Chen, S.-G.; et al. Negative-pressure wound therapy combined with artificial dermis (Terudermis) followed by split-thickness skin graft might be an effective treatment option for wounds exposing tendon and bone: A retrospective observation study. Medicine 2021, 100, e25395. [Google Scholar] [CrossRef]
- Imcha, M.; Liew, N.C.; McNally, A.; Zibar, D.; O’riordan, M.; Currie, A.; Styche, T.; Hughes, J.; Whittall, C. Single-use negative pressure wound therapy to prevent surgical site complications in high-risk patients undergoing caesarean sections: A real-world study. Int. J. Qual. Health Care 2023, 35, mzad089. [Google Scholar] [CrossRef]
- Tauseef, M.; Azam, F.; Iqbal, Y.; Ahmad, S.; Ahmad, F.; Masood, R.; Habib, S.R.; Rasheed, A.; Zafar, M.S.; Salam, A. Development and evaluation of silver-infused Biopolymer coated cotton wound dressings as possible wound healing material. Heliyon 2024, 10, e38407. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, X.; Zhao, N.; Chen, D. Study on the Effect and Mechanism of Antibacterial Adhesive Hydrogel on Wound Healing. Comput. Math. Methods Med. 2021, 2021, 8212518. [Google Scholar] [CrossRef] [PubMed]
- Tiscar-González, V.M.; Menor-Rodríguez, M.J.P.; Rabadán-Sainz, C.M.; Fraile-Bravo, M.P.; Styche, T.B.; Valenzuela-Ocaña, F.J.M.; Muñoz-García, L.P. Clinical and Economic Impact of Wound Care Using a Polyurethane Foam Multilayer Dressing. Adv. Ski. Wound Care 2021, 34, 23–30. [Google Scholar] [CrossRef]
- Broussard, K.C.; Powers, J.G. Wound Dressings: Selecting the Most Appropriate Type. Am. J. Clin. Dermatol. 2013, 14, 449–459. [Google Scholar] [CrossRef]
- Wang, P.-H.; Huang, B.-S.; Horng, H.-C.; Yeh, C.-C.; Chen, Y.-J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Bhandari, M.; Jeray, K.J.; Petrisor, B.A.; Devereaux, P.J.; Heels-Ansdell, D.; Schemitsch, E.H.; Anglen, J.; Della Rocca, G.J.; Jones, C.; Kreder, H.; et al. A Trial of Wound Irrigation in the Initial Management of Open Fracture Wounds. N. Engl. J. Med. 2015, 373, 2629–2641. [Google Scholar] [CrossRef]
- Hurlow, J.; Bowler, P.G. Acute and chronic wound infections: Microbiological, immunological, clinical and therapeutic distinctions. J. Wound Care 2022, 31, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Acosta, S.; Björck, M.; Wanhainen, A. Negative-pressure wound therapy for prevention and treatment of surgical-site infections after vascular surgery. Br. J. Surg. 2017, 104, e75–e84. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, J.M. Negative Pressure Wound Therapy for Chronic Wounds. Ann. Plast. Surg. 2024, 93, S19–S26. [Google Scholar] [CrossRef]
- Sinha, S. Management of post-surgical wounds in general practice. Aust. J. Gen. Pract. 2019, 48, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Anglen, J.O. Comparison of Soap and Antibiotic Solutions for Irrigation of Lower-Limb Open Fracture WoundsA Prospective, Randomized Study. J. Bone Joint Surg. Am. 2005, 87, 1415–1422. [Google Scholar] [CrossRef][Green Version]
- Arti, H.; Khorami, M.; Ebrahimi-Nejad, V.E.-N. Comparison of Negative Pressure Wound Therapy (NPWT) and Conventional Wound Dressings in the Open Fracture Wounds. Pak. J. Med. Sci. 2016, 32, 65–69. [Google Scholar] [CrossRef]
- Blackham, A.U.; Farrah, J.P.; McCoy, T.P.; Schmidt, B.S.; Shen, P. Prevention of surgical site infections in high-risk patients with laparotomy incisions using negative-pressure therapy. Am. J. Surg. 2013, 205, 647–654. [Google Scholar] [CrossRef]
- Blum, M.L.M.; Esser, M.M.; Richardson, M.M.; Paul, E.B.; Rosenfeldt, F.L.M. Negative Pressure Wound Therapy Reduces Deep Infection Rate in Open Tibial Fractures. J. Orthop. Trauma 2012, 26, 499–505. [Google Scholar] [CrossRef]
- Lawrentschuk, N.; Falkenberg, M.P.; Pirpiris, M. Wound blisters post hip surgery: A prospective trial comparingdressings. ANZ J. Surg. 2002, 72, 716–719. [Google Scholar] [CrossRef]
- Ondari, J.N.; Masika, M.M.; Ombachi, R.B.; Ating’a, J.E. Unblinded randomized control trial on prophylactic antibiotic use in gustilo II open tibia fractures at Kenyatta National Hospital, Kenya. Injury 2016, 47, 2288–2293. [Google Scholar] [CrossRef]
- Rezzadeh, K.S.; Nojan, M.; Buck, A.; Li, A.; Vardanian, A.; Crisera, C.; Festekjian, J.; Jarrahy, R. The use of negative pressure wound therapy in severe open lower extremity fractures: Identifying the association between length of therapy and surgical outcomes. J. Surg. Res. 2015, 199, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Stanirowski, P.J.; Bizoń, M.; Cendrowski, K.; Sawicki, W. Randomized Controlled Trial Evaluating Dialkylcarbamoyl Chloride Impregnated Dressings for the Prevention of Surgical Site Infections in Adult Women Undergoing Cesarean Section. Surg. Infect. 2016, 17, 427–435. [Google Scholar] [CrossRef]
- Stannard, J.P.; Robinson, J.T.; Anderson, E.R.; McGwin, G.; Volgas, D.A.; Alonso, J.E. Negative Pressure Wound Therapy to Treat Hematomas and Surgical Incisions Following High-Energy Trauma. J. Trauma 2006, 60, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Stannard, J.P.; Volgas, D.A.; Stewart, R.; McGwin, G.; Alonso, J.E. Negative Pressure Wound Therapy After Severe Open Fractures: A Prospective Randomized Study. J. Orthop. Trauma 2009, 23, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Stannard, J.P.; Volgas, D.A.; McGwin, G.; Stewart, R.L.; Obremskey, W.; Moore, T.; Anglen, J.O. Incisional Negative Pressure Wound Therapy After High-Risk Lower Extremity Fractures. J. Orthop. Trauma 2012, 26, 37–42. [Google Scholar] [CrossRef]
- Tauber, R.; Schmid, S.; Horn, T.; Thalgott, M.; Heck, M.; Haller, B.; Kübler, H.; Autenrieth, M.; Retz, M.; Gschwend, J.E.; et al. Inguinal lymph node dissection: Epidermal vacuum therapy for prevention of wound complications. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 390–396. [Google Scholar] [CrossRef]
- Vargo, D. Negative pressure wound therapy in the prevention of wound infection in high risk abdominal wound closures. Am. J. Surg. 2012, 204, 1021–1024, discussion 23–24. [Google Scholar] [CrossRef]
- Virani, S.R.; Dahapute, A.A.; Bava, S.; Muni, S.R. Impact of negative pressure wound therapy on open diaphyseal tibial fractures: A prospective randomized trial. J. Clin. Orthop. Trauma 2016, 7, 256–259. [Google Scholar] [CrossRef]
- Svensson-Björk, R.; Saha, S.; Acosta, S.; Gerdtham, U.-G.; Hasselmann, J.; Asciutto, G.; Zarrouk, M. Cost-effectiveness analysis of negative pressure wound therapy dressings after open inguinal vascular surgery—The randomised INVIPS-Trial. J. Tissue Viability 2021, 30, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Svensson-Björk, R.; Hasselmann, J.; Asciutto, G.; Zarrouk, M.; Björk, J.; Bilos, L.; Pirouzram, A.; Acosta, S. Negative Pressure Wound Therapy for the Prevention of Surgical Site Infections Using Fascia Closure After EVAR—A Randomized Trial. World J. Surg. 2022, 46, 3111–3120. [Google Scholar] [CrossRef]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625, quiz 25–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Tao, S.; Wang, Q.; Ma, P.-Q.; Li, Z.-B.; Wu, Y.-L.; Li, D.-W. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil. Med. Res. 2023, 10, 37. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Mende, K.; Stewart, L.; Shaikh, F.; Bradley, W.; Lu, D.; Krauss, M.R.; Greenberg, L.; Yu, Q.; Blyth, D.M.; Whitman, T.J.; et al. Microbiology of combat-related extremity wounds: Trauma Infectious Disease Outcomes Study. Diagn. Microbiol. Infect. Dis. 2019, 94, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Garner, M.R.; Sethuraman, S.A.; Schade, M.A.; Boateng, H. Antibiotic Prophylaxis in Open Fractures Evidence, Evolving Issues, and Recommendations. J. Am. Acad. Orthop. Surg. 2020, 28, 309–315. [Google Scholar] [CrossRef]
- Apelqvist, J.; Willy, C.; Fagerdahl, A.-M.; Fraccalvieri, M.; Malmsjö, M.; Piaggesi, A.; Probst, A.; Vowden, P. Ewma Document: Negative Pressure Wound Therapy. J. Wound Care 2017, 26 (Suppl. S3), S1–S154. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Da, J.; Wu, W.; Ma, F.; Tang, C.; Li, G.; Zhong, D.; Liao, B. A systematic review and meta-analysis of efficacy and safety of negative pressure wound therapy in the treatment of diabetic foot ulcer. Ann. Palliat. Med. 2021, 10, 10830–10839. [Google Scholar] [CrossRef]
- Iheozor-Ejiofor, Z.; Newton, K.; Dumville, J.C.; Costa, M.L.; Norman, G.; Bruce, J. Negative pressure wound therapy for open traumatic wounds. Cochrane Database Syst. Rev. 2018, 2018, CD012522. [Google Scholar] [CrossRef]
- De Pellegrin, L.; Feltri, P.; Filardo, G.; Candrian, C.; Harder, Y.; Galetti, K.; De Monti, M. Effects of negative pressure wound therapy with instillation and dwell time (NPWTi-d) versus NPWT or standard of care in orthoplastic surgery: A systematic review and meta-analysis. Int. Wound J. 2023, 20, 2402–2413. [Google Scholar] [CrossRef] [PubMed]
- Hulsker, C.C.C.; Kleinveld, S.; Zonnenberg, C.B.L.; Hogervorst, M.; Bekerom, M.P.J.v.D. Evidence-based treatment of open ankle fractures. Arch. Orthop. Trauma Surg. 2011, 131, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zou, R.; He, J.; Ouyang, K.; Piao, Z. Comparing osteogenic effects between concentrated growth factors and the acellular dermal matrix. Braz. Oral Res. 2018, 32, e29. [Google Scholar] [CrossRef]
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Sci. Rep. 2017, 7, 9692. [Google Scholar] [CrossRef] [PubMed]
- Al-Gharibi, K.A.; Sharstha, S.; Al-Faras, M.A. Cost-Effectiveness of Wound Care: A concept analysis. Sultan Qaboos Univ. Med. J. 2019, 18, 433–439. [Google Scholar] [CrossRef]
| Study | Bias 1 | Bias 2 | Bias 3 | Bias 4 | Bias 5 | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| Anglen et al., 2005 [17] |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |  Moderate Moderate |  Low Risk Low Risk |  Low Risk Low Risk |
| Arti et al., 2016 [18] |  Moderate Moderate |  Low Risk Low Risk |  Low Risk Low Risk |  Moderate Moderate |  Low Risk Low Risk |  Moderate risk Moderate risk |
| Blackham et al., 2013 [19] |  Low Risk Low Risk |  Moderate Moderate |  Low Risk Low Risk |  High Risk High Risk |  Moderate Moderate |  Moderate risk Moderate risk |
| Blum et al., 2012 [20] |  Moderate Moderate |  Low Risk Low Risk |  Low Risk Low Risk |  Moderate Moderate |  Low Risk Low Risk |  Moderate risk Moderate risk |
| Lawrentschuk et al., 2002 [21] |  High Risk High Risk |  Moderate Moderate |  Low Risk Low Risk |  High Risk High Risk |  Moderate Moderate |  High Risk High Risk |
| Ondari et al., 2016 [22] |  Low Risk Low Risk |  Moderate Moderate |  Low Risk Low Risk |  Moderate Moderate |  Low Risk Low Risk |  Moderate risk Moderate risk |
| Rezzadeh et al., 2015 [23] |  Moderate Moderate |  Moderate Moderate |  Low Risk Low Risk |  Moderate Moderate |  Moderate Moderate |  Moderate risk Moderate risk |
| Stanirowski et al., 2016 [24] |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |
| Stannard et al., 2006 [25] |  Moderate Moderate |  Moderate Moderate |  Low Risk Low Risk |  High Risk High Risk |  Moderate Moderate |  Moderate risk Moderate risk |
| Stannard et al., 2009 [26] |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |
| Stannard et al., 2012 [27] |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |
| Tauber et al., 2013 [28] |  Moderate Moderate |  Low Risk Low Risk |  Low Risk Low Risk |  Moderate Moderate |  Low Risk Low Risk |  Moderate risk Moderate risk |
| Vargo et al., 2012 [29] |  Low Risk Low Risk |  Moderate Moderate |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |
| Virani et al., 2016 [30] |  Moderate Moderate |  Low Risk Low Risk |  Moderate Moderate |  Moderate Moderate |  Low Risk Low Risk |  Moderate risk Moderate risk |
| Svensson-Björk et al., 2021 [31] |  Moderate Moderate |  Low Risk Low Risk |  Low Risk Low Risk |  Moderate Moderate |  Moderate Moderate |  Moderate risk Moderate risk |
| Svensson-Björk et al., 2022 [32] |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |  Low Risk Low Risk |
| Authors and Year | Design and Methodology | Key Results | Conclusions |
|---|---|---|---|
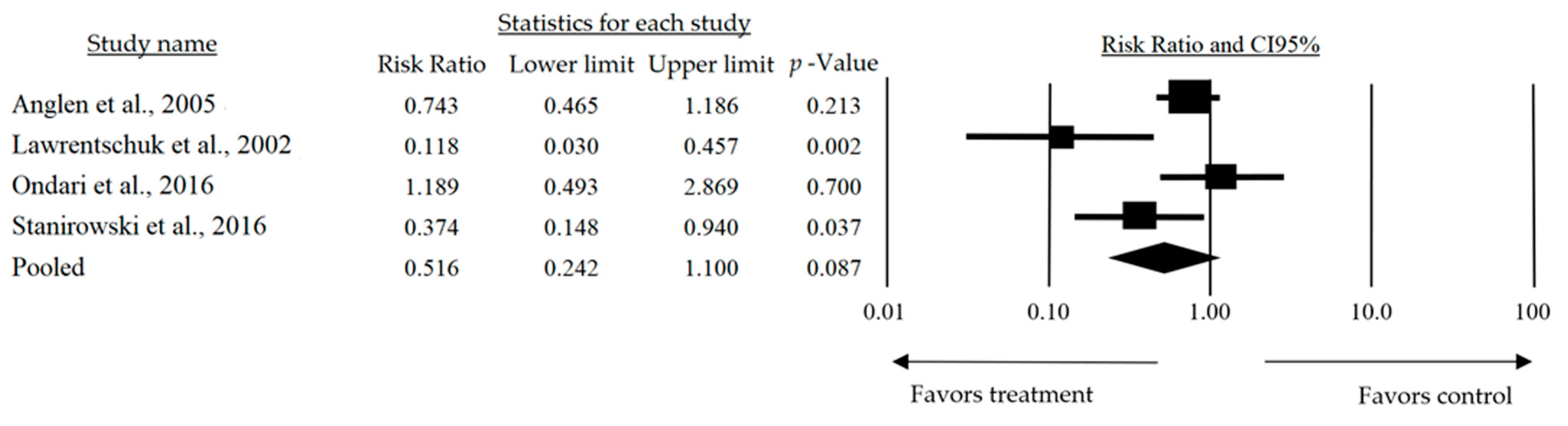
| Anglen et al., 2005 [17] | Experimental study with animal models and human patients (n = 50). | Low-pressure irrigation was more effective in reducing bacterial contamination without tissue damage. | Low-pressure irrigation is an efficient and safe alternative for managing open wounds. |
| Arti et al., 2016 [18] | Prospective randomized clinical trial with 90 patients treated and followed for one month. | NPWT showed shorter healing times but no significant differences in infection rates. | NPWT accelerates the healing of open wounds and may be more cost-effective than conventional dressings. |
| Blackham et al., 2013 [19] | Retrospective review of 200 patients treated with various topical agents on open wounds. | The use of topical antiseptics significantly reduced infections and healing times. | Topical agents are effective and improve clinical outcomes in open fractures. |
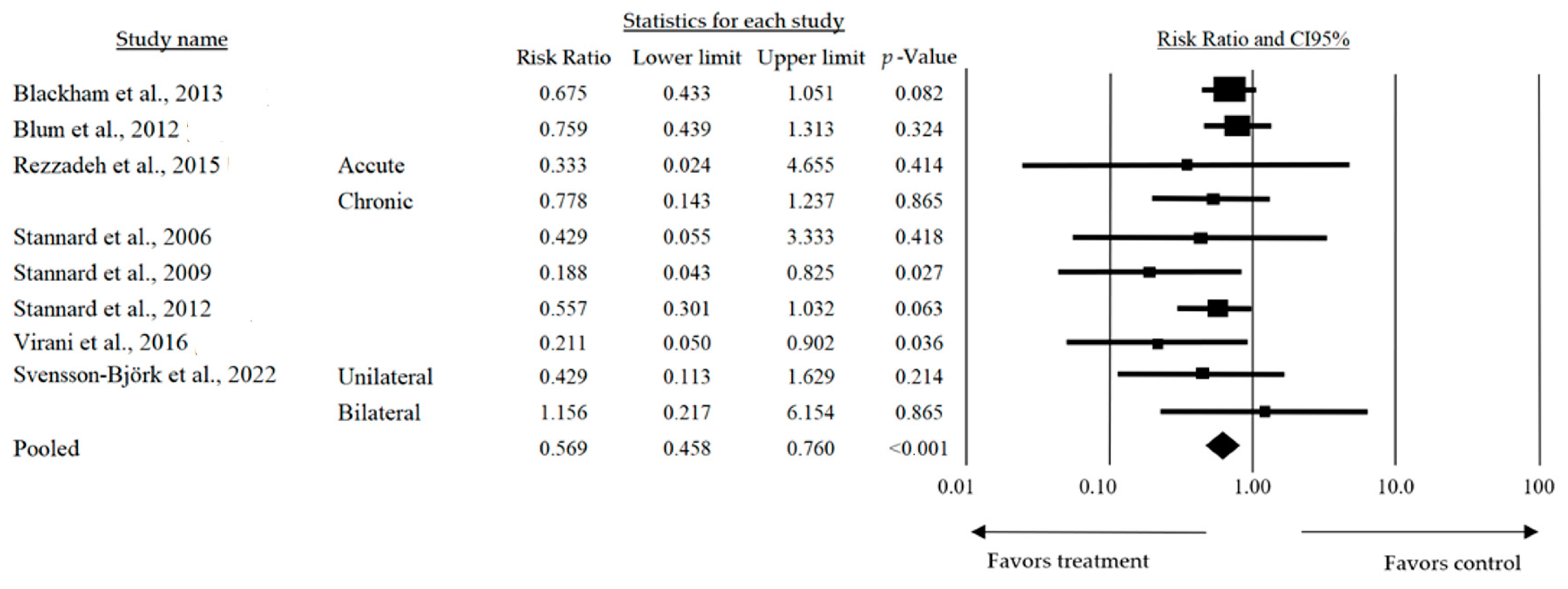
| Blum et al., 2012 [20] | Retrospective cohort study in two trauma centers with 229 open tibia fractures. | Negative pressure therapy reduced deep infection rates from 20.6% to 8.4% (p = 0.01). | Negative pressure therapy significantly reduces deep infection rates in open tibia fractures. |
| Lawrentschuk et al., 2002 [21] | Prospective randomized trial with 50 patients undergoing hip surgery. | PTG dressing significantly reduced blisters compared to NAA dressing (8% vs. 64%, p = 0.0028). | PTG produces fewer blisters compared to NAA, making it a more suitable option after hip surgery. |
| Ondari et al., 2016 [22] | Unblinded randomized clinical trial conducted in a Kenyan hospital with 84 patients divided into two groups. | No significant differences in infection rates were found between the two regimens (23% vs. 19%, p = 0.699). | Antibiotics for 24 h are adequate prophylaxis against infections in Gustilo II open fractures. |
| Rezzadeh et al., 2015 [23] | Randomized controlled trial in 120 patients divided into groups with and without growth factor treatment. | The growth factor-treated group showed greater granulation tissue formation and accelerated healing. | Growth factors significantly improve the healing of complex wounds. |
| Stanirowski et al., 2016 [24] | Prospective randomized study in 120 patients undergoing major surgery. | Pressure dressings significantly reduced healing times and postoperative infections. | Pressure dressings are effective in improving healing in complex postoperative wounds. |
| Stannard et al., 2006 [25] | Prospective study in 140 patients with traumatic wounds treated with VAC or standard dressings. | VAC reduced the healing time and the need for reoperations. | Vacuum-assisted closure is effective in accelerating healing and reducing complications. |
| Stannard et al., 2009 [26] | Prospective study in 160 patients with severe trauma treated in referral hospitals. | NPWT reduced complication rates by 25% compared to standard dressings. | NPWT is an effective option for managing severe lower limb trauma wounds. |
| Stannard et al., 2012 [27] | Systematic review of the recent literature on emerging technologies. | Technologies like NPWT and smart dressings showed promising results in wound healing. | Technological innovations can significantly transform the management of complex wounds. |
| Tauber et al., 2013 [28] | Retrospective cohort study with 180 patients in two trauma hospitals. | The use of topical antiseptics significantly reduced infection incidence (p < 0.05). | Topical antiseptics are effective in reducing infections in open fracture wounds. |
| Vargo et al., 2012 [29] | Longitudinal study with 5-year follow-up in 250 surgically treated patients. | Functionality was significantly better in patients with early intensive rehabilitation. | Intensive rehabilitation improves functional outcomes in severe lower limb fractures. |
| Virani et al., 2016 [30] | Multicenter prospective study with 200 patients treated with silver dressings or conventional bandages. | Silver-based dressings significantly reduced infection rates (12% vs. 25%, p < 0.05). | Silver-based dressings are effective in reducing infection rates in open fracture wounds. |
| Svensson-Björk et al., 2021 [31] | Multicenter randomized clinical trial with 377 incisions (uni- and bilateral), comparing negative pressure therapy and standard dressings. | No significant differences in infection incidence were found between the two groups at 90 days postoperatively. | The routine use of negative pressure therapy is not recommended for low-risk incisions after EVAR. |
| Svensson-Björk et al., 2022 [32] | Cost-effectiveness analysis based on data from the INVIPS randomized clinical trial, considering procedure-related costs and the quality of life. | Negative pressure therapy significantly reduced infection incidence, with an incremental cost of €1.853 per infection avoided. | Negative pressure therapy is a cost-effective strategy for reducing infections in open vascular inguinal surgeries. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millán-Reyes, M.J.; Afanador-Restrepo, D.F.; Carcelén-Fraile, M.d.C.; Aibar-Almazán, A.; Sánchez-Alcalá, M.; Cano-Sánchez, J.; Mesas-Aróstegui, M.A.; Castellote-Caballero, Y. Reducing Infections and Improving Healing in Complex Wounds: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 3237. https://doi.org/10.3390/jcm14093237
Millán-Reyes MJ, Afanador-Restrepo DF, Carcelén-Fraile MdC, Aibar-Almazán A, Sánchez-Alcalá M, Cano-Sánchez J, Mesas-Aróstegui MA, Castellote-Caballero Y. Reducing Infections and Improving Healing in Complex Wounds: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(9):3237. https://doi.org/10.3390/jcm14093237
Chicago/Turabian StyleMillán-Reyes, María Juana, Diego Fernando Afanador-Restrepo, María del Carmen Carcelén-Fraile, Agustín Aibar-Almazán, Marcelina Sánchez-Alcalá, Javier Cano-Sánchez, María Aurora Mesas-Aróstegui, and Yolanda Castellote-Caballero. 2025. "Reducing Infections and Improving Healing in Complex Wounds: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 9: 3237. https://doi.org/10.3390/jcm14093237
APA StyleMillán-Reyes, M. J., Afanador-Restrepo, D. F., Carcelén-Fraile, M. d. C., Aibar-Almazán, A., Sánchez-Alcalá, M., Cano-Sánchez, J., Mesas-Aróstegui, M. A., & Castellote-Caballero, Y. (2025). Reducing Infections and Improving Healing in Complex Wounds: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(9), 3237. https://doi.org/10.3390/jcm14093237