Matrix Metalloproteinase-9 and Postoperative Outcomes in Carotid Endarterectomy: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Study Selection and Data Extraction
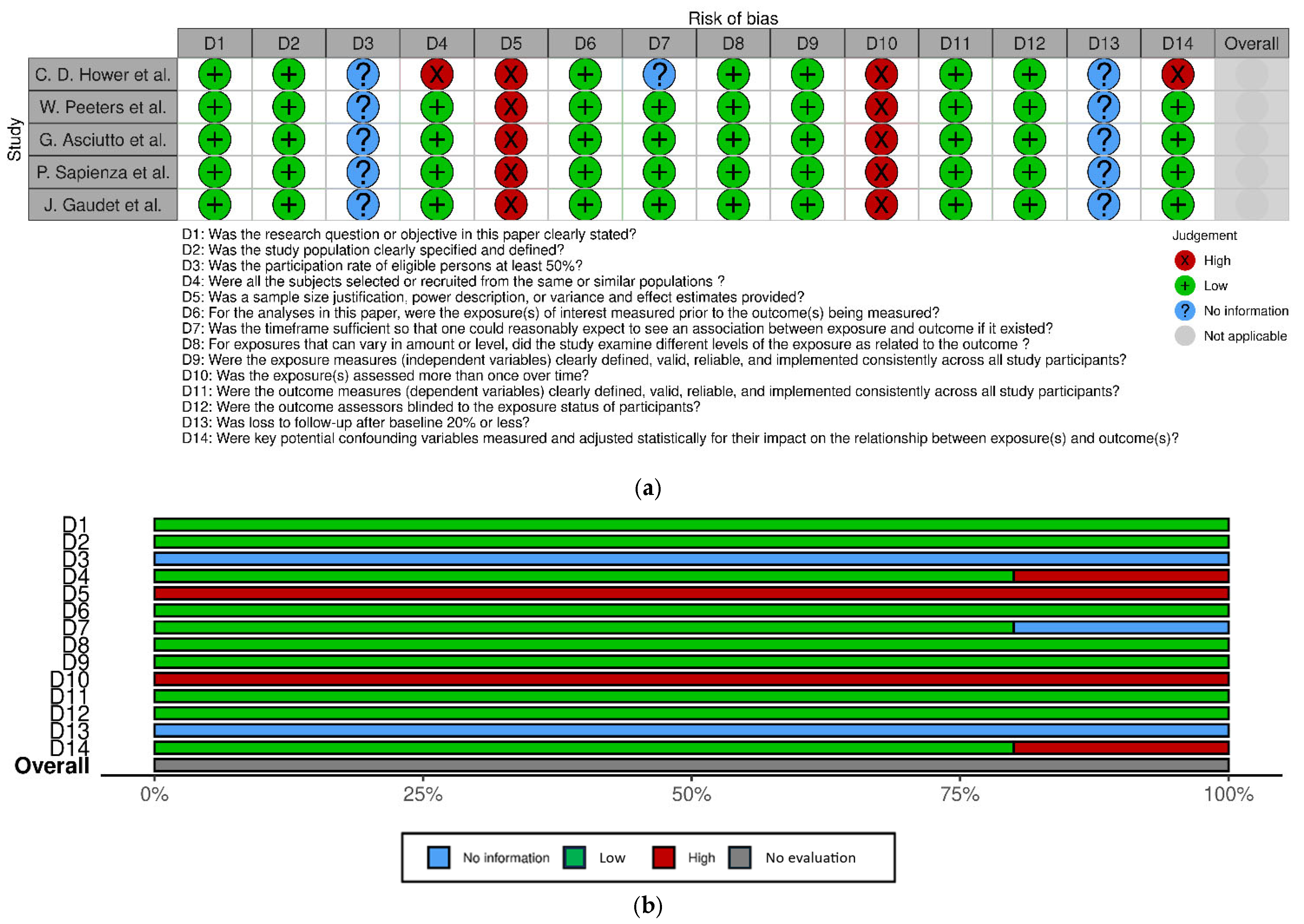
2.4. Assessment of Study Quality
3. Results
3.1. Search Results
3.2. Description of Studies
3.3. Study Quality
3.4. Main Findings
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- AbuRahma, A.F.; Avgerinos, E.D.; Chang, R.W.; Darling, R.C., 3rd; Duncan, A.A.; Forbes, T.L.; Malas, M.B.; Murad, M.H.; Perler, B.A.; Powell, J.D.; et al. Society for Vascular Surgery clinical practice guidelines for management of extracranial cerebrovascular disease. J. Vasc. Surg. 2022, 75, 4S–22S. [Google Scholar] [CrossRef] [PubMed]
- European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998, 351, 1379–1387. [Google Scholar] [CrossRef]
- Ouriel, K.; Hertzer, N.R.; Beven, E.G.; O’Hara, P.J.; Krajewski, L.P.; Clair, D.G.; Greenberg, R.K.; Sarac, T.P.; Olin, J.W.; Yadav, J.S. Preprocedural risk stratification: Identifying an appropriate population for carotid stenting. J. Vasc. Surg. 2001, 33, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Gaubatz, J.W.; Ballantyne, C.M.; Wasserman, B.A.; He, M.; Chambless, L.E.; Boerwinkle, E.; Hoogeveen, R.C. Association of circulating matrix metalloproteinases with carotid artery characteristics: The Atherosclerosis Risk in Communities Carotid MRI Study. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1034–1042. [Google Scholar] [CrossRef]
- Eldrup, N.; Gronholdt, M.L.; Sillesen, H.; Nordestgaard, B.G. Elevated matrix metalloproteinase-9 associated with stroke or cardiovascular death in patients with carotid stenosis. Circulation 2006, 114, 1847–1854. [Google Scholar] [CrossRef]
- Alvarez, B.; Ruiz, C.; Chacon, P.; Alvarez-Sabin, J.; Matas, M. Serum values of metalloproteinase-2 and metalloproteinase-9 as related to unstable plaque and inflammatory cells in patients with greater than 70% carotid artery stenosis. J. Vasc. Surg. 2004, 40, 469–475. [Google Scholar] [CrossRef]
- Loftus, I.M.; Naylor, A.R.; Bell, P.R.; Thompson, M.M. Plasma MMP-9-a marker of carotid plaque instability. Eur. J. Vasc. Endovasc. Surg. 2001, 21, 17–21. [Google Scholar] [CrossRef]
- Sef, D.; Milosevic, M.; Ostric, M.; Mestrovic, T.; Jernej, B.; Kovacic, S.; Skrtic, A.; Vidak, V. The role of magnetic resonance imaging and the expression of MMP-9 protein in the analysis of carotid atherosclerotic plaques in patients undergoing carotid endarterectomy: A prospective pilot study. Rev. Cardiovasc. Med. 2021, 22, 1611–1620. [Google Scholar] [CrossRef]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; PRISMA-DTA Group. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Henry, A.D.; Kristjansson, E.; Tugwell, P.; Welsch, V. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Gaudet, J.G.; Yocum, G.T.; Lee, S.S.; Granat, A.; Mikami, M.; Connolly, E.S.; Heyer, E.J.J. MMP-9 levels in elderly patients with cognitive dysfunction after carotid surgery. J. Clin. Neurosci. 2010, 17, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Hower, C.D.; Dassow, M.S.; Kajdacsy-Balla, A.; Seabrook, G.R.; Jean-Claude, J.; Towne, J.B.; Cambria, R.A. Metalloproteinase levels are decreased in symptomatic carotid plaques. J. Surg. Res. 2000, 88, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Sapienza, P.; Borrelli, V.; di Marzo, L.; Cavallaro, A. MMP and TIMP alterations in asymptomatic and symptomatic severe recurrent carotid artery stenosis. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Asciutto, G.; Dias, N.V.; Edsfeldt, A.; Nitulescu, M.; Persson, A.; Nilsson, M.; Dunner, P.; Goncalves, I. Low elastin content of carotid plaques is associated with increased risk of ipsilateral stroke. PLoS ONE 2015, 10, e0121086. [Google Scholar] [CrossRef]
- Peeters, W.; Moll, F.L.; Vink, A.; van der Spek, P.J.; de Kleijn, D.P.; de Vries, J.P.; Newby, A.C.; Pastercamp, G. Collagenase matrix metalloproteinase-8 expressed in atherosclerotic carotid plaques is associated with systemic cardiovascular outcome. Eur. Heart J. 2011, 32, 2314–2325. [Google Scholar] [CrossRef]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Edejer, T.T.T.; Liberati, A.; Hill, S. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar]
- Blankenberg, S.; Rupprecht, H.J.; Poirier, O.; Bickel, C.; Smieja, M.; Hafner, G.; Meyer, J.; Cambien, F.; Tiret, L.; Athero Gene Investigators. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 2003, 107, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Inokubo, Y.; Hanada, H.; Ishizaka, H.; Fukushi, T.; Kamada, T.; Okumura, K. Plasma levels of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 are increased in the coronary circulation in patients with acute coronary syndrome. Am. Heart J. 2001, 141, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L. Metalloproteinases in atherosclerosis. Eur. J. Pharmacol. 2017, 816, 93–106. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Feng, Y.; Dong, G.; Wang, Y.; Yang, J. The Role of Matrix Metalloproteinase-9 in Atherosclerotic Plaque Instability. Mediators Inflamm. 2020, 2020, 3872367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ezhov, M.; Safarova, M.; Afanasieva, O.; Mitroshkin, M.; Matchin, Y.; Pokrovsky, S. Matrix Metalloproteinase 9 as a Predictor of Coronary Atherosclerotic Plaque Instability in Stable Coronary Heart Disease Patients with Elevated Lipoprotein(a) Levels. Biomolecules 2019, 9, 129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silvello, D.; Narvaes, L.B.; Albuquerque, L.C.; Forgiarini, L.F.; Meurer, L.; Martinelli, N.C.; Andrades, M.E.; Clausell, N.; dos Santos, K.G.; Rohde, L.E. Serum levels and polymorphisms of matrix metalloproteinases (MMPs) in carotid artery atherosclerosis: Higher MMP-9 levels are associated with plaque vulnerability. Biomarkers 2014, 19, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Łacheta, D.; Kubiak-Tomaszewska, G. Matrix Metalloproteinases as Biomarkers of Atherosclerotic Plaque Instability. Int. J. Mol. Sci. 2020, 21, 3946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deleon-Pennell, K.Y.; Altara, R.; Yabluchanskiy, A.; Modesti, A.; Lindsey, M.L. The circular relationship between matrix metalloproteinase-9 and inflammation following myocardial infarction. IUBMB Life 2015, 67, 611–618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yabluchanskiy, A.; Li, Y.; Chilton, R.J.; Lindsey, M.L. Matrix metalloproteinases: Drug targets for myocardial infarction. Curr. Drug Targets 2013, 14, 276–286. [Google Scholar] [PubMed] [PubMed Central]
- Zhong, C.; Yang, J.; Xu, T.; Xu, T.; Peng, Y.; Wang, A.; Wang, J.; Peng, H.; Li, Q.; Ju, Z.; et al. CATIS Investigators. Serum matrix metalloproteinase-9 levels and prognosis of acute ischemic stroke. Neurology 2017, 89, 805–812. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Study (Year) | Study Design | Study Center | Recruitment Time | Sample Size (Patients) | GRADE Evaluation | No. CEA |
|---|---|---|---|---|---|---|
| Hower et al. [12] (2000) | Retrospective Cohort study | Multicenter (Medical College of Wisconsin and the Clement J. Zablocki Veterans Affairs Medical Center, Milwaukee, Wisconsin) | NA | 23 |  Low | 23 |
| Peeters et al. [15] (2011) | Retrospective Cohort study | Multicenter (St. Antonius Hospital Nieuwegein and University Medical Center Utrecht) | March 2002 and February 2006 | 543 |  Low Low | 543 |
| Asciutto et al. [14] (2015) | Retrospective Cohort study | Vascular Centre Malmö-Lund, Skåne University Hospital, Malmö, Sweden | October 2005 and October 2009 | 209 |  Low | 221 |
| Sapienza et al. [13] (2009) | Retrospective Cohort study | Department of Surgery “Pietro Valdoni”, University of Rome “La Sapienza” | January 1999 and December 2003 | 52 |  Low | 621 |
| Gaudet et al. [11] (2010) | Prospective Cohort study | New York Presbyterian Hospital, Columbia University | 2005–2007 | 64 |  Low | 73 |
| Study (Year) | Mean Age (Years) | Male n (%) | Arterial Hypertension n (%) | Dislipidemia n (%) | Diabetes Mellitus n (%) | Smoking History n (%) | Coronary Artery Disease n (%) | Symptomatic Carotid Disease n (%) |
|---|---|---|---|---|---|---|---|---|
| Hower et al. [12] (2000) | 73.0 | 13 (56.5%) | NA | NA | NA | NA | NA | NA |
| Peeters et al. [15] (2011) | 67.5 | 389 (71.6%) | 351 (65.0%) | 322 (59.4%) | 99 (18.3%) | 141 (26.7%) | 115 (21.3%) | 442 (81.4%) |
| Asciutto et al. [14] (2015) | 69.7 | NA | NA | NA | NA | NA | NA | NA |
| Sapienza et al. [13] (2009) | 73.9 | 19 (36.5%) | 29 (55.8%) | NA | 13 (25.0%) | 26 (50.0%) | 18 (34.6%) | 23 (44.2%) |
| Gaudet et al. [11] (2010) | NA | 47 (64.4%) | 50 (68.5%) | 53 (72.6%) | 11 (15.1%) | 46 (63.0%) | NA | 9 (12.3%) |
| Acute Cognitive Dysfunction n (%) | Acute Cognitive Dysfunction Definition | Follow-Up, Median (Range) | Long-Term Acute Myocardial Infarction, n (%) | Long-Term Amaurosis Fugax, n (%) | Long-Term Transient Ischemic Attack, n (%) | Long-Term Stroke n (%) | Long-Term Central Retinal Occlusion, n (%) | Long-Term All-Cause Mortality, n (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Hower et al. [12] (2000) | NA | NA | 6 weeks | NA | 4 (17.4%) | 2 (8.7%) | 1 (4.3%) | NA | NA |
| Peeters et al. [15] (2011) | NA | NA | 2.4 (0.97–3.0) years | 34 (6.3%) | NA | NA | 31 (5.7%) | NA | 22 (4.1%) |
| Asciutto et al. [14] (2015) | NA | NA | 39.6 (23.0–56.2) months | NA | NA | 5 (2.3%) | 12 (5.4%) | NA | NA |
| Sapienza et al. [13] (2009) | NA | NA | 74.0 (65.0–83.0) months | NA | 6 (11.5%) | 12 (23.1%) | 1 (1.9%) | 1 (1.9%) | NA |
| Gaudet et al. [11] (2010) | 12 (16.4%) | Patients with an average z-score of ≤−1.5 using 6 neurological tests for the assessment of cognitive dysfunction. | NA | NA | NA | NA | NA | NA | NA |
| Study (Year) | Outcome/Symptomatic Definition | MMP-9 Levels/Activity in Asymptomatic vs. Symptomatic Patients. (p Value) | MMP-9–Measurement Kit/Sample |
|---|---|---|---|
| Hower et al. [12] (2000) | Composite of endpoints: amaurosis fugax, TIAs and stroke. | Pro-MMP-9 levels: 17.42 (14.28–20.56) ng vs. 8.21 (5.86–10.56) ng; (p < 0.05) | ELISA kit (Biotrak assays) from Amersham Life Sciences (Buckinghamshire, England). Intraoperative-atheroscleortic plaque |
| Peeters et al. [15] (2011) | Composite of endpoints: any death of vascular origin, non-fatal stroke, non-fatal myocardial infarction and any arterial vascular intervention that had not already been planned at the time of inclusion. | 0.76 (0.40–1.42) AU vs. 0.95 (0.46–1.54) AU; (p = 0.14) | Biotrak activity assays (MMP-9 RPN-2634) from GE Healthcare Life-Sciences, Buckinghamshire, UK. Intraoperative-atheroscleortic plaque |
| Asciutto et al. [14] (2015) | Stroke; TIA. | Stroke: 921.36 (± 1247.18) AU/g vs. 1477.80 ± (2554.18) AU/g; (p = 0.683) TIA: 968.04 ± 1364.9 AU/g vs. 352.47 ± 162.5 AU/g; (p = 0.491) | MMP kit from Mesoscale (Gaithersburg, MD, USA), following the manufacturer’s instructions. Intraoperative-atheroscleortic plaque |
| Sapienza et al. [13] (2009) | Composite of endpoints: TIA, amaurosis fugax, central retinal artery occlusion and minor or major stroke. | Early symptoms (6 months to 3 years after CEA): 11 (8–14) ng/mL vs. 40 (34–46) ng/mL; (p < 0.0001) Late symptoms (over 3 years after CEA): 24 (16–32) ng/mL vs. 87 (77–97) ng/mL; (p < 0.0001) | ELISA technique provided by Amersham Pharmacia. Preoperative blood sample |
| Gaudet et al. [11] (2010) | Patients with an average z-score of ≤−1.5 on an assessment using six neurological tests representing a range of cognitive domains were considered to have cognitive disfunction. | 43.18 (38.74–47.62) ng/mL vs. 81.66 (69.41–91.91); (p < 0.005) | ELISA kit from R&D Systems (Minneapolis, MN, USA). Preoperative blood sample. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves-Silva, J.; Fragão-Marques, M.; Ribeiro, H.; Sá, S.I.; Rocha-Neves, J. Matrix Metalloproteinase-9 and Postoperative Outcomes in Carotid Endarterectomy: A Systematic Review. J. Clin. Med. 2025, 14, 3235. https://doi.org/10.3390/jcm14093235
Gonçalves-Silva J, Fragão-Marques M, Ribeiro H, Sá SI, Rocha-Neves J. Matrix Metalloproteinase-9 and Postoperative Outcomes in Carotid Endarterectomy: A Systematic Review. Journal of Clinical Medicine. 2025; 14(9):3235. https://doi.org/10.3390/jcm14093235
Chicago/Turabian StyleGonçalves-Silva, João, Mariana Fragão-Marques, Hugo Ribeiro, Susana I. Sá, and João Rocha-Neves. 2025. "Matrix Metalloproteinase-9 and Postoperative Outcomes in Carotid Endarterectomy: A Systematic Review" Journal of Clinical Medicine 14, no. 9: 3235. https://doi.org/10.3390/jcm14093235
APA StyleGonçalves-Silva, J., Fragão-Marques, M., Ribeiro, H., Sá, S. I., & Rocha-Neves, J. (2025). Matrix Metalloproteinase-9 and Postoperative Outcomes in Carotid Endarterectomy: A Systematic Review. Journal of Clinical Medicine, 14(9), 3235. https://doi.org/10.3390/jcm14093235







