Delayed Surgical Management of Congenital Syndactyly Improves Range of Motion: A Long-Term Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants and Eligibility Criteria
2.3. Intervention
2.4. Evaluated Variables
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AER | Apical ectodermal ridge |
| AP | Anteroposterior |
| BMPs | Bone Morphogenetic Proteins |
| DASH | Disabilities of the Arm, Shoulder, and Hand |
| DIP | Distal interphalangeal |
| ECDFs | Empirical cumulative distribution functions |
| FGFs | Fibroblast Growth Factors |
| FTSG | Full-thickness skin graft |
| I | Pollex |
| II | Index finger |
| III | Middle finger |
| IV | Ring finger |
| IQRs | Interquartile ranges |
| K-wire | Kirschner wire |
| MP | Metacarpophalangeal |
| PIP | Proximal interphalangeal |
| RA | Retinoic acid |
| ROM | Range of motion |
| SDs | Standard deviations |
| SHH | Sonic hedgehog |
| STSG | Split-thickness skin graft |
| V | Little finger |
| ZPA | Zone of polarizing activity |
References
- Castilla, E.E.; Paz, J.E.; Orioli-Parreiras, I.M.; Opitz, J.M.; Hermann, J. Syndactyly: Frequency of Specific Types. Am. J. Med. Genet. 1980, 5, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Giele, H.; Giele, C.; Bower, C.; Allison, M. The Incidence and Epidemiology of Congenital Upper Limb Anomalies: A Total Population Study. J. Hand Surg. 2001, 26, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.; Hindocha, S.; Dhital, M.; Saleh, M.; Khan, W. The Epidemiology, Genetics and Future Management of Syndactyly. Open Orthop. J. 2012, 6, 14–27. [Google Scholar] [CrossRef]
- Hay, S. Incidence of Selected Congenital Malformations in Iowa. Am. J. Epidemiol. 1971, 94, 572–584. [Google Scholar] [CrossRef]
- Andersson, G.-B.; Gillberg, C.; Fernell, E.; Johansson, M.; Nachemson, A. Children with Surgically Corrected Hand Deformities and Upper Limb Deficiencies: Self-Concept and Psychological Well-Being. J. Hand Surg. Eur. Vol. 2011, 36, 795–801. [Google Scholar] [CrossRef]
- Nietosvaara, N.N.; Sommarhem, A.J.; Puhakka, J.M.; Tan, R.E.S.; Schalamon, J.; Nietosvaara, A.Y. Appearance of Congenital Hand Anomalies. Scand. J. Surg. SJS Off. Organ Finn. Surg. Soc. Scand. Surg. Soc. 2021, 110, 434–440. [Google Scholar] [CrossRef]
- Luo, J.; Fu, C.; Yao, K.; Hu, R.; Du, Q.; Liu, Z. A case-control study on genetic and environmental factors regarding polydactyly and syndactyly. Zhonghua Liu Xing Bing Xue Za Zhi 2009, 30, 903–906. [Google Scholar]
- Zaib, T.; Rashid, H.; Khan, H.; Zhou, X.; Sun, P. Recent Advances in Syndactyly: Basis, Current Status and Future Perspectives. Genes 2022, 13, 771. [Google Scholar] [CrossRef]
- Cassim, A.; Hettiarachchi, D.; Dissanayake, V.H.W. Genetic Determinants of Syndactyly: Perspectives on Pathogenesis and Diagnosis. Orphanet J. Rare Dis. 2022, 17, 198. [Google Scholar] [CrossRef]
- Temtamy, S.A.; McKusick, V.A. Syndactyly. In The Genetics of Hand Malformations; Alan R. Liss: New York, NY, USA, 1978; Volume 14, pp. 301–322. [Google Scholar]
- Malik, S. Syndactyly: Phenotypes, Genetics and Current Classification. Eur. J. Hum. Genet. 2012, 20, 817–824. [Google Scholar] [CrossRef]
- Dao, K.D.; Shin, A.Y.; Billings, A.; Oberg, K.C.; Wood, V.E. Surgical Treatment of Congenital Syndactyly of the Hand. JAAOS—J. Am. Acad. Orthop. Surg. 2004, 12, 39. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, S.; Li, N.; Feng, Z.; Liu, Q. Dorsal Hexagon Local Flap Without Skin Graft for Web Reconstruction of Congenital Syndactyly. J. Hand Surg. 2020, 45, e1–e63. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.L.; Trost, J.G.; Pederson, W.C. Syndactyly Release. Semin. Plast. Surg. 2016, 30, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Little, K.J.; Cornwall, R. Congenital Anomalies of the Hand—Principles of Management. Orthop. Clin. N. Am. 2016, 47, 153–168. [Google Scholar] [CrossRef]
- Hinkley, J.R.; Fallahi, A.-K.M. Syndactyly. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557704/ (accessed on 12 February 2025).
- Lumenta, D.B.; Kitzinger, H.B.; Beck, H.; Frey, M. Long-Term Outcomes of Web Creep, Scar Quality, and Function after Simple Syndactyly Surgical Treatment. J. Hand Surg. 2010, 35, 1323–1329. [Google Scholar] [CrossRef]
- Ferrari, B.R.; Werker, P.M.N. A Cross-Sectional Study of Long-Term Satisfaction after Surgery for Congenital Syndactyly: Does Skin Grafting Influence Satisfaction? J. Hand Surg. Eur. Vol. 2019, 44, 296–303. [Google Scholar] [CrossRef]
- Dao, K.D.; Wood, V.E.; Billings, A. Treatment of Syndactyly. Tech. Hand Up. Extrem. Surg. 1998, 2, 166–177. [Google Scholar] [CrossRef]
- Jester, A. Timing of Surgery in Congenital Hand Differences: A Consensus Proposal. J. Hand Surg. Eur. Vol. 2023, 48, 1249–1250. [Google Scholar] [CrossRef]
- Withey, S.J.; Kangesu, T.; Carver, N.; Sommerlad, B.C. The Open Finger Technique for the Release of Syndactyly. J. Hand Surg. Br. Eur. Vol. 2001, 26, 4–7. [Google Scholar] [CrossRef]
- Hudak, P.L.; Amadio, P.C.; Bombardier, C. Development of an Upper Extremity Outcome Measure: The DASH (Disabilities of the Arm, Shoulder and Hand) [Corrected]. The Upper Extremity Collaborative Group (UECG). Am. J. Ind. Med. 1996, 29, 602–608. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, S.H.; Kim, K.S.; Shin, J.H.; Hwang, J.H.; Lee, S.Y. Palmar Reconstruction Using Full-Thickness Skin Grafts from the Groin and Lateral Malleolus Regions: A Comparison of Long-Term Outcomes. Medicine 2023, 102, e36487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Pandya, S.; Alessandri Bonetti, M.; Costantino, A.; Egro, F.M. Comparison of Split Thickness Skin Graft versus Full Thickness Skin Graft for Radial Forearm Flap Donor Site Closure: A Systematic Review and Meta-Analysis. Am. J. Otolaryngol. 2024, 45, 104156. [Google Scholar] [CrossRef] [PubMed]
- De Smet, L.; Van Ransbeeck, H.; Deneef, G. Syndactyly Release: Results of the Flatt Technique. Acta Orthop. Belg. 1998, 64, 301–305. [Google Scholar] [PubMed]
- Phan, M.D.; Truong, Q.H.; Ho Nguyen, T.T.; Ngo Thi, D.M.; Nguyen, P.D. Optimizing Surgical Outcomes in Congenital Syndactyly: Evaluation of Flap and Graft Techniques in Older Pediatric Patients. Cureus 2024, 16, e64559. [Google Scholar] [CrossRef]

| Outcome | Variable | Unit (Total) | Count | Percentage | Notes |
|---|---|---|---|---|---|
| sex distribution | boy | patients (n = 20) | 12 | 60.00 | p = 0.371, non-significant |
| girl | 8 | 40.00 | |||
| boy | fingers (n = 86) | 60 | 69.76 | ||
| girl | 26 | 30.24 | p < 0.001 | ||
| laterality | bilateral | patients (n = 20) | 7 | 35.00 | bilateral vs. unilateral: p = 0.263 |
| unilateral | 13 | 65.00 | |||
| left sided | 9 | 69.23 | unilateral cases: p = 0.267 | ||
| right sided | 4 | 30.77 | |||
| digit involvement | IV | fingers (n = 85) | 25 | 29.41 | p = 0.026 vs. I, II, V |
| III | 24 | 28.23 | p = 0.039 vs. I, II, V | ||
| I | 12 | 14.12 | |||
| II | 12 | 14.12 | |||
| V | 12 | 14.12 | |||
| interdigital connections | III–IV | connections (n = 50) | 14 | 28.00 | p = 0.940 between groups |
| IV–V | 13 | 26.00 | |||
| I–II | 12 | 24.00 | |||
| II–III | 11 | 22.00 | |||
| severity | simple, complete | fingers (n = 86) | 40 | 45.52 | p < 0.028 vs. others |
| simple, incomplete | 25 | 29.07 | p < 0.001 vs. complicated | ||
| complex, complete | 17 | 19.77 | p = 0.005 vs. complicated | ||
| complicated | 4 | 4.65 | |||
| wound closure | FTSG | fingers (n = 85) | 72 | 84.71 | p < 0.001 vs. direct |
| direct closure | 13 | 15.29 | four patients, 21.05% of all patients | ||
| FTSG donor site | cubital region | fingers (n = 72; 84.88% of all) | 53 | 73.97 | one patient had both sites |
| wrist crease | 19 | 26.03 | utilized, in six children | ||
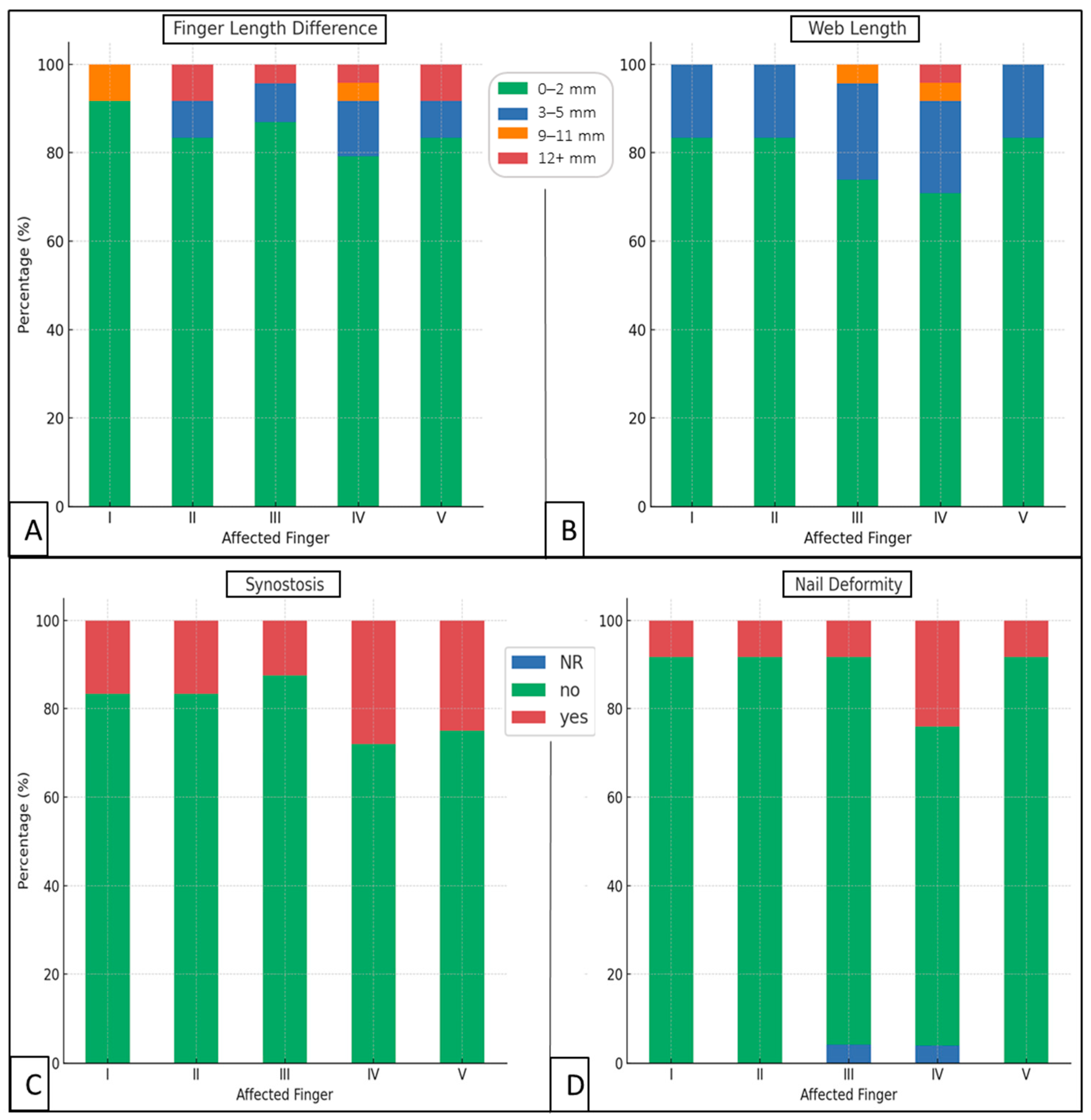
| nail deformities | affected | fingers (n = 83) | 11 | 13.25 | |
| synostosis | affected | fingers (n = 85) | 17 | 20.00 | p = 0.034 vs. nail deformities |
| Continuous Outcomes (Unit) | Count | Mean | SD | Min | Max | Median | IQR | IQR25 | IQR75 |
|---|---|---|---|---|---|---|---|---|---|
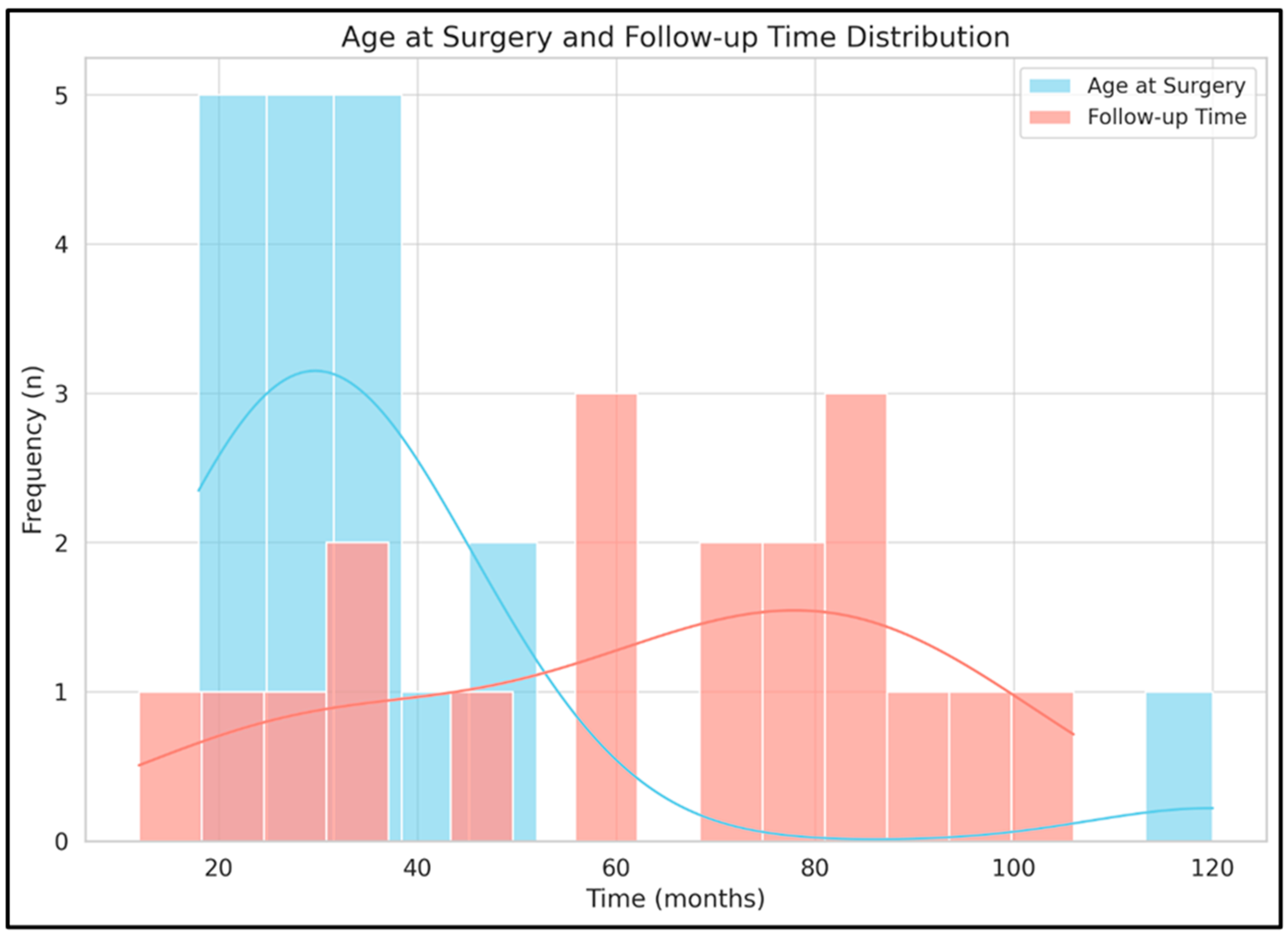
| age at surgery (months) | 86 | 33.87 | 24.63 | 18 | 120 | 31 | 11.75 | 24.75 | 36.5 |
| follow-up time (months) | 86 | 67.82 | 22.82 | 12 | 106 | 72 | 44.25 | 42 | 86.25 |
| age at follow-up (years) | 86 | 8.28 | 2.82 | 3.67 | 16 | 8.25 | 3.79 | 6.13 | 9.92 |
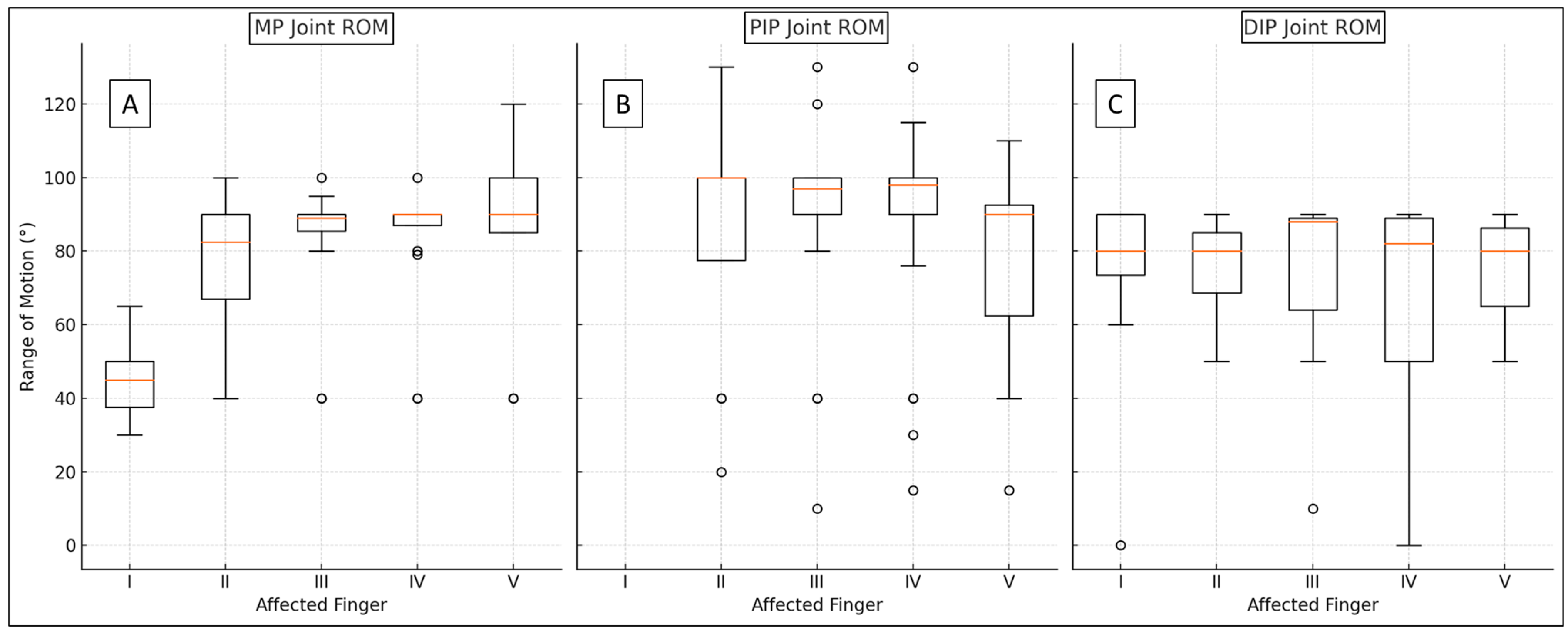
| II–V MP (°) | 71 | 83.44 | 18.08 | 40 | 120 | 89 | 5 | 85 | 90 |
| pollex MP (°) | 11 | 45.91 | 11.58 | 30 | 65 | 45 | 13 | 38 | 50 |
| PIP (°) | 71 | 86.39 | 28.33 | 10 | 130 | 98 | 10 | 90 | 100 |
| DIP (°) | 83 | 72.34 | 22.79 | 0 | 90 | 80 | 27 | 63 | 89 |
| web (mm) | 83 | 1.43 | 2.74 | 0 | 15 | 0 | 2 | 0 | 2 |
| web * (mm) | 25 | 4.76 | 3.02 | 2 | 15 | 5 | 2 | 3 | 5 |
| finger length difference (mm) | 83 | 1.40 | 3.68 | 0 | 15 | 0 | 0 | 0 | 0 |
| finger length disparity * (mm) | 14 | 8.29 | 4.87 | 2 | 15 | 5 | 8.75 | 5 | 13.75 |
| DASH (x/100) | 75 | 0.32 | 0.90 | 0 | 3.3 | 0 | 0 | 0 | 0 |
| satisfaction (1–3) | 76 | 2.76 | 0.56 | 1 | 3 | 3 | 0 | 3 | 3 |
| Continuous Outcomes (Unit) | Early (Average ± SD (n)) | Delayed (Average ± SD (n)) | p-Value |
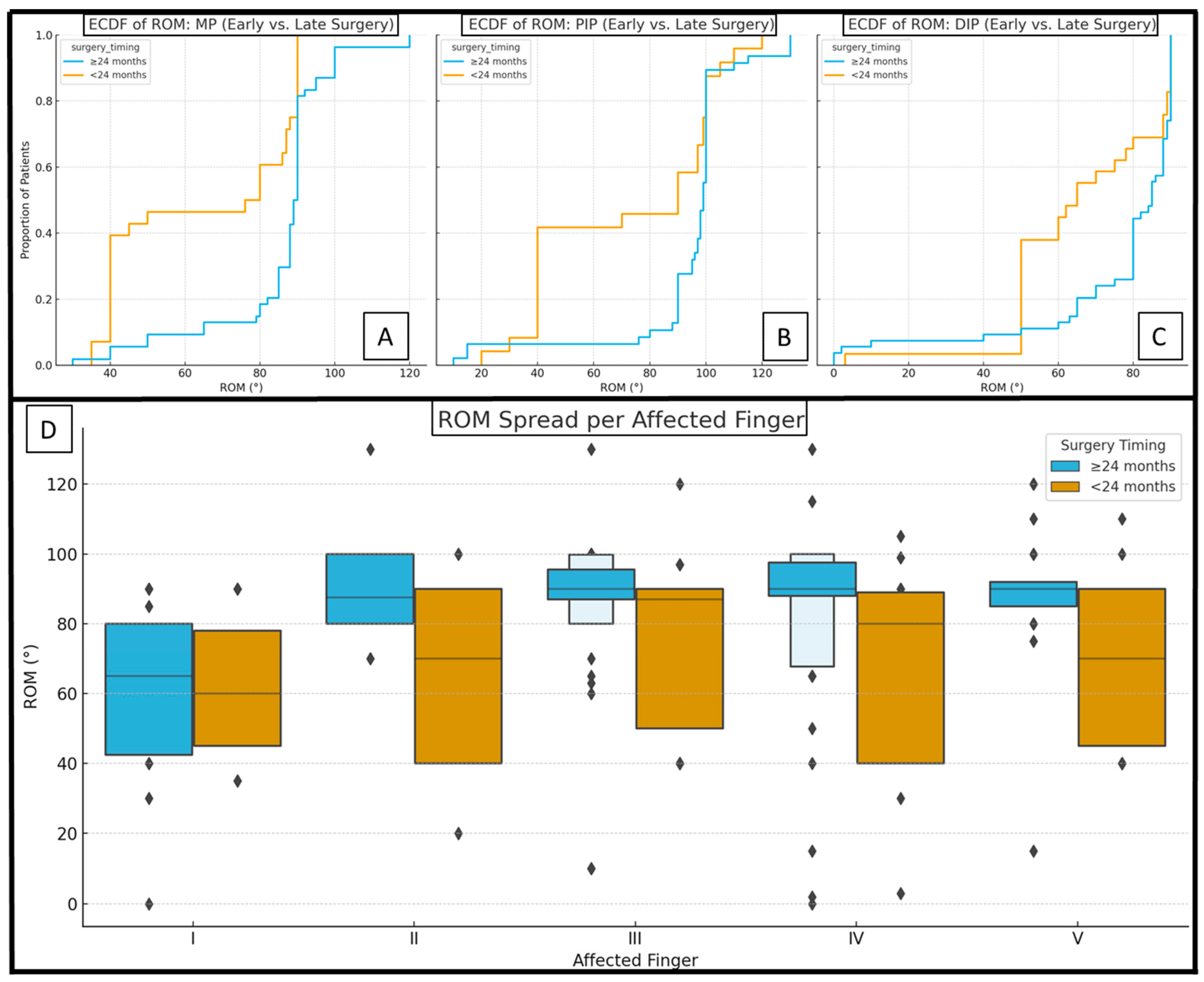
| age at surgery (months) | 20.31 ± 2.57 (36) | 46.08 ± 28.95 (50) | <0.001 * |
| follow-up time (months) | 64.39 ± 18.26 (36) | 70.90 ± 26.11 (50) | 0.059 |
| II–V MP (°) | 73.13 ± 22.37 (30) | 90.98 ± 8.44 (41) | 0.004 * |
| pollex MP (°) | 41.00 ± 6.52 (5) | 50.00 ± 13.78 (6) | 0.215 |
| PIP (°) | 77.37 ± 30.29 (30) | 93.00 ± 25.18 (41) | 0.075 |
| DIP (°) | 67.19 ± 22.78 (36) | 76.28 ± 22.24 (47) | 0.028 * |
| web (mm) | 1.56 ± 3.07 (36) | 1.34 ± 2.48 (47) | 0.937 |
| finger length difference (mm) | 1.25 ± 3.02 (36) | 1.51 ± 4.14 (47) | 0.662 |
| DASH (x/100) | 0.00 ± 0.00 (26) | 0.24 ± 0.81 (49) | 0.141 |
| satisfaction (1–3) | 3.00 ± 0.00 (27) | 2.80 ± 0.50 (49) | 0.028 * |
| Discrete Outcomes | Early (yes/n (%)) | Delayed (yes/n (%)) | p-Value |
| finger | 36/86 (41.86) | 50/86 (58.14) | 0.404 |
| female | 14/36 (38.88) | 12/50 (24.00) | 0.232 |
| grafted | 18/29 (62.07) | 48/50 (96.00) | <0.001 * |
| synostosis | 14/36 (38.88) | 3/50 (6.00) | 0.002 * |
| nail deformity | 3/36 (8.33) | 8/50 (16.00) | 0.229 |
| Continuous Outcome (Unit) | Elbow (Average ± SD (n)) | Wrist (Average ± SD (n)) | Direct Closure (Average ± SD (n)) | p-Value |
| age at surgery (months) | 40.28 ± 30.55 (53) | 28.11 ± 7.19 (19) | 21.69 ± 9.01 (13) | 0.027 * |
| follow-up time (month) | 64.23 ± 20.54 (53) | 89.42 ± 12.04 (19) | 45.15 ± 9.86 (13) | <0.001 * |
| II–V MP (°) | 89.27 ± 11.18 (45) | 88.12 ± 4.79 (16) | 49.70 ± 20.45 (10) | <0.001 * |
| pollex MP (°) | 42.50 ± 7.58 (6) | 60.00 ± 8.66 (3) | 35.00 ± 0.00 (2) | 0.011 * |
| PIP (°) | 93.27 ± 22.91 (45) | 88.75 ± 29.86 (16) | 51.70 ± 24.67 (10) | <0.001 * |
| DIP (°) | 74.80 ± 24.00 (51) | 73.74 ± 21.47 (19) | 60.62 ± 16.78 (13) | 0.128 |
| web (mm) | 1.61 ± 2.66 (51) | 1.42 ± 3.42 (19) | 0.77 ± 1.88 (13) | <0.001 * |
| finger length difference (mm) | 1.20 ± 3.53 (51) | 1.84 ± 4.78 (19) | 1.54 ± 2.40 (13) | 0.802 |
| DASH (x/100) | 0.22 ± 0.78 (53) | 0.00 ± 0.00 (19) | 0.00 ± 0.00 (3) | 0.434 |
| satisfaction (1–3) | 2.77 ± 0.52 (44) | 3.00 ± 0.00 (19) | 3.00 ± 0.00 (13) | 0.057 |
| Discrete Outcomes | Elbow (yes/n (%)) | Wrist (yes/n (%)) | Direct Closure (yes/n (%)) | p-Value |
| fingers | 53/85 (62.35%) | 19/85 (22.35%) | 13/85 (15.30%) | <0.001 * |
| female | 20/53 (37.74%) | 2/19 (10.53%) | 3/13 (23.08%) | 0.071 |
| synostosis | 4/53 (7.55%) | 3/19 (15.79%) | 10/13 (76.92%) | <0.001 * |
| nail deformity | 8/53 (15.09%) | 3/19 (15.79%) | 0/13 (0.00%) | 0.319 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lőrincz, A.; Nudelman, H.; Kormos, E.I.; Józsa, G. Delayed Surgical Management of Congenital Syndactyly Improves Range of Motion: A Long-Term Follow-Up. J. Clin. Med. 2025, 14, 3200. https://doi.org/10.3390/jcm14093200
Lőrincz A, Nudelman H, Kormos EI, Józsa G. Delayed Surgical Management of Congenital Syndactyly Improves Range of Motion: A Long-Term Follow-Up. Journal of Clinical Medicine. 2025; 14(9):3200. https://doi.org/10.3390/jcm14093200
Chicago/Turabian StyleLőrincz, Aba, Hermann Nudelman, Edina Ilona Kormos, and Gergő Józsa. 2025. "Delayed Surgical Management of Congenital Syndactyly Improves Range of Motion: A Long-Term Follow-Up" Journal of Clinical Medicine 14, no. 9: 3200. https://doi.org/10.3390/jcm14093200
APA StyleLőrincz, A., Nudelman, H., Kormos, E. I., & Józsa, G. (2025). Delayed Surgical Management of Congenital Syndactyly Improves Range of Motion: A Long-Term Follow-Up. Journal of Clinical Medicine, 14(9), 3200. https://doi.org/10.3390/jcm14093200





