The Impact of Nutritional Support on Outcomes of Lung Cancer Surgery—Narrative Review
Abstract
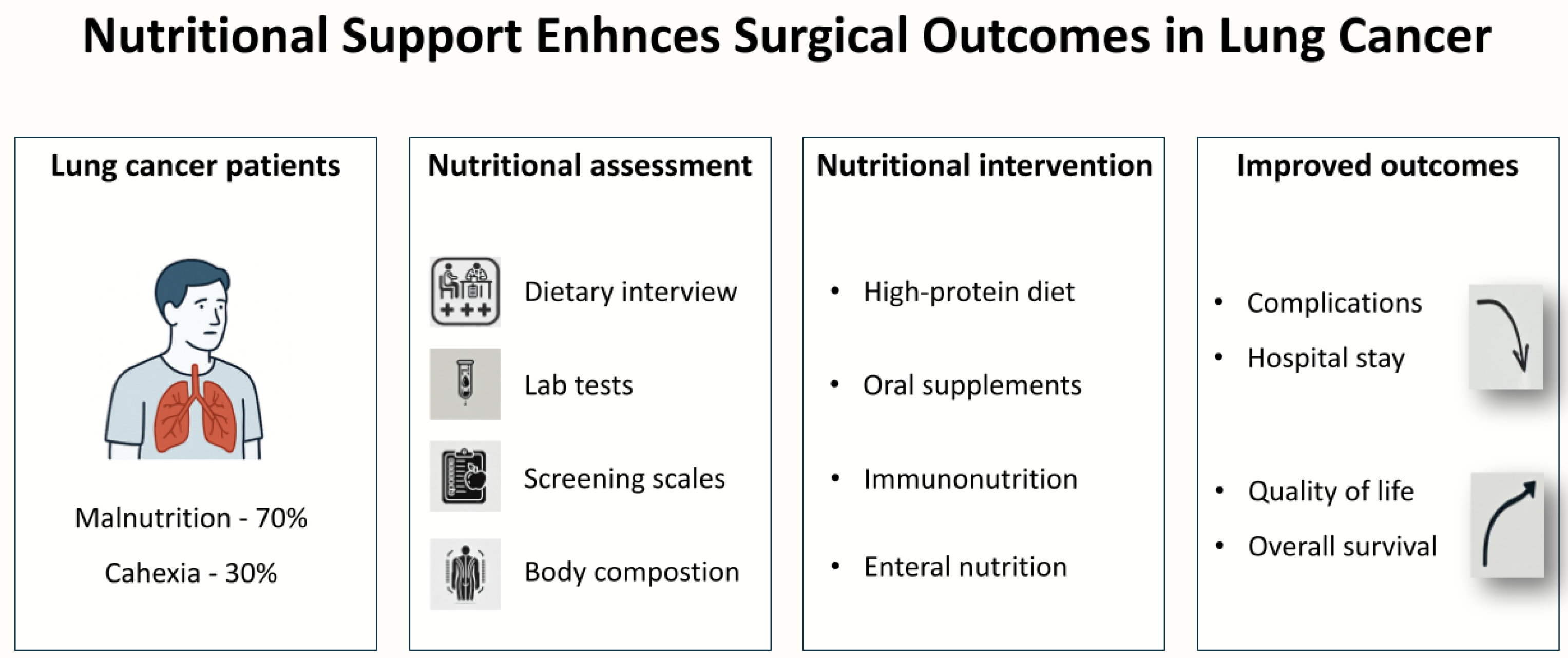
1. Introduction
2. Nutritional Status Assessment in Patients with Lung Cancer
2.1. Nutritional Status of Cancer Patients
2.2. Methods of Nutritional Status Assessment
- Dietary interview: The dietary interview is the basic tool for assessing nutritional status. It includes a detailed conversation with the patient about eating habits, such as the frequency and types of meals consumed, dietary preferences, and the use of dietary supplements [28]. Additionally, the interview includes questions about eating difficulties, such as swallowing problems (dysphagia), nausea, vomiting, diarrhea, constipation, and changes in taste and smell perception [28,29]. An important element is also the assessment of body weight changes, especially losses exceeding 10% of initial body weight within the last 3–6 months [22]. The dietary interview allows for the initial identification of patients at risk of malnutrition and the adaptation of nutritional interventions to their individual needs [20].
- Physical examination: Physical examination is essential for assessing the overall condition of a patient undergoing surgery for lung cancer and detecting signs of malnutrition or cancer cachexia [30,31,32]. It includes the following:
- Clinical signs of malnutrition: dry skin, brittle hair, edema, changes in mucous membranes, and muscle weakness [6].
- Laboratory tests: laboratory tests provide objective data regarding nutritional status; however, their interpretations necessitates consideration of inflammatory conditions and comorbidities, which can influence the results [36]. Key parameters include the following:
- Albumin (half-life 20 days): low albumin levels (<3.5 g/dL) correlate with poorer prognosis but exhibit low sensitivity to short-term nutritional changes due to its long half-life [37];
- Prealbumin: with a short half-life (2–3 days), prealbumin is more sensitive marker of current nutritional status; however, its deficiency can also result from the acute phase response, and levels below 10 mg/dL suggest malnutrition [38];
- Transferrin: low transferrin levels (<200 mg/dL) may indicate iron and protein deficiency, but its concentration is dependent on inflammatory status [39];
- Peripheral lymphocytes: reduced lymphocyte count (<1500/μL) is associated with impaired immune function but may be associated with chemotherapy or infections [40];
- Cholesterol: low cholesterol levels (<97 mg/dL) increase mortality risk in patients [41];
- CRP (C-reactive protein): levels above 10 mg/L indicate inflammation, often associated with cancer cachexia [42].Laboratory tests are useful in monitoring the response to nutritional interventions, but they should be interpreted in conjunction with clinical assessment (e.g., nutritional history, anthropometric measurements, etc.) and screening tools (e.g., NRS 2002, PG-SGA, etc.) to avoid misdiagnosis.
- Scales and questionnaires: scales and questionnaires are simple tools for assessing nutritional status and the risk of malnutrition [21]. Some of the most commonly used include the following:
- Mini Nutritional Assessment (MNA): A tool primarily for older adults, assessing nutritional status based on interview, physical examination, and laboratory parameters. A score < 17 indicates malnutrition, 17–23.5 indicates risk of malnutrition [43];
- NRS 2002 (Nutritional Risk Screening 2002): A screening tool for assessing malnutrition risk in hospitalized patients, covering weight loss, food intake, and disease severity. A score ≥ 3 indicates malnutrition risk [48];
- MUST (Malnutrition Universal Screening Tool): A simple tool for assessing malnutrition risk, applicable to various patient groups, including oncology patients. A score ≥ 2 indicates high malnutrition risk [49];
- Global Leadership Initiative on Malnutrition (GLIM): This diagnostic framework utilizes a two-step approach for identifying malnutrition. The process begins with initial screening using validated tools such as NRS 2002 or MUST. For patients identified as at-risk, a comprehensive assessment evaluates both phenotypic and etiologic criteria.For diagnosis, patients must meet at least one phenotypic criterion: unintended weight loss exceeding 5% within six months or 10% over a longer period, low body mass index (BMI below 18.5 for patients under 70 years or below 20 for those 70 and older), or clinically evident reduction in muscle mass. Additionally, at least one etiologic criterion must be present: either significantly reduced food intake (consuming less than 50% of nutritional requirements for more than one week) or presence of inflammation as evidenced by markers like elevated CRP in cancer patients [17].The GLIM framework not only facilitates malnutrition diagnosis but also enables severity stratification (Stage 1 or 2) and has demonstrated particular utility in oncology populations through validation studies. This standardized approach enhances consistency in nutritional assessment across clinical settings while addressing the complex interplay between nutritional status and disease processes [17].
- Body composition analysis: body composition analysis provides detailed information about muscle and fat mass, which is important in detecting malnutrition or cancer cachexia [20,30]. Common methods include the following:
- Bioelectrical impedance analysis (BIA) represents a non-invasive and readily accessible body composition assessment modality that is gaining increasing clinical relevance in the nutritional monitoring of oncological patients. This technology enables comprehensive quantitative and qualitative evaluation of muscle mass through measurement of fat-free mass (FFM) and skeletal muscle mass (SMM), with normative values corresponding to 75–85% of FFM in healthy adult populations. A particularly significant parameter is the phase angle (PA), which serves as a biomarker of cellular membrane integrity. Reference values for PA typically range between 5–7° in healthy individuals, while diminished values (<4.5°) demonstrate significant correlation with poorer prognostic outcomes in neoplastic disease [50]. In pulmonary carcinoma specifically, phase angle measurements below 3.8° are associated with a 2.5-fold increase in mortality risk, establishing this parameter as a valuable prognostic indicator. BIA facilitates early detection of muscle mass depletion (>5% reduction in FFM over a 3-month period) and enables identification of sarcopenia, including in patients with normal or elevated body mass index (BMI) [50]. The skeletal muscle mass index (SMI) proves particularly clinically relevant, with values below 7.26 kg/m2 in male patients and 5.45 kg/m2 in female patients correlating with a threefold increased risk of postoperative pulmonary complications. This methodology also proves instrumental in monitoring therapeutic outcomes of nutritional interventions and prehabilitation programs through objective assessment of muscle mass accrual. Beyond muscular evaluation, BIA provides critical data regarding adipose tissue content (reference ranges: 10–20% in males; 20–30% in females) [51];
- Dual-energy X-ray absorptiometry (DXA) currently represents the gold standard for body composition assessment, utilizing the differential absorption of X-rays at two distinct energy levels. This technology enables simultaneous and precise evaluation of fat-free mass with 1–2% accuracy, adipose tissue with differentiation between subcutaneous and visceral depots, and bone mineral content [52]. In oncological practice, DXA has proven particularly valuable for early detection of cancer cachexia, where a >5% loss of muscle mass over 3 months is considered clinically significant, and for diagnosing sarcopenia using established thresholds of FFM <17 kg/m2 in men and <15 kg/m2 in women [52]. Characterized by low radiation exposure (1–10 μSv) and rapid scan times (10–15 min), this method also permits regional fat distribution analysis, which is crucial for monitoring body composition changes during therapy. However, results may be influenced by hydration status, and limitations exist in assessing individuals with body weight exceeding 130 kg [53];
- CT and MRI: For cases requiring more precise evaluation, particularly in surgical planning, computed tomography (CT) and magnetic resonance imaging (MRI) techniques are employed. CT, especially when analyzing a single slice at the third lumbar vertebra (L3), is considered the reference standard for quantitative muscle mass assessment, offering measurement error below 2% while simultaneously evaluating muscle quality through Hounsfield unit (HU) analysis. In lung cancer, established skeletal muscle index (SMI) thresholds of <52.4 cm2/m2 for men and <38.5 cm2/m2 for women are particularly relevant, along with myosteatosis criteria defined as <41 HU for men and <38 HU for women. Research demonstrates that >8.3% muscle mass loss on CT correlates with a 4.1-fold increased risk of postoperative complications, which is critical for thoracic surgery patient selection [54].MRI, as a non-ionizing radiation modality, offers even broader diagnostic capabilities, including whole-body muscle volumetry with ±1.5% accuracy and quantitative fat infiltration assessment (PDFF) with ±0.5% precision. Advanced MRI protocols incorporate diffusion tensor imaging for muscle microstructure evaluation and spectroscopy for metabolic assessment through ATP/PCr ratio measurement. Clinically, the detection of even early muscular changes is particularly significant, where fat infiltration exceeding 5% on PDFF correlates with substantially worse prognosis. Importantly, results obtained through different modalities show strong correlations—for instance, reduced phase angle values in BIA (<4.5°) correlate with decreased SMI on CT (r = 0.82), while increased BIA resistance corresponds to greater fat infiltration visible on MRI [54]. In clinical practice, a stepped approach is therefore recommended, with BIA serving as a screening tool, while DXA, CT, or MRI are utilized for more detailed assessment based on specific diagnostic needs and technical availability. This integrated methodology optimizes the unique advantages of each technique while minimizing their respective limitations [55].
- CONUT (Controlling Nutritional Status): The CONUT index is a simple tool based on laboratory parameters such as albumin levels, peripheral lymphocyte count, and cholesterol levels. A high CONUT score (≥6) is associated with worse prognosis, including lower overall survival (OS) and disease-free survival (DFS). A low CONUT score may indicate better prognosis [26,56].
- CALLY (C-reactive protein-albumin-lymphocyte): The CALLY index is specifically designed for assessing nutritional status in cancer patients. A score <3.0 is associated with worse DFS and OS. The index includes the assessment of inflammatory markers—C-reactive protein, albumin, and lymphocytes. The CALLY index helps in the early detection of cancer cachexia and the implementation of appropriate nutritional interventions [27,57].
3. The Impact of Malnutrition on Outcomes of Lung Cancer Surgery
4. Preoperative Nutritional Strategies
4.1. Nutritional Support Strategies for Lung Cancer Patients
4.1.1. Short-Term Goals
4.1.2. Long-Term Goals
4.2. Forms of Nutritional Support
4.2.1. High-Protein Diet
4.2.2. Oral Nutritional Supplements
4.2.3. Enteral Nutrition
4.2.4. Parenteral Nutrition
4.2.5. Perioperative Immunomodulation
4.3. The Role of ERAS in Optimizing Nutritional Support
5. Practical Guidance for Pulmonologist, Oncologists, and Thoracic Surgeons
- Nutritional and Sarcopenia Assessment
- Mandatory nutritional screening and standardized diagnosis: This includes performing systematic nutritional risk screening at surgical qualification using validated tools (e.g., NRS 2002 and MUST). In patients identified as at-risk, the Global Leadership Initiative on Malnutrition (GLIM) criteria should be applied to confirm the diagnosis of malnutrition, requiring at least one phenotypic and one etiologic criterion;
- Comprehensive nutritional assessment: this includes conducting detailed evaluations, including dietary interview, physical examination (BMI, calf and arm circumference, handgrip strength), laboratory investigations (albumin, prealbumin, CRP), and body composition analysis (BIA, DXA, CT, or MRI when available);
- Early detection of sarcopenia: this includes assessing skeletal muscle mass and strength systematically, utilizing validated thresholds (e.g., SMI values on CT or DXA and handgrip strength measures) to diagnose sarcopenia in its early stages;
- Continuous perioperative monitoring: this includes reassesses nutritional status and muscle mass throughout the perioperative period;
- Multidisciplinary team approach: this includes integrating dietitians, physiotherapists, and rehabilitation specialists into the oncological care team to optimize nutritional support and enhance functional recovery.
- High-Protein Diet
- Individualize protein requirements: age, body weight, health status, and physical activity levels should be considered when determining daily protein needs. For most patients, 1.2–1.5 g/kg body weight/day is optimal, but elderly or severely malnourished patients may require 1.5–2.0 g/kg body weight/day;
- Diverse protein sources: a variety of protein sources should be recommended, including animal-based (poultry, fish, eggs, and dairy) and plant-based (legumes, tofu, and quinoa).
- Oral Nutritional Supplements
- Early intervention: oral nutritional supplements should be implemented early for patients with appetite loss, swallowing difficulties, or other issues preventing adequate food intake;
- Combine with physical activity: if possible, combining protein supplementation with resistance exercises should be recommended to improve muscle mass and strength before surgery;
- Monitor outcomes: body weight and composition should be regularly assessed to evaluate the effectiveness of supplementation and adjust dosages as needed.
- Enteral Nutrition
- Choose the right access method: Nasogastric tubes or PEG should be considered for patients with swallowing difficulties or chronic malnutrition. PEG is preferred for long-term nutritional support;
- Early implementation: enteral nutrition should be initiated 7–10 days before surgery in malnourished patients to reduce infection risks and improve wound healing;
- Monitor for complications: patients should be watched for risks such as diarrhea, tube obstruction, or aspiration pneumonia, and they should be monitored regularly.
- Parenteral Nutrition
- Limited use: parenteral nutrition should be used only when enteral nutrition is not feasible, such as in cases of severe malabsorption, intestinal obstruction, or short bowel syndrome;
- Intensive monitoring: due to risks such as sepsis, metabolic disturbances, and liver damage, parenteral nutrition requires regular laboratory monitoring and tailored therapy.
- Perioperative Immunomodulation
- Arginine supplementation: arginine supplementation should be considered before surgery to improve blood flow and wound healing, reducing the risk of infectious complications;
- Omega-3 fatty acids: omega-3 supplementation should be recommended for patients with chronic conditions (e.g., cancer) to reduce inflammation and improve preoperative outcomes;
- Glutamine: glutamine supplementation should be considered for patients at high risk of inflammation, as it can reduce markers such as C-reactive protein and interleukin-6;
- Combine with probiotics: increasing evidence suggests that combining immunomodulatory supplements with probiotics can enhance immune function and shorten hospital stays.
6. Summary
- Areas for Future Research:
- Lack of standardized nutritional assessment methods: despite the availability of various tools, there is a lack of unified guidelines for their use across different disease stages and patient groups;
- Limitations in body composition assessment: methods such as bioelectrical impedance analysis (BIA) and dual-energy X-ray absorptiometry (DXA) are unavailable in many centers, making precise assessment of muscle and fat mass difficult;
- Insufficient understanding of cancer cachexia: the mechanisms driving cachexia and its impact on treatment outcomes require further research, particularly in the context of nutritional and pharmacological interventions;
- Lack of long-term studies on nutritional interventions: most available data focus on short-term effects, while the long-term benefits of nutritional optimization remain unclear;
- Limitations in immunomodulation use: despite promising results, there are no clear guidelines on dosing and duration for immunomodulatory supplements (e.g., arginine and omega-3 fatty acids).
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Rami-Porta, R.; Bolejack, V.; Crowley, J.; Ball, D.; Kim, J.; Lyons, G.; Rice, T.; Suzuki, K.; Thomas, C.F.; Travis, W.D.; et al. The IASLC Lung Cancer Staging Project: Proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2015, 10, 990–1003. [Google Scholar] [CrossRef]
- Lembicz, M.; Gabryel, P.; Brajer-Luftmann, B.; Dyszkiewicz, W.; Batura-Gabryel, H. Comorbidities with non-small cell lung cancer: Is there an interdisciplinary consensus needed to qualify patients for surgical treatment? Ann. Thorac. Med. 2018, 13, 101–107. [Google Scholar] [CrossRef]
- Gabryel, P.; Skrzypczak, P.; Campisi, A.; Kasprzyk, M.; Roszak, M.; Piwkowski, C. Predictors of long-term survival of thoracoscopic lobectomy for stage IA non-small cell lung cancer: A large retrospective cohort study. Cancers 2023, 15, 3877. [Google Scholar] [CrossRef]
- Arends, J. Malnutrition in cancer patients: Causes, consequences and treatment options. Eur. J. Surg. Oncol. 2024, 50, 107074. [Google Scholar] [CrossRef]
- Oscanoa, T.J.; Cieza, E.C.; Pourhassan, M.; Romero-Ortuno, R. Frequency of malnutrition in older adults according to different types of cancer. Nowotw. J. Oncol. 2024, 74, 159–165. [Google Scholar] [CrossRef]
- Kiss, N.; Curtis, A. Current insights in nutrition assessment and intervention for malnutrition or muscle loss in people with lung cancer: A narrative review. Adv. Nutr. 2022, 13, 2420–2434. [Google Scholar] [CrossRef]
- The Problem of Hospital Malnutrition and Its Consequences. Termedia. Available online: https://www.termedia.pl/The-problem-of-hospital-malnutrition-and-its-consequences,67,40247,1,1.html (accessed on 26 February 2025).
- Kim, D.; Lee, J.W. Current status of lung cancer and surgery based on studies using a nationwide database. J. Chest Surg. 2022, 55, 1–9. [Google Scholar] [CrossRef]
- Sigona, A.; Richman, D.C. Identifying and reducing risks of postoperative pulmonary complications. J. Oral Maxillofac. Anesth. 2023, 2, 30. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Xiu, Z.; Guo, J.; Wang, L.; Zhou, Y.; Jiao, Y.; Sun, M.; Cai, J. Combination of immune checkpoint inhibitors with chemotherapy in lung cancer. Onco Targets Ther. 2020, 13, 7229–7241. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hu, Y.; Chen, J. Effectiveness of an ERAS-based exercise-nutrition management model in enhancing postoperative recovery for thoracoscopic radical resection of lung cancer: A randomized controlled trial. Medicine 2024, 103, e37667. [Google Scholar] [CrossRef]
- Kaya, S.O.; Akcam, T.I.; Ceylan, K.C.; Samancliar, O.; Ozturk, O.; Usluer, O. Is preoperative protein-rich nutrition effective on postoperative outcome in non-small cell lung cancer surgery? A prospective randomized study. J. Cardiothorac. Surg. 2016, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.; Ferreira, V.; Carli, F.; Chevalier, S. Effects of multimodal prehabilitation on muscle size, myosteatosis, and dietary intake of surgical patients with lung cancer: A randomized feasibility study. Appl. Physiol. Nutr. Metab. 2021, 46, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Hao, Y.; Rao, B.; Cao, Y. Prognostic impact of the pre-treatment controlling nutritional status score in patients with non-small cell lung cancer: A meta-analysis. Medicine 2021, 100, e26488. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. ESPEN Endorsed Recommendation: GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report From the Global Clinical Nutrition Community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Bonomi, P.D.; Crawford, J.; Dunne, R.F.; Roeland, E.J.; Smoyer, K.E.; Siddiqui, M.K.; McRae, T.D.; Rossulek, M.I.; Revkin, J.H.; Tarasenko, L.C. Mortality burden of pre-treatment weight loss in patients with non-small-cell lung cancer: A systematic literature review and meta-analysis. J. Cachexia Sarcopenia Muscle 2024, 15, 1226–1238. [Google Scholar] [CrossRef]
- Sheas, M.N.; Ali, S.R.; Safdar, W.; Tariq, M.R.; Ahmed, S.; Ahmad, N.; Hameed, A.; Qazi, A.S. Nutritional assessment in cancer patients. Cancer Treat. Res. 2023, 185, 285–310. [Google Scholar] [CrossRef]
- Van Bokhorst-de van der Schueren, M.A.E.; Guaitoli, P.R.; Jansma, E.P.; de Vet, H.C.W. Nutrition screening tools: Does one size fit all? A systematic review of screening tools for the hospital setting. Clin. Nutr. 2014, 33, 39–58. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Wang, P.-P.; Soh, K.L.; Khazaai, H.B.; Ning, C.-Y.; Huang, X.-L.; Yu, J.-X.; Liao, J.-L. Nutritional assessment tools for patients with cancer: A narrative review. Curr. Med. Sci. 2024, 44, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Crestani, M.S.; Grassi, T.; Steemburgo, T. Methods of nutritional assessment and functional capacity in the identification of unfavorable clinical outcomes in hospitalized patients with cancer: A systematic review. Nutr. Rev. 2022, 80, 786–811. [Google Scholar] [CrossRef] [PubMed]
- Gil-Andrés, D.; Cabañas-Alite, L. A narrative review comparing nutritional screening tools in outpatient management of cancer patients. Nutrients 2024, 16, 752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, X.-K.; Cong, Z.-Z.; Zheng, C.; Luo, C.; Xie, K.; Xu, Y.; Gu, W.-F.; Qiang, Y.; Shen, Y. Controlling nutritional status is a prognostic factor for patients with lung cancer: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 3896–3905. [Google Scholar] [CrossRef]
- Matsui, S.; Kato, Y.; Ohgi, K.; Ashida, R.; Yamada, M.; Otsuka, S.; Uesaka, K.; Sugiura, T. Prognostic impact of the CALLY index in patients with resectable pancreatic cancer. Surg. Oncol. Insight 2025, 2, 100119. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Hesketh, P.J.; Kris, M.G.; Basch, E.; Bohlke, K.; Barbour, S.Y.; Clark-Snow, R.A.; Danso, M.A.; Dennis, K.; Dupuis, L.L.; Dusetzina, S.B.; et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 3240–3261. [Google Scholar] [CrossRef]
- de Andrade, G.K.P.; Carvalho, J.B.; do Nascimento, L.A.; Rodrigues, A.L.C.C.; Severine, A.N. Nutritional assessment team: Body composition assessment protocol in hospitalized patients. Clin. Nutr. Open Sci. 2022, 42, 119–129. [Google Scholar] [CrossRef]
- Pinto, A.C.; Sousa, A.S.; Amaral, T.F.; Guerra, R.S. Association between anthropometric indicators of nutrition status and length of hospital stay in hospitalized patients. J. Parenter. Enteral. Nutr. 2021, 45, 381–393. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Gonzalez, M.C.; Schulzke, J.D.; Pirlich, M. Hand grip strength: Outcome predictor and marker of nutritional status. Clin. Nutr. 2011, 30, 135–142. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile And Cut Off Points; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- World Health Organization. Malnutrition in Women. Available online: https://www.who.int/data/nutrition/nlis/info/malnutrition-in-women (accessed on 3 February 2025).
- Kim, S.Y.; Kim, M.J.; Shin, D.W.; Won, C.W.; Shim, H.Y.; Cho, B.L. Mid-upper arm circumference as a screening tool for identifying physical frailty in community-dwelling older adults: The Korean Frailty and Aging Cohort Study. Geriatr. Gerontol. Int. 2024, 24, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Keller, U. Nutritional laboratory markers in malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef]
- Beck, F.K.; Rosenthal, T.C. Prealbumin: A marker for nutritional evaluation. Am. Fam. Physician 2002, 65, 1575–1579. [Google Scholar]
- Benjamin, S.; Assounga, A. Transferrin levels are associated with malnutrition markers in hemodialysis patients in KwaZulu-Natal, South Africa. Ren. Fail. 2024, 46, 2337292. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Zheng, H.; Wang, X.; Liu, Z.; Sun, T. Prognostic role of serum albumin, total lymphocyte count, and mini nutritional assessment on outcomes after geriatric hip fracture surgery: A meta-analysis and systematic review. J. Arthroplasty 2019, 34, 1287–1296. [Google Scholar] [CrossRef]
- Kim, S.; Kim, G.; Cho, S.H.; Oh, R.; Kim, J.Y.; Lee, Y.-B.; Jin, S.-M.; Hur, K.Y.; Kim, J.H. Association between total cholesterol levels and all-cause mortality among newly diagnosed patients with cancer. Sci. Rep. 2024, 14, 58. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Heimbürger, O.; Paultre, F.; Diczfalusy, U.; Wang, T.; Berglund, L.; Jogestrand, T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999, 55, 1899–1911. [Google Scholar] [CrossRef]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.-L. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? J. Parenter. Enteral. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef]
- Duerksen, D.R.; Laporte, M.; Jeejeebhoy, K. Evaluation of nutrition status using the subjective global assessment: Malnutrition, cachexia, and sarcopenia. Nutr. Clin. Pract. 2021, 36, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Leipold, C.E.; Bertino, S.B.; L’Huillier, H.M.; Howell, P.M.; Rosenkotter, M. Validation of the Malnutrition Screening Tool for use in a community rehabilitation program. Nutr. Diet. 2018, 75, 117–122. [Google Scholar] [CrossRef]
- Ferguson, M.; Bauer, J.; Gallagher, B.; Capra, S.; Christie, D.; Mason, B. Validation of a malnutrition screening tool for patients receiving radiotherapy. Australas. Radiol. 1999, 43, 325–327. [Google Scholar] [CrossRef]
- Hersberger, L.; Bargetzi, L.; Bargetzi, A.; Tribolet, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; et al. Nutritional risk screening (NRS 2002) is a strong and modifiable predictor risk score for short-term and long-term clinical outcomes: Secondary analysis of a prospective randomised trial. Clin. Nutr. 2020, 39, 2720–2729. [Google Scholar] [CrossRef]
- Sharma, Y.; Avina, P.; Ross, E.; Horwood, C.; Hakendorf, P.; Thompson, C. Validity of the Malnutrition Universal Screening Tool for evaluation of frailty status in older hospitalized patients. Gerontol. Geriatr. Med. 2022, 8, 23337214221107816. [Google Scholar] [CrossRef]
- Bilir, C.; Engin, H. Body composition and bioelectrical impedance analysis; Prognostic significance of measurements in patients with locally advanced or metastatic cancer. Ann. Oncol. 2014, 25 (Suppl. 4), iv533. [Google Scholar] [CrossRef]
- Alexio, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Battaglini, C.L.; Williams, G.R. Bioelectrical Impedance Analysis for the Assessment of Sarcopenia in Patients with Cancer: A Systematic Review. Oncologist 2020, 25, 170–182. [Google Scholar] [CrossRef]
- Valensise, H.; Andreoli, A.; Lello, S.; Magnani, F.; Romanini, C.; De Lorenzo, A. Total-body skeletal muscle mass: Development and cross-validation of anthropometric prediction models. Am. J. Clin. Nutr. 2000, 72, 796–803. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Baracos, V.E. Dual-Energy X-ray Absorptiometry in Cancer Patients: Research Applications and Clinical Utility. Appl. Physiol. Nutr. Metab. 2010, 35, 571–588. [Google Scholar]
- Bennett, J.P.; Ford, K.L.; Siervo, M.; Gonzalez, M.C.; Lukaski, H.C.; Sawyer, M.B.; Mourtzakis, M.; Deutz, N.E.; Shepherd, J.A.; Prado, C.M. Advancing body composition assessment in patients with cancer: First comparisons of traditional versus multicompartment models. Nutrition 2024, 125, 112494. [Google Scholar] [CrossRef]
- Thoresen, M.; Rønning, P.A.; Haugnes, H.S.; Kleven, O.C.; Sørhaug, S.; Paulsen, T.; Roislien, J.; Jordal, M.; Oldervoll, L.M.; Brustad, M. Computed Tomography and Magnetic Resonance Imaging for the Assessment of Cancer-Related Sarcopenia and Myosteatosis: Current Evidence and Future Perspectives. Acta Oncol. 2020, 59, 1133–1145. [Google Scholar]
- Akamine, T.; Toyokawa, G.; Matsubara, T.; Kozuma, Y.; Haratake, N.; Takamori, S.; Katsura, M.; Takada, K.; Shoji, F.; Okamoto, T.; et al. Significance of the preoperative CONUT score in predicting postoperative disease-free and overall survival in patients with lung adenocarcinoma with obstructive lung disease. Anticancer Res. 2017, 37, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Iida, H.; Tani, M.; Komeda, K.; Nomi, T.; Matsushima, H.; Tanaka, S.; Ueno, M.; Nakai, T.; Maehira, H.; Mori, H.; et al. Superiority of CRP-albumin-lymphocyte index (CALLY index) as a non-invasive prognostic biomarker after hepatectomy for hepatocellular carcinoma. HPB 2022, 24, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Polański, J.; Chabowski, M.; Świątoniowska-Lonc, N.; Dudek, K.; Jankowska-Polańska, B.; Zabierowski, J.; Mazur, G. Relationship between nutritional status and clinical outcome in patients treated for lung cancer. Nutrients 2021, 13, 3332. [Google Scholar] [CrossRef]
- Duan, P.; Hu, C.; Quan, C.; Yi, X.; Zhou, W.; Yuan, M.; Yu, T.; Kourouma, A.; Yang, K. Body mass index and risk of lung cancer: Systematic review and dose-response meta-analysis. Sci. Rep. 2015, 5, 16938. [Google Scholar] [CrossRef]
- Goins, E.C.; Weber, J.M.; Truong, T.; Moss, H.A.; Previs, R.A.; Davidson, B.A.; Havrilesky, L.J. Malnutrition as a risk factor for post-operative morbidity in gynecologic cancer: Analysis using a national surgical outcomes database. Gynecol. Oncol. 2022, 165, 309–316. [Google Scholar] [CrossRef]
- Meissner, C.; Otto, R.; Fahlke, J.; Mueller, M.; Ridwelski, K. Relationship between nutritional status and length of hospital stay in oncological surgery. J. Clin. Oncol. 2020, 38, e13591. [Google Scholar] [CrossRef]
- Wallace, A.F.; Weksler, B. Commentary: Food for thought: Assessing the influence of malnutrition in patients with lung cancer. J. Thorac. Cardiovasc. Surg. 2021, 162, 1269–1270. [Google Scholar] [CrossRef]
- Bagan, P.; Berna, P.; De Dominicis, F.; Pereira, J.C.D.N.; Mordant, P.; De La Tour, B.; Le Pimpec-Barthes, F.; Riquet, M. Nutritional status and postoperative outcome after pneumonectomy for lung cancer. Ann. Thorac. Surg. 2013, 95, 392–396. [Google Scholar] [CrossRef]
- França, T.G.D.; Ishikawa, L.L.W.; Zorzella-Pezavento, S.F.G.; Chiuso-Minicucci, F.; Da Cunha, M.L.R.S.M.; Sartori, A. Impact of malnutrition on immunity and infection. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009, 15, 374–390. [Google Scholar] [CrossRef]
- Zellner, H.K.; Moss, O.A.; Peterson, S.J.; Hicks-McGarry, S.; Moran, E.; Becker, E.; Foley, S. Differences in respiratory muscle strength measures in well-nourished and malnourished hospitalized patients. J. Acad. Nutr. Diet. 2019, 119, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Demarest-Litchford, M.; Munoz, N.; Strange, N.; Casirati, A.; Cereda, E. The impact of malnutrition on skin integrity and wound healing. Adv. Skin Wound Care 2024, 37, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Knight, S.R.; Ghosh, D.; A Kingsley, P.; Lapitan, M.C.; Parreno-Sacdalan, M.D.; Sundar, S.; Qureshi, A.U.; Valparaiso, A.P.; Pius, R.; et al. Impact of malnutrition on early outcomes after cancer surgery: An international, multicentre, prospective cohort study. Lancet Glob. Health 2023, 11, e341–e349. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Schwartz, B. The relationship between nutrition and the immune system. Front. Nutr. 2022, 9, 1082500. [Google Scholar] [CrossRef]
- Cachexia (Wasting Syndrome): Symptoms & Treatment. Cleveland Clinic. Available online: https://my.clevelandclinic.org/health/diseases/cachexia-wasting-syndrome (accessed on 3 February 2025).
- Yue, M.; Qin, Z.; Hu, L.; Ji, H. Understanding cachexia and its impact on lung cancer and beyond. Chin. Med. J. Pulm. Crit. Care Med. 2024, 2, 95–105. [Google Scholar] [CrossRef]
- Shirado, K.; Kawamitsu, K.; Okuno, S.; Kido, T.; Eto, T.; Yamashita, T. Impact of pre-operative cachexia and sarcopenia on post-operative physical function and skeletal muscle mass in lung cancer patients. Clin. Nutr. ESPEN 2023, 54, 726–732. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, C.; Wan, Q.; Zhao, R.; Han, L.; Wang, X. Impact of sarcopenia on postoperative pulmonary complications after gastric cancer surgery: A retrospective cohort study. Front. Surg. 2023, 9, 1013665. [Google Scholar] [CrossRef]
- Cancer Cachexia: Symptoms, Treatment & Prognosis. Cleveland Clinic. Available online: https://my.clevelandclinic.org/health/diseases/cancer-cachexia?utm_source=chatgpt.com (accessed on 3 February 2025).
- Mariean, C.R.; Tiucă, O.M.; Mariean, A.; Cotoi, O.S. Cancer cachexia: New insights and future directions. Cancers 2023, 15, 5590. [Google Scholar] [CrossRef]
- Watanabe, H.; Oshima, T. The latest treatments for cancer cachexia: An overview. Anticancer Res. 2023, 43, 511–521. [Google Scholar] [CrossRef]
- Pourhassan, M.; Rommersbach, N.; Lueg, G.; Klimek, C.; Schnatmann, M.; Liermann, D.; Janssen, G.; Wirth, R. The impact of malnutrition on acute muscle wasting in frail older hospitalized patients. Nutrients 2020, 12, 1387. [Google Scholar] [CrossRef]
- Kadakia, K.C.; Hamilton-Reeves, J.M.; Baracos, V.E. Current therapeutic targets in cancer cachexia: A pathophysiologic approach. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389942. [Google Scholar] [CrossRef] [PubMed]
- Gaafer, O.U.; Zimmers, T.A. Nutrition challenges of cancer cachexia. J. Parenter. Enteral. Nutr. 2021, 45, S16–S25. [Google Scholar] [CrossRef]
- Kasprzyk, A.; Bilmin, K.; Chmielewska-Ignatowicz, T.; Pawlikowski, J.; Religioni, U.; Merks, P. The role of nutritional support in malnourished patients with lung cancer. In Vivo 2021, 35, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Q.; Wang, X. Role of nutritional support for postoperative recovery of respiratory function in patients with primary lung cancer. Oncol. Lett. 2018, 16, 5978–5984. [Google Scholar] [CrossRef] [PubMed]
- Lis, C.G.; Gupta, D.; Lammersfeld, C.A.; Markman, M.; Vashi, P.G. Role of nutritional status in predicting quality of life outcomes in cancer: A systematic review of the epidemiological literature. Nutr. J. 2012, 11, 27. [Google Scholar] [CrossRef]
- Martínez-Ortega, A.J.; Piñar-Gutiérrez, A.; Serrano-Aguayo, P.; González-Navarro, I.; Remón-Ruíz, P.J.; Pereira-Cunill, J.L.; García-Luna, P.P. Perioperative nutritional support: A review of current literature. Nutrients 2022, 14, 1601. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Ford, K.L.; Arends, J.; Atherton, P.J.; Engelen, M.P.; Gonçalves, T.J.; Laviano, A.; Lobo, D.N.; Phillips, S.M.; Ravasco, P.; Deutz, N.E.; et al. The importance of protein sources to support muscle anabolism in cancer: An expert group opinion. Clin. Nutr. 2022, 41, 192–201. [Google Scholar] [CrossRef]
- Jachnis, A.; Słodkowski, M. The importance of proper use of oral nutritional supplements in oncological patients undergoing surgery. Pol. Przegl. Chir. 2021, 94, 60–68. [Google Scholar] [CrossRef]
- Orsso, C.E.; Caretero, A.; Poltronieri, T.S.; Arends, J.; de van der Schueren, M.A.; Kiss, N.; Laviano, A.; Prado, C.M. Effects of high-protein supplementation during cancer therapy: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2024, 120, 1311–1322. [Google Scholar] [CrossRef]
- Sa-nguansai, S.; Pintasiri, P.; Tienchaiananda, P. Efficacy of oral nutritional supplement in cancer patients receiving chemotherapy: A systematic review and meta-analysis of randomized controlled trials. Ann. Palliat. Med. 2024, 13, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Vudayagiri, L.; Hoilat, G.J.; Gemma, R. Percutaneous Endoscopic Gastrostomy Tube; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Löser, C.; Aschl, G.; Hébuterne, X.; Mathus-Vliegen, E.M.H.; Muscaritoli, M.; Niv, Y.; Rollins, H.; Singer, P.; Skelly, R.H. ESPEN guidelines on artificial enteral nutrition--percutaneous endoscopic gastrostomy (PEG). Clin. Nutr. 2005, 24, 848–861. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. J. Parenter. Enteral. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.A.; Andriolo, R.B.; Bennett, C.; Lustosa, S.A.; Matos, D.; Waisberg, D.R.; Waisberg, J. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database Syst. Rev. 2015, 2015, CD008096. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Austin, P.; Boeykens, K.; Chourdakis, M.; Cuerda, C.; Jonkers-Schuitema, C.; Lichota, M.; Nyulasi, I.; Schneider, S.M.; Stanga, Z.; et al. ESPEN practical guideline: Home enteral nutrition. Clin. Nutr. 2022, 41, 468–488. [Google Scholar] [CrossRef]
- Rustom, I.K.; Jebreel, A.; Tayyab, M.; England, R.J.A.; Stafford, N.D. Percutaneous endoscopic, radiological and surgical gastrostomy tubes: A comparison study in head and neck cancer patients. J. Laryngol. Otol. 2006, 120, 463–466. [Google Scholar] [CrossRef]
- Doley, J. Enteral nutrition overview. Nutrients 2022, 14, 2180. [Google Scholar] [CrossRef]
- Pironi, L.; Arends, J.; Bozzetti, F.; Cuerda, C.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN guidelines on chronic intestinal failure in adults. Clin. Nutr. 2016, 35, 247–307. [Google Scholar] [CrossRef]
- Elke, G.; van Zanten, A.R.; Lemieux, M.; McCall, M.; Jeejeebhoy, K.N.; Kott, M.; Jiang, X.; Day, A.G.; Heyland, D.K. Enteral versus parenteral nutrition in critically ill patients: An updated systematic review and meta-analysis of randomized controlled trials. Crit. Care 2016, 20, 117. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Guglielmi, F.; Boggio-Bertinet, D.; Federico, A.; Forte, G.; Guglielmi, A.; Loguercio, C.; Mazzuoli, S.; Merli, M.; Palmo, A.; Panella, C.; et al. Total parenteral nutrition-related gastroenterological complications. Dig. Liver Dis. 2006, 38, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, X.; Yu, W.; Li, J. Early enteral nutrition in critically ill patients with hemodynamic instability: An evidence-based review and practical advice. Nutr. Clin. Pract. 2014, 29, 90–96. [Google Scholar] [CrossRef] [PubMed]
- ESICM Working Group on Gastrointestinal Function; Blaser, A.R.; Starkopf, J.; Alhazzani, W.; Berger, M.M.; Casaer, M.P.; Deane, A.M.; Fruhwald, S.; Hiesmayr, M.; Ichai, C.; et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017, 43, 380–398. [Google Scholar] [CrossRef]
- Hübner, M.; Cerantola, Y.; Schäfer, M.; Demartines, N. Perioperative immunonutrition in major abdominal surgery. In Diet and Nutrition in Critical Care; Springer: New York, NY, USA, 2015; pp. 189–201. [Google Scholar] [CrossRef]
- García-Malpartida, K.; Aragón-Valera, C.; Botella-Romero, F.; Ocón-Bretón, M.J.; López-Gómez, J.J. Effects of immunonutrition on cancer patients undergoing surgery: A scoping review. Nutrients 2023, 15, 1776. [Google Scholar] [CrossRef]
- Słotwiński, R.; Sarnecka, A.; Dąbrowska, A.; Kosałka, K.; Wachowska, E.; Bałan, B.J.; Jankowska, M.; Korta, T.; Niewiński, G.; Kański, A.; et al. Innate immunity gene expression changes in critically ill patients with sepsis and disease-related malnutrition. Cent. Eur. J. Immunol. 2015, 40, 311–318. [Google Scholar] [CrossRef]
- Mohsen, G.; Stroemer, A.; Mayr, A.; Kunsorg, A.; Stoppe, C.; Wittmann, M.; Velten, M. Effects of omega-3 fatty acids on postoperative inflammatory response: A systematic review and meta-analysis. Nutrients 2023, 15, 3414. [Google Scholar] [CrossRef]
- Wibowo, A.A.; Willyanto, N.A. The efficacy of omega-3 fatty acids (O3FAs) as a complementary in colorectal cancer patients: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2024, 61, 322–332. [Google Scholar] [CrossRef]
- Anthony, R.; Macartney, M.J.; Heileson, J.L.; McLennan, P.L.; Peoples, G.E. A review and evaluation of study design considerations for omega-3 fatty acid supplementation trials in physically trained participants. Nutr. Res. Rev. 2024, 37, 1–13. [Google Scholar] [CrossRef]
- Martinez, J.L.; Bosco-Garate, I.; Souza-Gallardo, L.M.; Méndez, J.D.; Juárez-Oropeza, M.A.; Román-Ramos, R.; Ferat-Osorio, E. Effect of preoperative administration of oral arginine and glutamine in patients with enterocutaneous fistula submitted to definitive surgery: A prospective randomized trial. J. Gastrointest. Surg. 2020, 24, 426–434. [Google Scholar] [CrossRef]
- Trone, K.; Rahman, S.; Green, C.H.; Venegas, C.; Martindale, R.; Stroud, A. Synbiotics and surgery: Can prebiotics and probiotics affect inflammatory surgical outcomes? Curr. Nutr. Rep. 2023, 12, 238–246. [Google Scholar] [CrossRef]
- Sauro, K.M.; Smith, C.; Ibadin, S.; Thomas, A.; Ganshorn, H.; Bakunda, L.; Bajgain, B.; Bisch, S.P.; Nelson, G. Enhanced recovery after surgery guidelines and hospital length of stay, readmission, complications, and mortality: A meta-analysis of randomized clinical trials. JAMA Netw. Open 2024, 7, e2417310. [Google Scholar] [CrossRef] [PubMed]
- Moningi, S.; Patki, A.; Padhy, N.; Ramachandran, G. Enhanced recovery after surgery: An anesthesiologist’s perspective. J. Anaesthesiol. Clin. Pharmacol. 2019, 35 (Suppl. 1), S5–S13. [Google Scholar] [CrossRef] [PubMed]
- Nunns, M.; Shaw, L.; Briscoe, S.; Coon, J.T.; Hemsley, A.; McGrath, J.S.; Lovegrove, C.J.; Thomas, D.; Anderson, R. Multicomponent hospital-led interventions to reduce hospital stay for older adults following elective surgery: A systematic review. Health Soc. Care Deliv. Res. 2019, 7, 1–178. [Google Scholar] [CrossRef] [PubMed]
| Assessment Method | Description | Advantages | Disadvantages |
|---|---|---|---|
| Dietary Interview | Detailed conversation with the patient about dietary habits, preferences, eating problems, and changes in body weight. | Easy to conduct, inexpensive, and allows for initial identification of patients at risk of malnutrition. | Subjective; depends on the patient’s memory and ability to report accurately. |
| Physical Examination | Measurement of body weight, height, calf and arm circumference, assessment of muscle strength, and clinical signs of malnutrition. | Quick and allows for detection of malnutrition and cancer cachexia. | Effectiveness depends on the examiner’s experience; may be subjective. |
| Laboratory Tests | Assessment of albumin, prealbumin, transferrin, peripheral lymphocyte count, cholesterol, and C – reactive Protein-CRP levels. | Objective data on nutritional status. | Results may be influenced by inflammatory conditions and comorbidities. |
| Scales and Questionnaires | Mini Nutritional Assessment-MNA, Subjective Global Assessment-SGA, Patient-Generated Subjective Global Assessment-PG-SGA, Nutritional Risk Screening 2002-NRS 2002, and Malnutrition Universal Screening Tool-MUST. | Quick, non-invasive, and easy to use. | May not consider all aspects of nutritional status. |
| Body Composition Analysis | Bioelectrical impedance analysis-BIA, Dual-energy X-ray absorptiometry-DXA, computed tomography -CT, and magnetic resonance imaging -MRI. | Precise data on muscle and fat mass. | Requires specialized equipment and expertise. |
| Controlling Nutritional Status-CONUT and C-reactive protein-albumin-lymphocyte-CALLY Index | Assessment of albumin, peripheral lymphocyte count, cholesterol, and CRP levels. | Simple, based on laboratory parameters, and useful for prognosis assessment. | Do not consider anthropometric parameters. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werblińska, A.; Zielińska, D.; Szlanga, L.; Skrzypczak, P.; Bryl, M.; Piwkowski, C.; Gabryel, P. The Impact of Nutritional Support on Outcomes of Lung Cancer Surgery—Narrative Review. J. Clin. Med. 2025, 14, 3197. https://doi.org/10.3390/jcm14093197
Werblińska A, Zielińska D, Szlanga L, Skrzypczak P, Bryl M, Piwkowski C, Gabryel P. The Impact of Nutritional Support on Outcomes of Lung Cancer Surgery—Narrative Review. Journal of Clinical Medicine. 2025; 14(9):3197. https://doi.org/10.3390/jcm14093197
Chicago/Turabian StyleWerblińska, Alicja, Dominika Zielińska, Lidia Szlanga, Piotr Skrzypczak, Maciej Bryl, Cezary Piwkowski, and Piotr Gabryel. 2025. "The Impact of Nutritional Support on Outcomes of Lung Cancer Surgery—Narrative Review" Journal of Clinical Medicine 14, no. 9: 3197. https://doi.org/10.3390/jcm14093197
APA StyleWerblińska, A., Zielińska, D., Szlanga, L., Skrzypczak, P., Bryl, M., Piwkowski, C., & Gabryel, P. (2025). The Impact of Nutritional Support on Outcomes of Lung Cancer Surgery—Narrative Review. Journal of Clinical Medicine, 14(9), 3197. https://doi.org/10.3390/jcm14093197








