Exploring Antibiotic Resistance Patterns in Escherichia coli Isolates from Urinary Tract Infections: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Subjects
2.2. Data Collection
2.3. Identification of Resistance Pattern for Empiric Antibiotics
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UTI | Urinary Tract Infection |
| Escherichia coli | E. coli |
| ESBLs | Extended-Spectrum Beta-Lactamases |
| MDR | Multidrug-Resistant |
| JUH | Jordan University Hospital |
| WHO | World Health Organization |
| ICU | Intensive Care Unit |
References
- Polse, R.F.; Yousif, S.Y.; Assafi, M.S. Prevalence and antimicrobial susceptibility patterns of uropathogenic E. coli among people in Zakho, Iraq. Int. J. Res. Med. Sci. 2016, 4, 1219–1223. [Google Scholar] [CrossRef]
- Khalil, A.; Raja, I.; Hussain, I.; Jan, M.; Nafees, M.A.; Jahan, Z.; Javeed, M.; Shah, G.; Latif, A. Prevalence of Escherichia coli in suspected urinary tract infected patients and their sensitivity pattern against various antibiotics in Gilgit-Baltistan, Pakistan. Pak. J. Zool. 2014, 46, 1783–1788. [Google Scholar]
- Glover, E.K.; Sheerin, N.S. Urinary tract infection. Medicine 2023, 51, 239–243. [Google Scholar] [CrossRef]
- Kline, K.A.; Lewis, A.L. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. In Urinary Tract Infections Molecular Pathogenesis and Clinical Management; Wiley: Hoboken, NJ, USA, 2017; pp. 459–502. [Google Scholar]
- Behzadi, P.; Urbán, E.; Matuz, M.; Benkő, R.; Gajdács, M. The role of gram-negative bacteria in urinary tract infections: Current concepts and therapeutic options. Adv. Microbiol. Infect. Dis. Public Health: Vol. 15 2021, 1323, 35–69. [Google Scholar]
- Córdoba, G.; Holm, A.; Hansen, F.; Hammerum, A.M.; Bjerrum, L. Prevalence of antimicrobial resistant Escherichia coli from patients with suspected urinary tract infection in primary care, Denmark. BMC Infect. Dis. 2017, 17, 760. [Google Scholar] [CrossRef]
- Mehta, M.; Bhardwaj, S.; Sharma, J. Prevalence and antibiotic susceptibility pattern of multi-drug resistant Escherichia coli isolates from urinary tract infection (UTI) patients. Int. J. Life Sci. Pharma Res. 2012, 2, 6–11. [Google Scholar]
- Odongo, I.; Ssemambo, R.; Kungu, J.M. Prevalence of Escherichia coli and its antimicrobial susceptibility profiles among patients with UTI at Mulago Hospital, Kampala, Uganda. Interdiscip. Perspect. Infect. Dis. 2020, 2020, 8042540. [Google Scholar] [CrossRef]
- Daoud, N.; Hamdoun, M.; Hannachi, H.; Gharsallah, C.; Mallekh, W.; Bahri, O. Antimicrobial susceptibility patterns of Escherichia coli among Tunisian outpatients with community-acquired urinary tract infection (2012–2018). Curr. Urol. 2020, 14, 200–205. [Google Scholar] [CrossRef]
- Luty, R.S.; Fadil, A.G.; Najm, J.M.; Abduljabbar, H.H.; Kashmar, S.A.A. Uropathogens antibiotic susceptibility as an indicator for the empirical therapy used for urinary tract infections: A retrospective observational study. Iran. J. Microbiol. 2020, 12, 395. [Google Scholar] [CrossRef]
- Alkhawaldeh, R.; Abu Farha, R.; Abu Hammour, K.; Alefishat, E. The appropriateness of empiric treatment of urinary tract infections in a tertiary teaching hospital in Joran: A cross-sectional study. Antibiotics 2022, 11, 629. [Google Scholar] [CrossRef]
- Alkhawaldeh, R.; Abu Farha, R.; Abu Hammour, K.; Alefishat, E. Optimizing antimicrobial therapy in urinary tract infections: A focus on urine culture and sensitivity testing. Front. Pharmacol. 2022, 13, 1058669. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S., II; Mathers, A.J.; Bobenchik, A.M.; Bryson, A.L.; Campeau, S.; Cullen, S.K.; Dingle, T.; Esparza, G.; Humphries, R.M.; Lutgring, J.; et al. Performance Standards for Antimicrobial Susceptibility Testing, 35th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2025. [Google Scholar]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmeister, A.S.; Jones, R.N. The Importance of Antimicrobial Resistance Monitoring Worldwide and the Origins of SENTRY Antimicrobial Surveillance Program. Open Forum Infect. Dis. 2019, 6, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef]
- Nairoukh, Y.R.; Mahafzah, A.M.; Irshaid, A.; Shehabi, A.A. Molecular Characterization of Multidrug Resistant Uropathogenic E. Coli Isolates from Jordanian Patients. Open Microbiol. J. 2018, 12, 1–7. [Google Scholar] [CrossRef]
- National AMR Surveillance Report. Available online: https://www.moh.gov.jo/ebv4.0/root_storage/ar/eb_list_page/jordan_national_amr_surveillance_report_2022_(final).pdf (accessed on 22 May 2022).
- Rowe, T.A.; Juthani-Mehta, M. Urinary tract infection in older adults. Aging Health 2013, 9, 519–528. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: Treatment of urinary tract infections in nonpregnant women. Obs. Gynecol. 2008, 111, 785–794. [Google Scholar] [CrossRef]
- Prasad, S.; Sung, B.; Aggarwal, B.B. Age-associated chronic diseases require age-old medicine: Role of chronic inflammation. Prev. Med. 2012, 54 Suppl., S29–S37. [Google Scholar] [CrossRef]
- Murthy, R. Implementation of strategies to control antimicrobial resistance. Chest 2001, 119, 405s–411s. [Google Scholar] [CrossRef]
- Esparcia, A.; Artero, A.; Eiros, J.M.; Balaguer, M.; Madrazo, M.; Alberola, J.; Nogueira, J.M. Influence of inadequate antimicrobial therapy on prognosis in elderly patients with severe urinary tract infections. Eur. J. Intern. Med. 2014, 25, 523–527. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Y.; Hang, Y.; Luo, H.; Fang, X.; Xiao, Y.; Cao, X.; Zou, S.; Hu, X.; Hu, L.; et al. Impact of inappropriate empirical antibiotic treatment on clinical outcomes of urinary tract infections caused by Escherichia coli: A retrospective cohort study. J. Glob. Antimicrob. Resist. 2021, 26, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Kusin, S.B.; Fan, E.M.; Prokesch, B.C.; Christie, A.L.; Zimmern, P.E. Empiric versus culture-based antibiotic therapy for UTIs in menopausal women. World J. Urol. 2023, 41, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Shani, V.; Muchtar, E.; Kariv, G.; Robenshtok, E.; Leibovici, L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob. Agents Chemother. 2010, 54, 4851–4863. [Google Scholar] [CrossRef]
- Kulkarni, S.R.; Peerapur, B.V.; Sailesh, K.S. Isolation and Antibiotic Susceptibility Pattern of Escherichia coli from Urinary Tract Infections in a Tertiary Care Hospital of North Eastern Karnataka. J. Nat. Sci. Biol. Med. 2017, 8, 176–180. [Google Scholar] [CrossRef]
- Shirvani, M.; Keramati, A.; Esmaeli, M. Evaluating the pattern of antibiotic resistance of urinary tract infection (UTI)-causing bacteria in the urine culture samples of patients in the infectious ward of Imam Khomeini Hospital, Kermanshah, in Iran from 2016–2018. Afr. J. Urol. 2023, 29, 32. [Google Scholar] [CrossRef]
- Mansour, G.H.; Fararjeh, A.-F.S.; Shawagfeh, M.T.; Abu Laban, N.M.; Alsarhan, A.A.; Al-Shawabkeh, J.D.; Abd Wahid, M.E. The Hidden Threat of Antimicrobial Resistance: A Case Study from A Private Hospital in Jordan. J. Pure Appl. Microbiol. 2024, 18, 2570–2581. [Google Scholar] [CrossRef]
- Gu, J.; Chen, X.; Yang, Z.; Bai, Y.; Zhang, X. Gender differences in the microbial spectrum and antibiotic sensitivity of uropathogens isolated from patients with urinary stones. J. Clin. Lab. Anal. 2022, 36, e24155. [Google Scholar] [CrossRef]
- Linhares, I.; Raposo, T.; Rodrigues, A.; Almeida, A. Frequency and antimicrobial resistance patterns of bacteria implicated in community urinary tract infections: A ten-year surveillance study (2000–2009). BMC Infect. Dis. 2013, 13, 19. [Google Scholar] [CrossRef]
- Alrabayah, M.; Nadi, N.; Suleiman, A.; Abbad, A.; Ghanem, H.; Obeidat, M.; Alaqrabawi, M.; Yousef, M.; Harb, T.A.; Bsisu, I. Trends of antimicrobial resistance in Escherichia coli isolates from urine cultures of women in Jordan: A 10-year retrospective study. Int. Arab. J. Antimicrob. Agents 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Mareș, C.; Petca, R.-C.; Popescu, R.-I.; Petca, A.; Geavlete, B.F.; Jinga, V. Uropathogens’ antibiotic resistance evolution in a female population: A sequential multi-year comparative analysis. Antibiotics 2023, 12, 948. [Google Scholar] [CrossRef]
- Tabibian, J.H.; Gornbein, J.; Heidari, A.; Dien, S.L.; Lau, V.H.; Chahal, P.; Churchill, B.M.; Haake, D.A. Uropathogens and host characteristics. J. Clin. Microbiol. 2008, 46, 3980–3986. [Google Scholar] [CrossRef] [PubMed]
- World Medical, A. World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
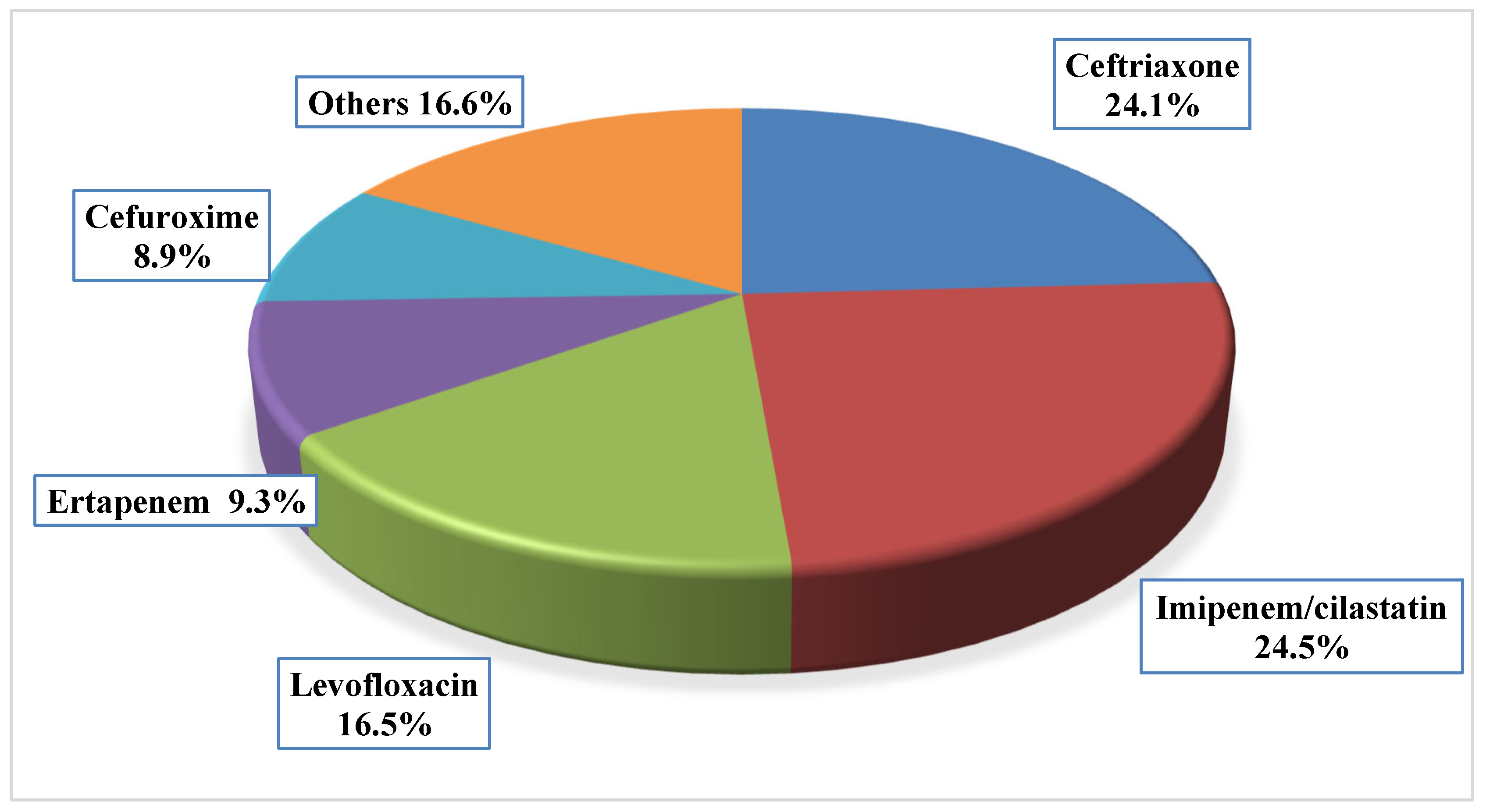
| Parameter | Median (IQR) | n (%) |
|---|---|---|
| Age (years) | 64.0 (25.0) | |
| Age categories (years) | ||
| 4 (1.7) | |
| 22 (9.6) | |
| 16 (7) | |
| 31 (13.5) | |
| 44 (19.1) | |
| 113 (49.1) | |
| Gender | ||
| 166 (72.2) | |
| 64 (27.8) | |
| Number of chronic medications | ||
| 50 (21.7) | |
| 58 (25.2) | |
| 122 (53) | |
| Length of Stay | 10.0 (9.0) |
| Parameter | Mean ± SD | n (%) |
|---|---|---|
| Number of microorganisms per culture | ||
| 187 (81.3) | |
| 40 (17.4) | |
| 3 (1.3) | |
| Total number of identified micro-organisms | 276 | |
| Mean number of identified microorganisms per patient | 1.2 ± 0.4 | |
| Number of prescribed antibiotics per patient | ||
| 29 (12.6) | |
| 170 (73.9) | |
| 27 (11.7) | |
| 4 (1.7) | |
| Total number of prescribed empiric antibiotics | 236 | |
| Number of empiric antibiotics prescribed per patient | 1 ± 0.5 |
| Parameter | n (%) |
|---|---|
| Patients without empiric treatment | 29 (12.6) |
| Patients prescribed antibiotics not covering E. coli | 8 (3.5) |
| Availability of susceptibility reporting among patients receiving antibiotics covering E. coli | |
| 136 (59.1) |
| 59 (24.8) |
| Susceptibility of E. coli among patients | |
| 62 (27.0) |
| 74 (32.1) |
| Parameter | n (%) |
|---|---|
| Antibiotics without sensitivity reports | 72/236 (30.5) |
| Antibiotics not covering E. coli | 8/236 (3.4) |
Antibiotics to which E. coli is sensitive
| 89/236 (37.7) 43/89 11/89 9/89 7/89 19/89 |
Antibiotics to which E. coli is sensitive
| 67/236 (28.4) 32/67 15/67 10/67 2/67 8/67 |
| Outcome | Sensitivity Test Present (n = 136) | Sensitivity Test Absent (n = 57) | p-Value # |
|---|---|---|---|
| Length of stay (days), median (IQR) | 10.0 (10.0) | 8.0 (6.0) | 0.032 |
| Variables | Resistant Isolates | Sensitive Isolates | p-Value Statistical Test |
|---|---|---|---|
| Gender | |||
| 12 (30.8%) | 27 (69.2%) | 0.036 |
| 50 (51.5%) | 47 (48.5%) | Pearson Chi-Square |
| Length of stay (median, IQR) | 11 (10) | 9.5 (8) | 0.190 Mann–Whitney U Test |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhawaldeh, R.; Gharaibeh, L.; Khan, A.H.; Abu Hammour, K.; Zawiah, M.; Shilbayeh, S.A.; Abu-Farha, R.K. Exploring Antibiotic Resistance Patterns in Escherichia coli Isolates from Urinary Tract Infections: A Retrospective Study. J. Clin. Med. 2025, 14, 3196. https://doi.org/10.3390/jcm14093196
Alkhawaldeh R, Gharaibeh L, Khan AH, Abu Hammour K, Zawiah M, Shilbayeh SA, Abu-Farha RK. Exploring Antibiotic Resistance Patterns in Escherichia coli Isolates from Urinary Tract Infections: A Retrospective Study. Journal of Clinical Medicine. 2025; 14(9):3196. https://doi.org/10.3390/jcm14093196
Chicago/Turabian StyleAlkhawaldeh, Rama, Lobna Gharaibeh, Amer Hayat Khan, Khawla Abu Hammour, Mohammed Zawiah, Sireen AR. Shilbayeh, and Rana K. Abu-Farha. 2025. "Exploring Antibiotic Resistance Patterns in Escherichia coli Isolates from Urinary Tract Infections: A Retrospective Study" Journal of Clinical Medicine 14, no. 9: 3196. https://doi.org/10.3390/jcm14093196
APA StyleAlkhawaldeh, R., Gharaibeh, L., Khan, A. H., Abu Hammour, K., Zawiah, M., Shilbayeh, S. A., & Abu-Farha, R. K. (2025). Exploring Antibiotic Resistance Patterns in Escherichia coli Isolates from Urinary Tract Infections: A Retrospective Study. Journal of Clinical Medicine, 14(9), 3196. https://doi.org/10.3390/jcm14093196






