Efficacy and Safety of Ab Externo Open Conjunctiva XEN® 63 µm Implantation with a 30G Needle Scleral Tract in Primary Open-Angle Glaucoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Statement
2.2. Study Participants and Data Collection
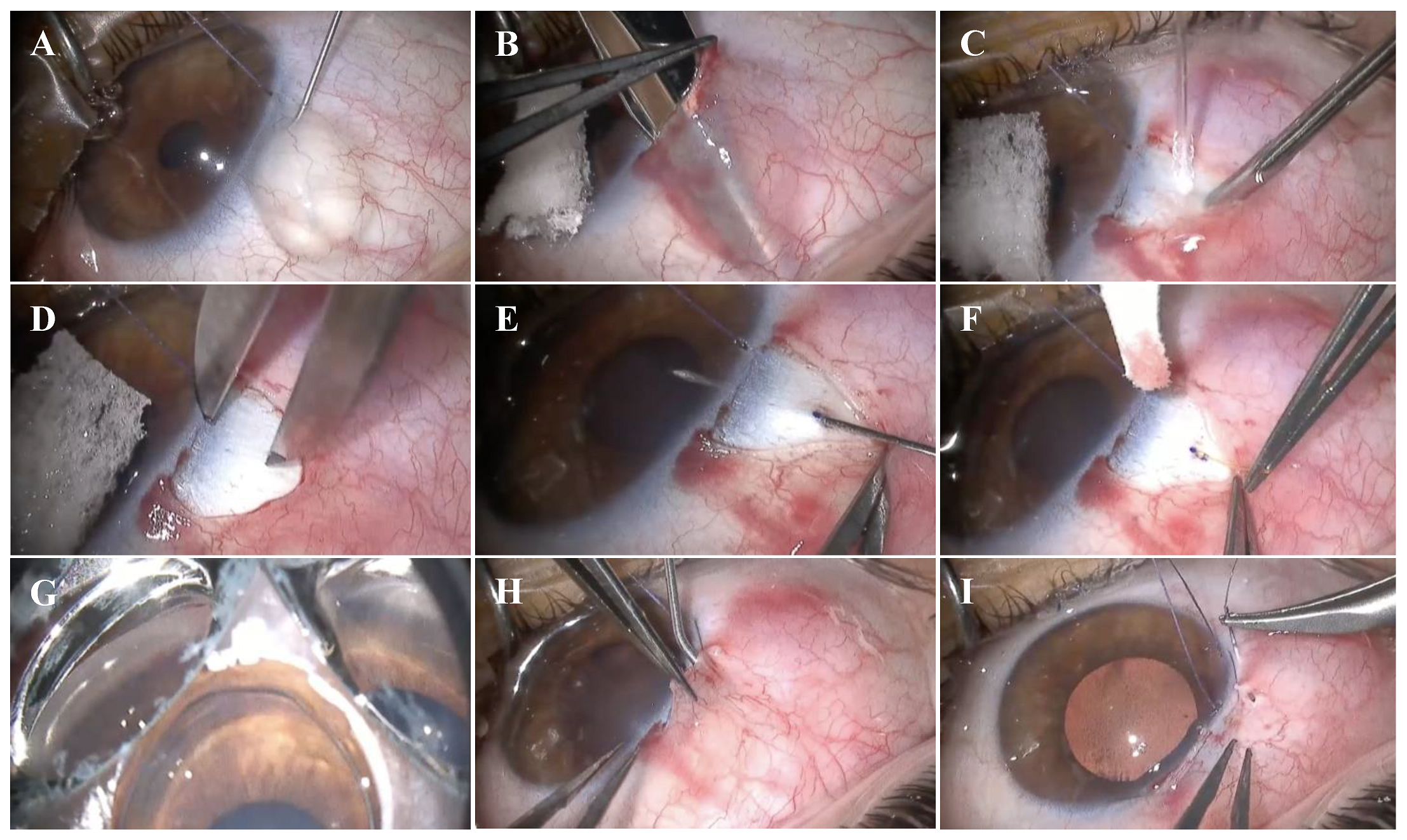
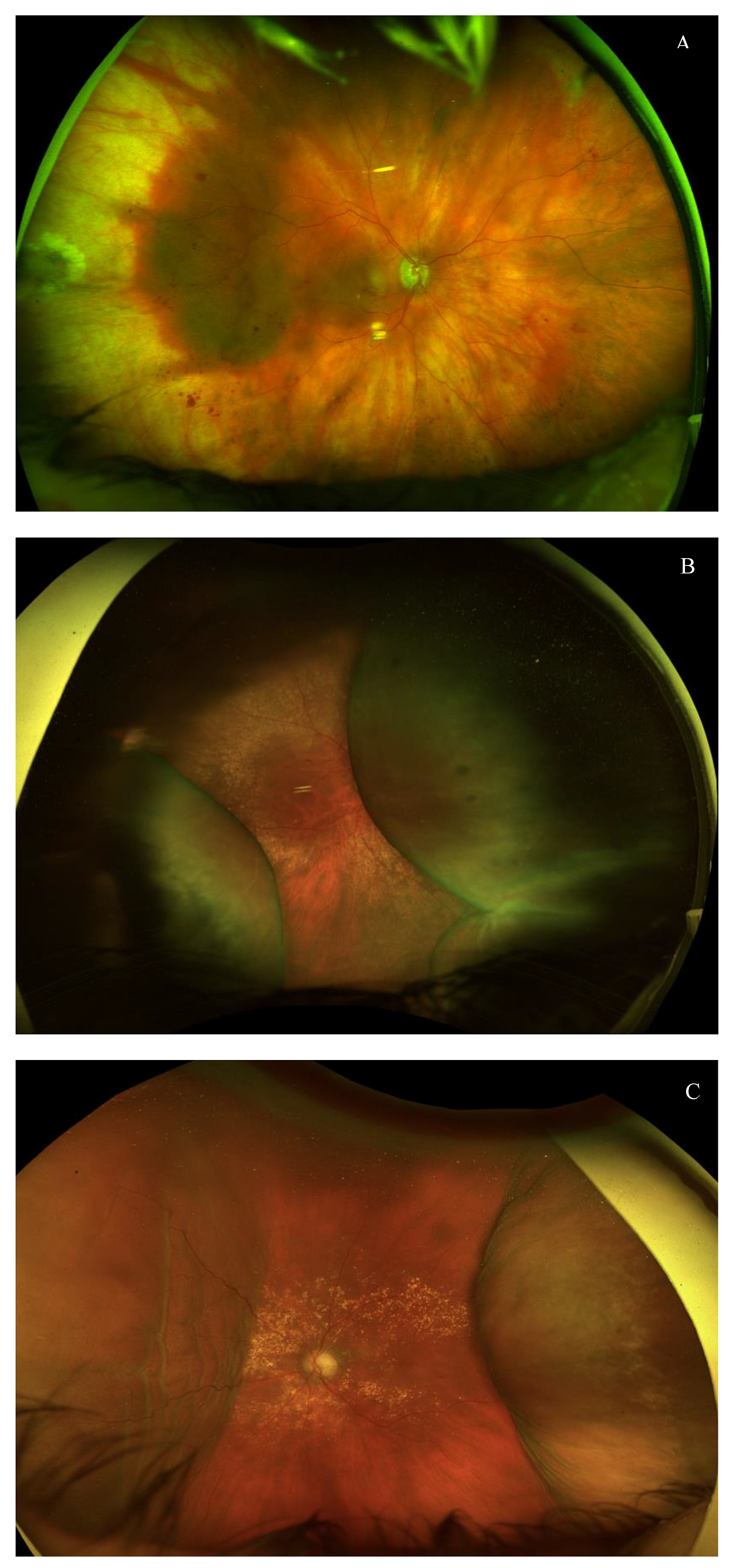
2.3. Surgical Technique
2.4. Postoperative Regime, Procedures, and Care
2.5. Surgical Success and Outcomes
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
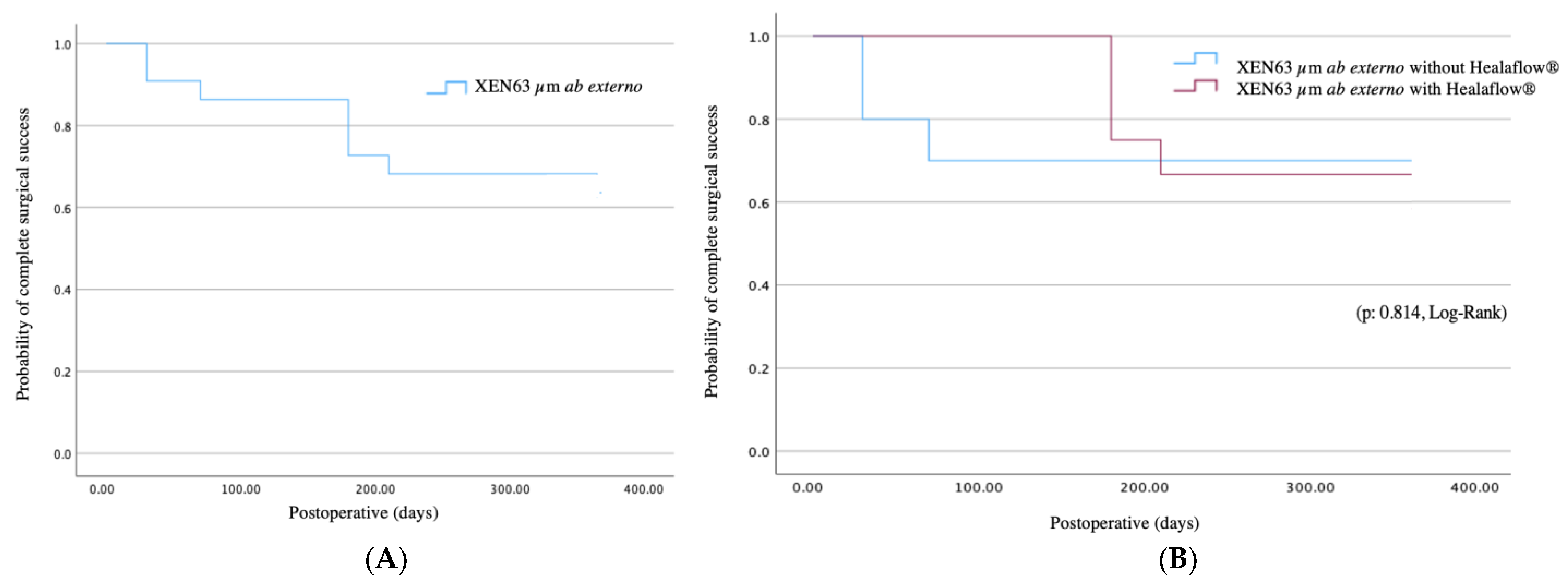
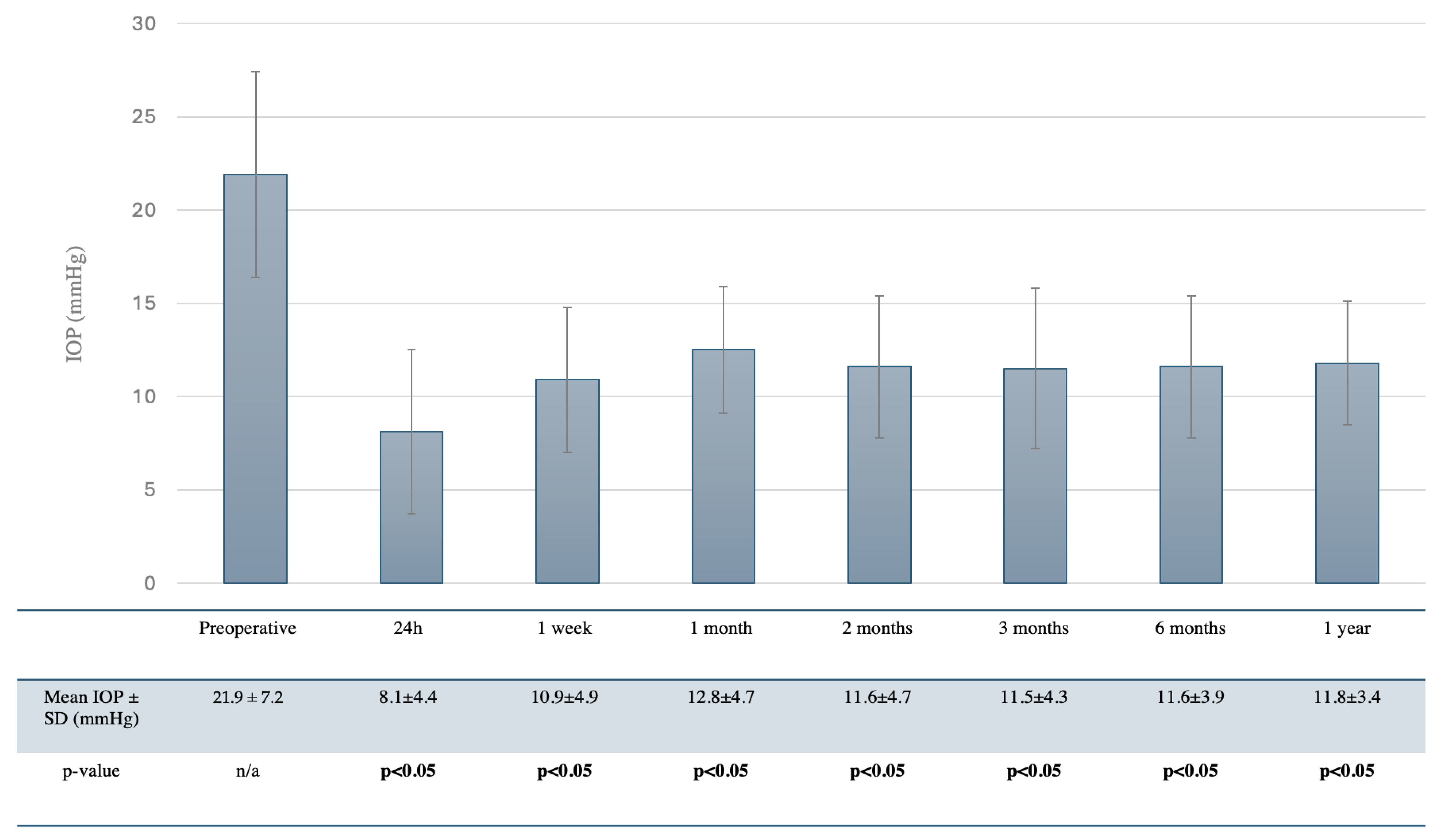
3.2. Efficacy
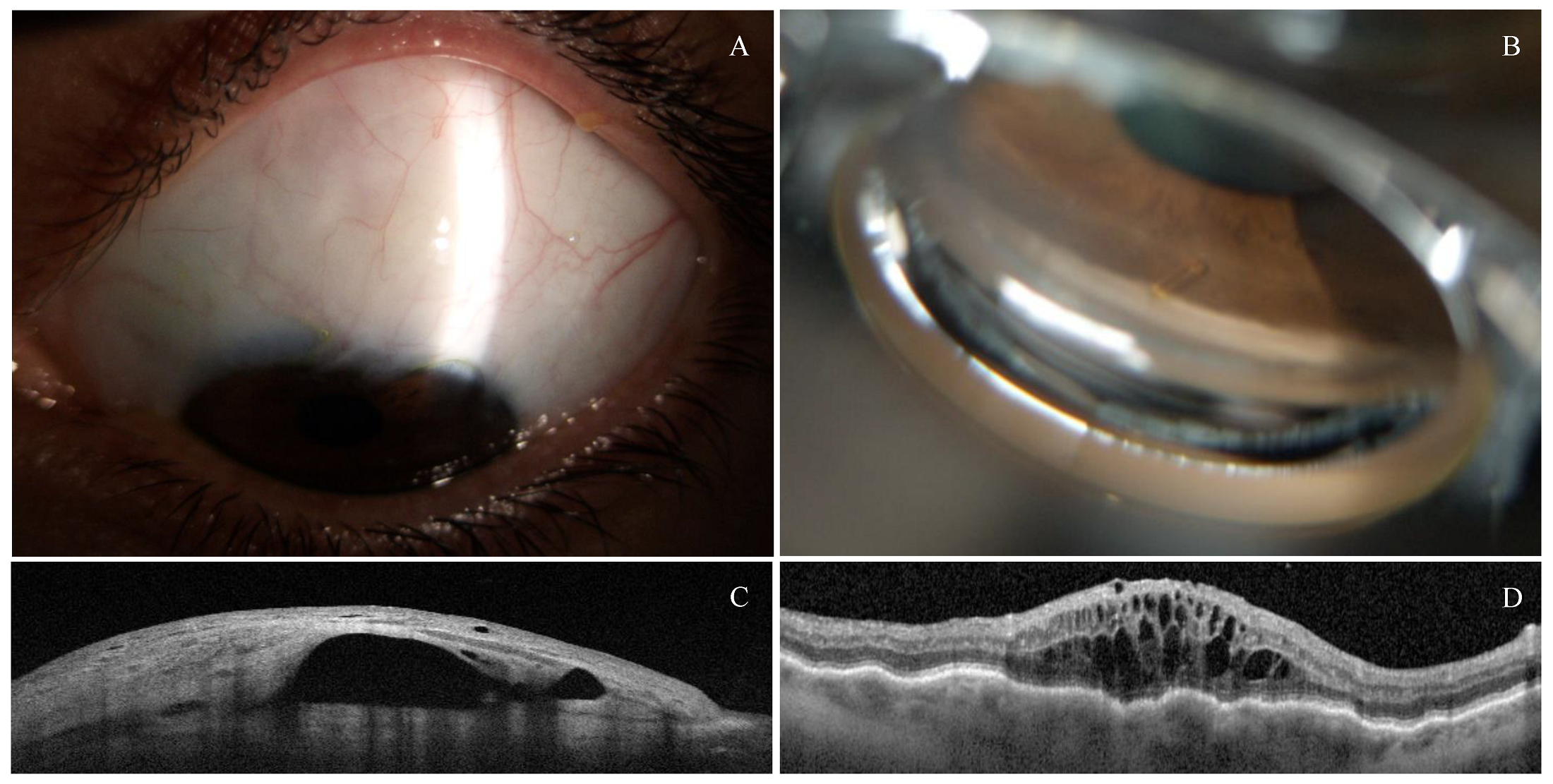
3.3. Safety Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEO | Ab Externo With open conjunctiva |
| AIC | Ab Interno with closed conjunctiva |
| AS-OCT | Anterior Segment Optical Coherence Tomography |
| BSS | Balanced Salt Solution |
| BVCA | Best-Corrected Visual Acuity |
| CME | Cystoid Macular Edema |
| DB | Decibel |
| GATT | Gonioscopy-Assisted Transluminal Trabeculotomy |
| IOP | Intraocular Pressure |
| LASIK | Laser-Assisted In Situ Keratomileusis |
| logMAR | Logarithm of the Minimum Angle of Resolution |
| MD | Mean Deviation |
| MIBS | Minimally Invasive Bleb Surgery |
| MIGS | Minimally Invasive Glaucoma Surgery |
| MMC | Mitomycin C |
| NPDS | Non-Penetrating Deep Sclerectomy |
| OCT | Optical Coherence Tomography |
| ONH-OCT | Optical Coherence Tomography of the Optic Nerve Head |
| POAG | Primary Open-Angle Glaucoma |
| SLT | Selective Laser Trabeculoplasty |
| SPSS | Statistical Package for the Social Sciences |
| TBT | Trabeculectomy |
| VF | Visual Field |
References
- Spaeth, G.L. European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br. J. Ophthalmol. 2021, 105, 1–169. [Google Scholar] [CrossRef]
- Vinod, K.; Gedde, S.J.; Feuer, W.J.; Panarelli, J.F.; Chang, T.C.; Chen, P.P.; Parrish, R.K. Practice Preferences for Glaucoma Surgery. J. Glaucoma 2017, 26, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.F.; Lockwood, A.J.; Shah, P.; Macleod, A.; Broadway, D.C.; King, A.J.; McNaught, A.I.; Agrawal, P. Trabeculectomy in the 21st Century: A Multicenter Study. Ophthalmology 2013, 120, 2532–2539. [Google Scholar] [CrossRef]
- Suñer, I.J.; Greenfield, D.S.; Miller, M.P.; Nicolela, M.T.; Palmberg, P.F. Hypotony maculopathy after filtering surgery with mitomycin-C. Incidence and treatment. Ophthalmology 1997, 104, 207–214. [Google Scholar] [CrossRef]
- Lavia, C.; Dallorto, L.; Maule, M.; Ceccarelli, M.; Fea, A.M. Minimally-Invasive Glaucoma Surgeries (MIGS) for Open Angle Glaucoma: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0183142. [Google Scholar] [CrossRef]
- Bui, T.T.; Rosdahl, J.A. Systematic Review of MIGS and Non-Penetrating Glaucoma Procedures for Uveitic Glaucoma. Semin. Ophthalmol. 2022, 37, 830–838. [Google Scholar] [CrossRef]
- Traverso, C.E.; Carassa, R.G.; Fea, A.M.; Figus, M.; Astarita, C.; Piergentili, B.; Vera, V.; Gandolfi, S. Effectiveness and Safety of Xen Gel Stent in Glaucoma Surgery: A Systematic Review of the Literature. J. Clin. Med. 2023, 12, 5339. [Google Scholar] [CrossRef]
- Balas, M.; Mathew, D.J. Minimally Invasive Glaucoma Surgery: A Review of the Literature. Vision 2023, 7, 54. [Google Scholar] [CrossRef]
- Pirani, V.; Cavallero, E.; Cesari, C.; Virgili, F.; Ramovecchi, V. Ab Externo Transconjunctival XEN® 45 Gel Stent Implantation: Efficacy and Safety of a New Surgical Technique. J. Curr. Glaucoma Pract. 2024, 18, 94–97. [Google Scholar] [CrossRef]
- Yuan, L.; Rana, H.S.; Lee, I.; Lai, G.; Raiciulescu, S.; Kim, W. Short-Term Outcomes of Xen-45 Gel Stent Ab Interno Versus Ab Externo Transconjunctival Approaches. J. Glaucoma 2023, 32, 71–79. [Google Scholar] [CrossRef]
- El Helwe, H.; Ingram, Z.; Neeson, C.E.; Falah, H.; Trzcinski, J.; Lin, J.B.; Solá-Del Valle, D.A. Comparing Outcomes of 45 Xen Implantation Ab Interno with Closed Conjunctiva to Ab Externo with Open Conjunctiva Approaches. J. Glaucoma 2024, 33, 116–125. [Google Scholar] [CrossRef]
- Tan, S.Y.; Md Din, N.; Mohd Khialdin, S.; Wan Abdul Halim, W.H.; Tang, S.F. Ab-Externo Implantation of XEN Gel Stent for Refractory Steroid-Induced Glaucoma After Lamellar Keratoplasty. Cureus 2021, 13, e13320. [Google Scholar] [CrossRef]
- To, L.K.; Dhoot, R.K.; Chuang, A.Z.; Karimaghaei, S.; Guevara-Abadia, F.; Shah, R.D.; Feldman, R.M. Defining the role of ab externo Xen gel stent in glaucomatous eyes with prior failed surgical intervention. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 779–789. [Google Scholar] [CrossRef]
- Martínez-de-la-Casa, J.M.; Marcos-Parra, M.T.; Millá-Griñó, E.; Laborda, T.; Giménez-Gomez, R.; Larrosa, J.M.; Urcola, A.; Teus, M.Á.; Perucho-Martínez, S. Effectiveness and safety of XEN63 in patients with primary-open-angle glaucoma. Sci. Rep. 2024, 14, 4561. [Google Scholar] [CrossRef]
- Fea, A.M.; Menchini, M.; Rossi, A.; Posarelli, C.; Malinverni, L.; Figus, M. Early Experience with the New XEN63 Implant in Primary Open-Angle Glaucoma Patients: Clinical Outcomes. J. Clin. Med. 2021, 10, 1628. [Google Scholar] [CrossRef]
- Lavin-Dapena, C.; Cordero-Ros, R.; D’Anna, O.; Mogollón, I. XEN 63 gel stent device in glaucoma surgery: A 5-years follow-up prospective study. Eur. J. Ophthalmol. 2021, 31, 1829–1835. [Google Scholar] [CrossRef]
- Fea, A.M.; Menchini, M.; Rossi, A.; Posarelli, C.; Malinverni, L.; Figus, M. Outcomes of XEN 63 Device at 18-Month Follow-Up in Glaucoma Patients: A Two-Center Retrospective Study. J. Clin. Med. 2022, 11, 3801. [Google Scholar] [CrossRef]
- Voykov, B.; Nasyrov, E.; Neubauer, J.; Gassel, C.J. New XEN63 Gel Stent Implantation in Open-Angle Glaucoma: A Two-Year Follow-Up Pilot Study. Clin. Ophthalmol. 2023, 17, 2243–2249. [Google Scholar] [CrossRef]
- Thatcher, M.D.; Coupal, D.J.; Cheng, Y.; Podbielski, D.W. Short-Term Efficacy and Safety of Open Conjunctiva Ab Externo XEN45 Gel Stent Implantation in Glaucoma Patients. J. Glaucoma 2022, 31, 757–762. [Google Scholar] [CrossRef]
- Panarelli, J.F.; Yan, D.B.; Francis, B.; Craven, E.R. XEN Gel Stent Open Conjunctiva Technique: A Practical Approach Paper. Adv. Ther. 2020, 37, 2538–2549. [Google Scholar] [CrossRef]
- Ruda, R.C.; Yuan, L.; Lai, G.M.; Raiciulescu, S.; Kim, W.I. Clinical Outcomes of Ab Interno Placement versus Ab Externo Placement of XEN45 Gel Stents. Ophthalmol. Glaucoma 2023, 6, 4–10. [Google Scholar] [CrossRef]
- Tan, N.E.; Tracer, N.; Terraciano, A.; Parikh, H.A.; Panarelli, J.F.; Radcliffe, N.M. Comparison of Safety and Efficacy Between Ab Interno and Ab Externo Approaches to XEN Gel Stent Placement. Clin. Ophthalmol. 2021, 15, 299–305. [Google Scholar] [CrossRef]
- Han, K.; Lee, J.; Moon, S. One-Year Outcomes of Ab Externo XEN45 Gel Stent Implantation with an Open Conjunctiva Approach in Patients with Open-Angle Glaucoma. Korean J. Ophthalmol. 2023, 37, 353–364. [Google Scholar] [CrossRef]
- Wy, S.; Shin, Y.I.; Kim, Y.K.; Jeoung, J.W.; Park, K.H. Bleb Morphology on Anterior-Segment Optical Coherence Tomography after XEN Gel Stent Implantation. J. Clin. Med. 2023, 12, 6740. [Google Scholar] [CrossRef]
- Villarreal, E.; Berkowitz, E.; Tiosano, B. XEN45 Gel Stent Combined with Healaflow Injectable Viscoelastic Implant. Case Rep. Ophthalmol. Med 2023, 22, 7096406. [Google Scholar] [CrossRef]
- Papaconstantinou, D.; Diagourtas, A.; Petrou, P.; Rouvas, A.; Vergados, A.; Koutsandrea, C.; Georgalas, I. Trabeculectomy with Healaflow versus Trabeculectomy for the Treatment of Glaucoma: A Case-Control Study. J. Ophthalmol. 2015, 15, 836269. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.; Chang, X.; Zheng, Y. The therapeutic effect of Healaflow in glaucoma surgery. Am. J. Transl. Res. 2021, 13, 9729–9735. [Google Scholar]
| Characteristics | Value (n = 22) |
|---|---|
| Follow-up time (days) | 397 ± 25.4 |
| Age, mean (y) | 70.1 ± 18.0 |
| Male sex | 12 (54.5%) |
| Eye laterality (right) | 12 (54.5%) |
| Preoperative mean BCVA (logMAR) | 0.2 ± 0.4 |
| Preoperative mean IOP (mmHg) | 21.9 ± 7.2 |
Glaucoma staging
| 8 (36.4%) 6 (27.3%) 8 (36.4%) |
| Mean number of glaucoma medications | 2.4 ± 0.9 |
| Preoperative lens status (phakic) | 13 (59.1%) |
| Mean visual field deviation (dB) | −6.36 ± 3.13 |
| Previous selective laser trabeculoplasty (SLT) | 2 (9.1%) |
Previous ophthalmological procedures (time apart from XEN implant)
| 1 (4.5%) 1 (4.5%) 9 (40.9%) |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Sex (male = 1) | 0.764 (0.474–1.123) | 0.435 | - | - |
| Preoperative lens status (pseudophakic = 1) | 0.921 (0.765–1.034) | 0.765 | - | - |
| Age (years) | 1.025 (0.970–1.083) | 0.376 | - | - |
| Baseline VF MD (º) | 1.112 (0.754–1.243) | 0.567 | - | - |
| Preoperative BVCA (logMAR) | 0.984 (0.864–1.102) | 0.430 | - | - |
| Preoperative SLT | 0.867 (0.645–1.429) | 0.865 | - | - |
| Number of preoperative medications | 0.987 (0.433–2.249) | 0.976 | - | - |
| Use of Healaflow® | 1.183 (0.281–4.972) | 0.819 | - | - |
| Preoperative IOP (mmHg) | 0.840 (0.713–1.189) | 0.336 | - | - |
| 24 h postoperative IOP (mmHg) | 1.039 (0.888–1.217) | 0.630 | - | - |
| 1 week postoperative IOP (mmHg) | 1.154 (1.029–1.294) | 0.014 | - | - |
| 1 month postoperative IOP (mmHg) | 1.137 (1.038–1.245) | 0.005 | 1.113 (0.986–1.256) | 0.083 |
| Secondary needling | 4.361 (1.502–9.936) | 0.012 | 2.753 (0.800–6.243) | 0.538 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertolani, Y.; Rigo-Quera, J.; Sánchez-Vela, L.; Pujol-Carreras, O.; Amilburu, M.; Dou, A.; Castany, M. Efficacy and Safety of Ab Externo Open Conjunctiva XEN® 63 µm Implantation with a 30G Needle Scleral Tract in Primary Open-Angle Glaucoma. J. Clin. Med. 2025, 14, 3195. https://doi.org/10.3390/jcm14093195
Bertolani Y, Rigo-Quera J, Sánchez-Vela L, Pujol-Carreras O, Amilburu M, Dou A, Castany M. Efficacy and Safety of Ab Externo Open Conjunctiva XEN® 63 µm Implantation with a 30G Needle Scleral Tract in Primary Open-Angle Glaucoma. Journal of Clinical Medicine. 2025; 14(9):3195. https://doi.org/10.3390/jcm14093195
Chicago/Turabian StyleBertolani, Yann, Jaume Rigo-Quera, Laura Sánchez-Vela, Olivia Pujol-Carreras, Manuel Amilburu, Antonio Dou, and Marta Castany. 2025. "Efficacy and Safety of Ab Externo Open Conjunctiva XEN® 63 µm Implantation with a 30G Needle Scleral Tract in Primary Open-Angle Glaucoma" Journal of Clinical Medicine 14, no. 9: 3195. https://doi.org/10.3390/jcm14093195
APA StyleBertolani, Y., Rigo-Quera, J., Sánchez-Vela, L., Pujol-Carreras, O., Amilburu, M., Dou, A., & Castany, M. (2025). Efficacy and Safety of Ab Externo Open Conjunctiva XEN® 63 µm Implantation with a 30G Needle Scleral Tract in Primary Open-Angle Glaucoma. Journal of Clinical Medicine, 14(9), 3195. https://doi.org/10.3390/jcm14093195







