Navigating the Intricacies of Robotic Pylorus-Preserving Pancreaticoduodenectomy Using the da Vinci SP (Single Port) System
Abstract
1. Introduction
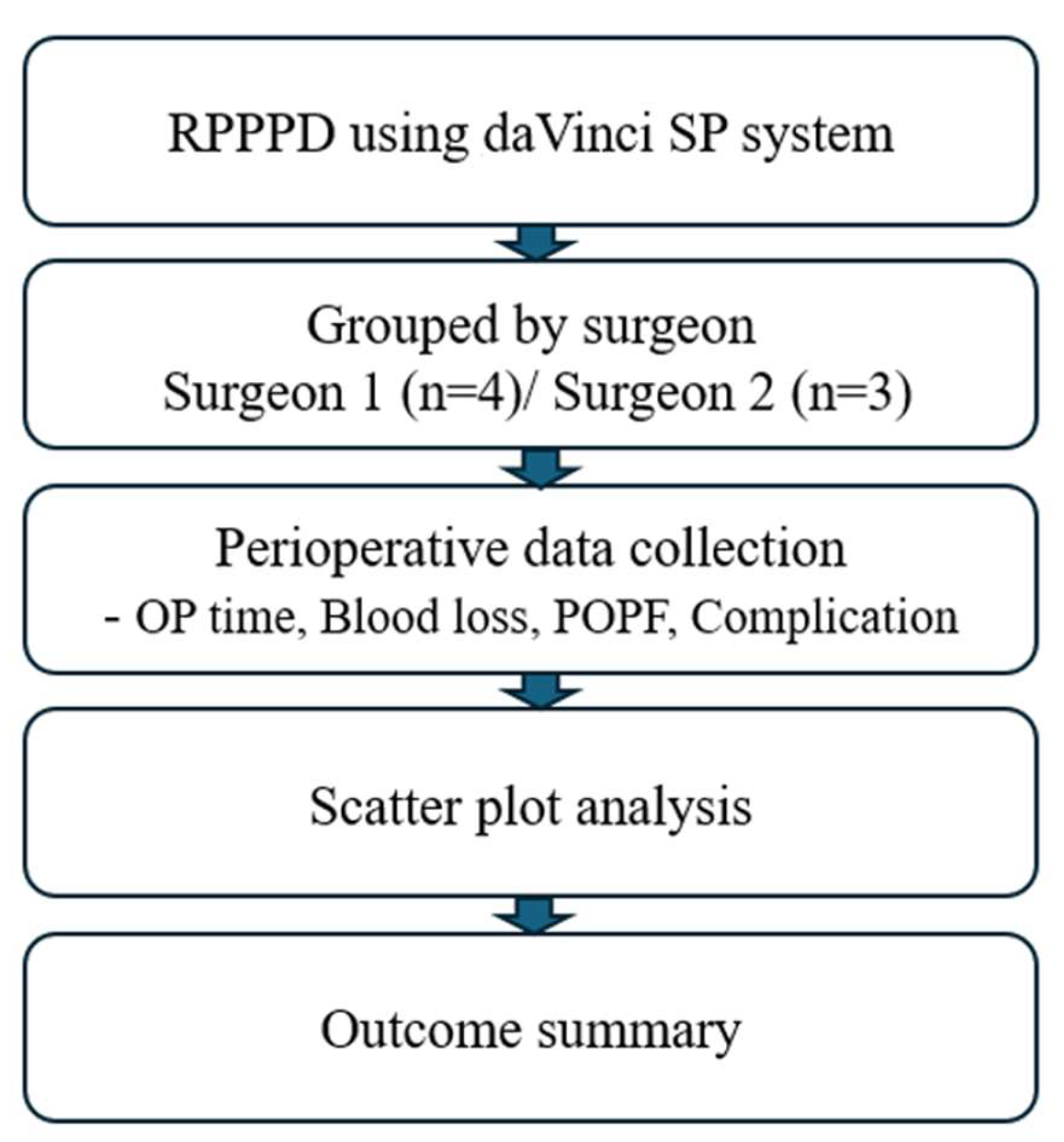
2. Materials and Methods
2.1. Study Participants and Design
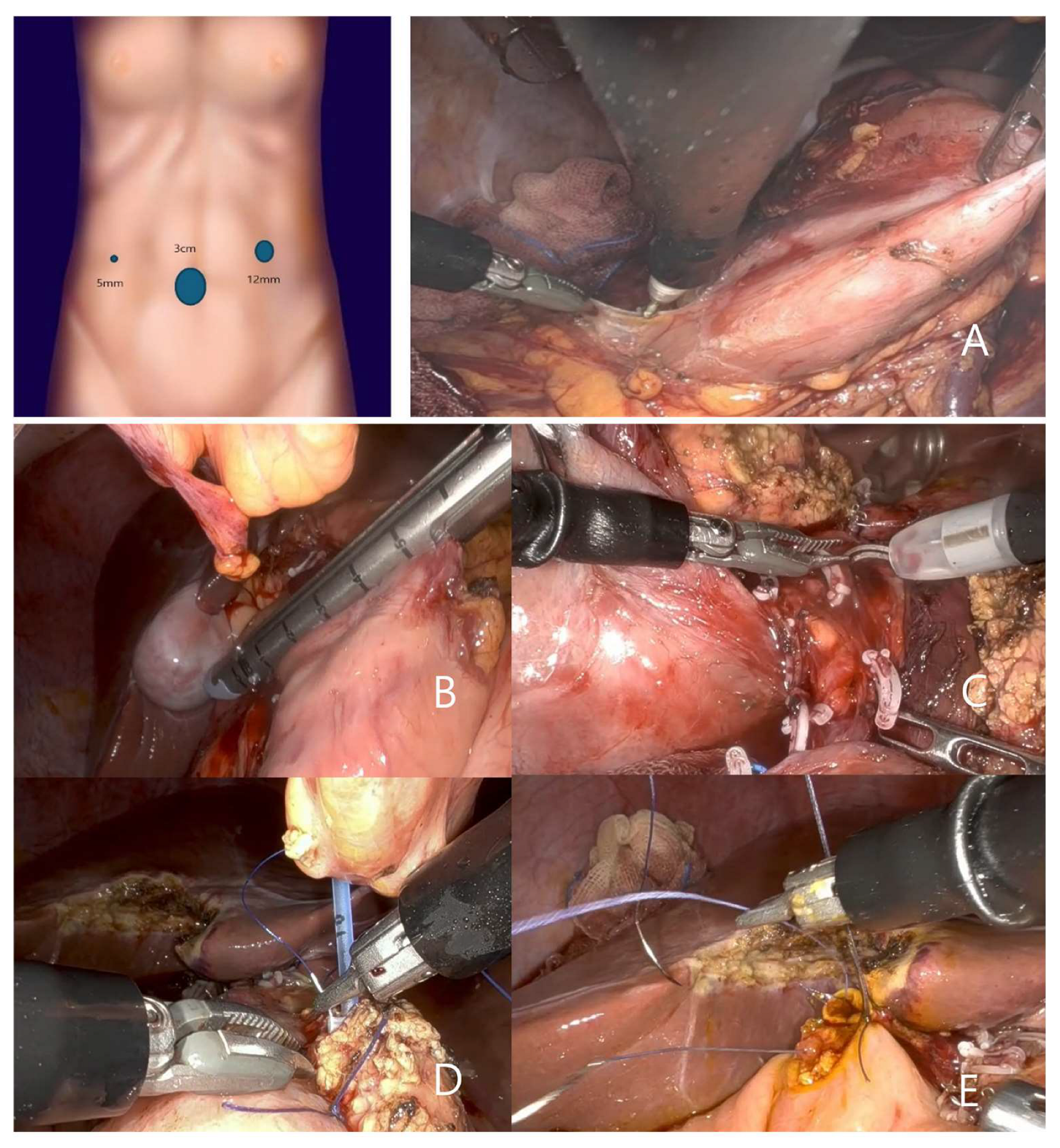
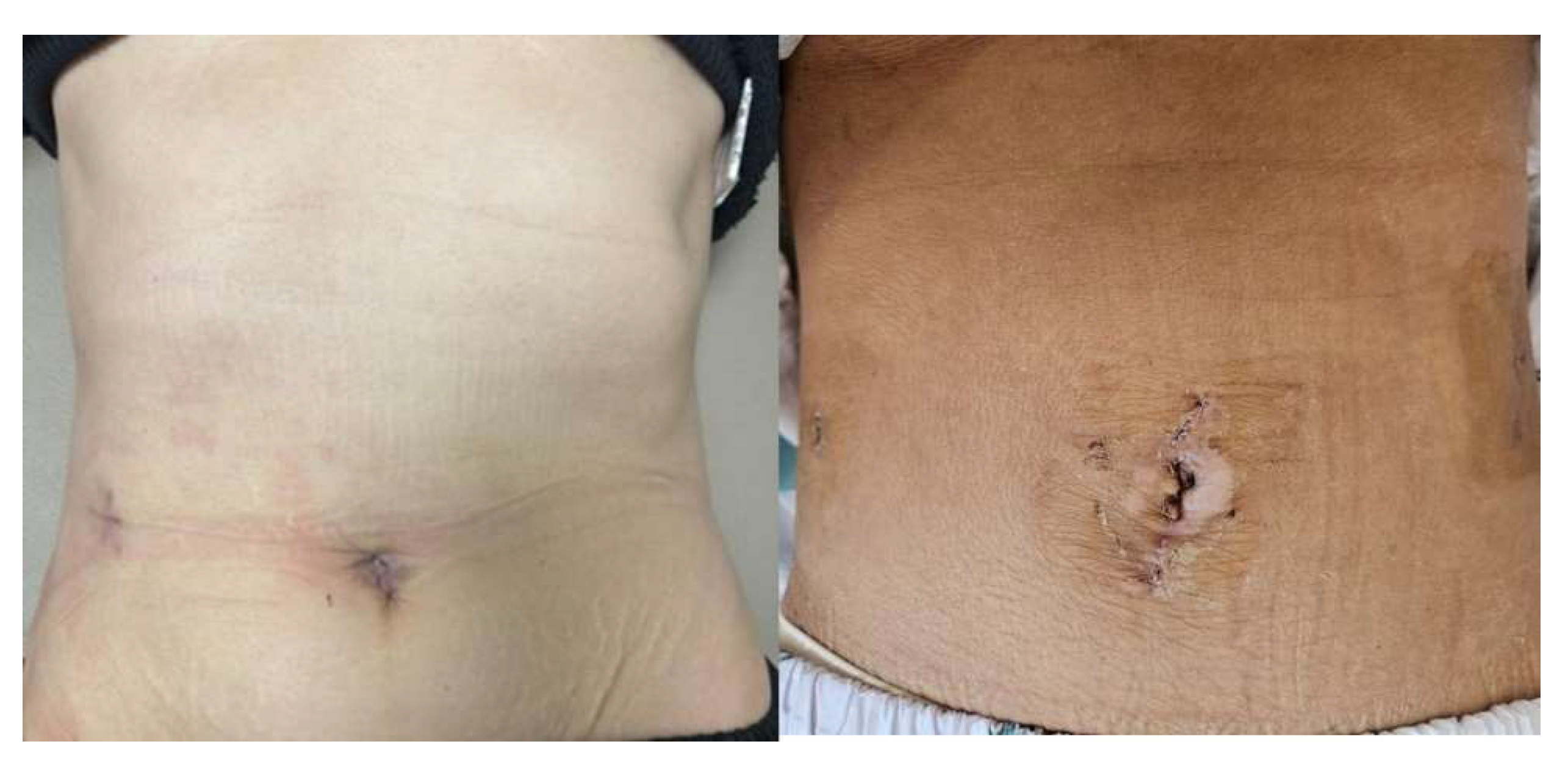
2.2. Surgical Techniques and Technical Modifications
3. Results
3.1. Clinical Characteristics of Patients with RPPPD Using da Vinci SP System
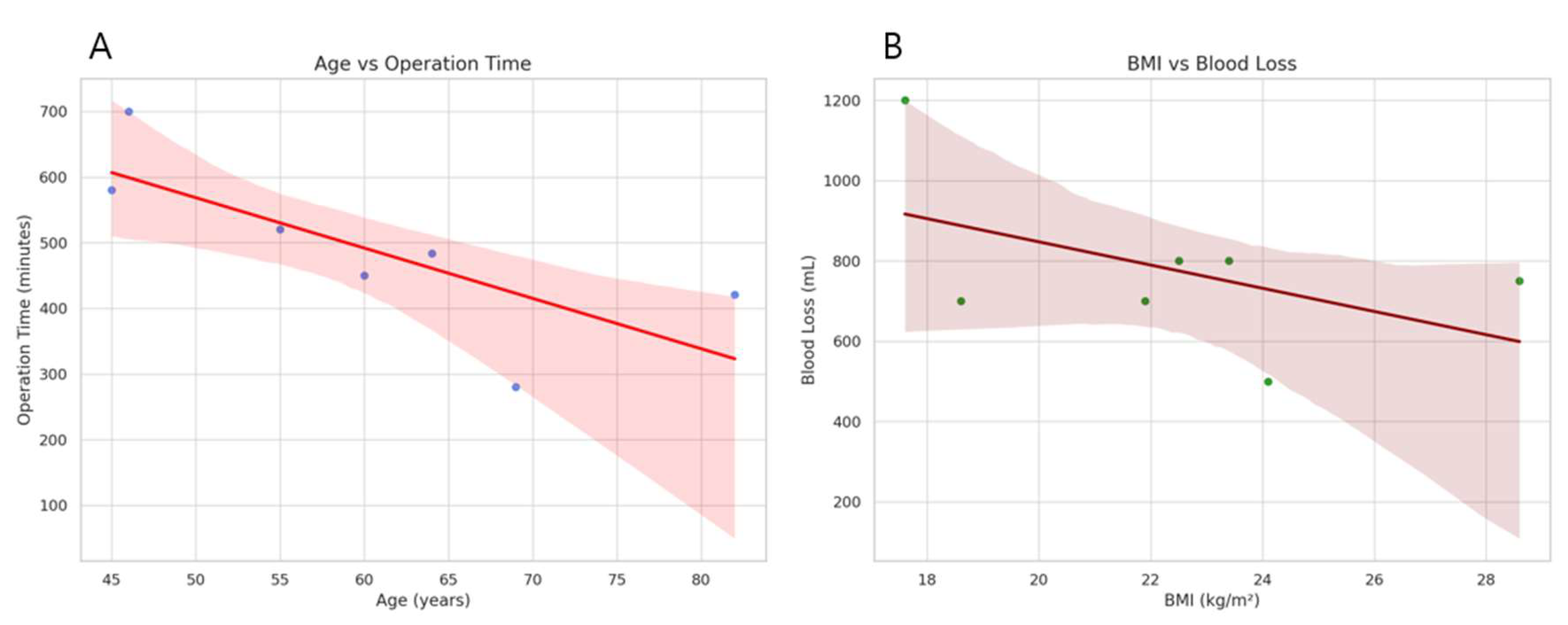
3.2. Perioperative and Postoperative Outcomes of Patients with RPPPD Using da Vinci SP System
3.3. Conparison of Clinical Outcomes Between Multiport RPPPD and RPPPD Using da Vinci SP System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RPPPD | Robotic pylorus-preserving pancreaticoduodenectomy |
| BMI | Body mass index |
| POPF | Postoperative pancreatic fistula |
| SCN | Serous cystic adenoma |
| AOV | Ampulla of Vater |
| CBD | Common bile duct |
| SPN | Solid pseudopapillary neoplasm |
References
- Hartman, V.; Bracke, B.; Chapelle, T.; Hendrikx, B.; Liekens, E.; Roeyen, G. Robotic Pancreaticoduodenectomy for Pancreatic Head Tumour: A Single-Centre Analysis. Cancers 2024, 16, 4243. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.; Shi, Z.; Zhai, S.; Li, J.; Qian, H.; Tang, X.; Weng, Y.; Shi, Y.; Wang, L.; Wang, Y.; et al. Predicting Selection Preference of Robotic Pancreaticoduodenectomy (RPD) in a Chinese Single Center Population: Development and Assessment of a New Predictive Nomogram. Med Sci. Monit. 2019, 25, 8034–8042. [Google Scholar] [CrossRef] [PubMed]
- Kalabin, A.; Mani, V.R.; Kruse, R.L.; Schlesselman, C.; Li, K.Y.; Staveley-O’Carroll, K.F.; Kimchi, E.T. New perspectives on robotic pancreaticoduodenectomy: An analysis of the National Cancer Database. World J. Gastrointest. Surg. 2023, 15, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-Y.; Shyr, B.-S.; Chen, S.-C.; Shyr, Y.-M.; Wang, S.-E. Surgical and survival outcomes after robotic and open pancreaticoduodenectomy with positive margins. J. Chin. Med. Assoc. 2021, 84, 698–703. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, N.; Tian, E.; Li, M.; Zhang, H.; Zhao, G.; Tan, X.; Wang, W.; Han, B.; Yuan, J.; et al. Short-term outcomes of robotic versus open pancreaticoduodenectomy in elderly patients: A multicenter retrospective cohort study. Int. J. Surg. 2022, 104, 106819. [Google Scholar] [CrossRef]
- Mungo, B.; Hammad, A.; AlMasri, S.; Dogeas, E.; Nassour, I.; Singhi, A.D.; Zeh, H.J., III; Hogg, M.E.; Lee, K.K.W.; Zureikat, A.H.; et al. Pancreaticoduodenectomy for benign and premalignant pancreatic and ampullary disease: Is robotic surgery the better approach? Surg. Endosc. 2023, 37, 1157–1165. [Google Scholar] [CrossRef]
- Nakamura, S.; Nakata, K.; Nagakawa, Y.; Kozono, S.; Wakabayashi, G.; Wakabayashi, T.; Uyama, I.; Takahara, T.; Takeda, Y.; Ohmura, Y.; et al. The safety and feasibility of robotic pancreaticoduodenectomy: A multicenter retrospective assessment of 425 patients in Japan. J. Hepatobiliary Pancreat. Sci. 2024, 32, 124–131. [Google Scholar] [CrossRef]
- Cannoletta, D.; Mazzone, E.; Dell’oglio, P.; Pettenuzzo, G.; Pacini, M.; Lambertini, L.; Pellegrino, A.A.; Sauer, R.C.; Torres-Anguiano, J.R.; Stabile, A.; et al. Development and validation of a novel comorbidity score specific for prostate cancer patients treated with robotic platform and its implication on DaVinci single-port system. J. Robot. Surg. 2024, 18, 400. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, W.R. Early Single-Center Experience of DaVinci((R)) Single-Port (SP) Robotic Surgery in Colorectal Patients. J. Clin. Med. 2024, 13, 2989. [Google Scholar] [CrossRef]
- Park, S.E.; Hong, T.H. Gasless robotic single-port cholecystectomy using the DaVinci SP system: A feasible way to minimise surgical derangement while obtaining critical view of safety. Int. J. Med. Robot. 2023, 20, e2547. [Google Scholar] [CrossRef]
- Zhang, K.; Yuan, Z.; Lu, Y.; Chen, L.; Lu, C. Totally robotic surgery for rectal cancer with the daVinci(R) single-port platform. Br. J. Surg. 2023, 110, 1890. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Hong, J.I.; Jang, Y.J.; Kim, H.K. Single-port plus one port subcostal robotic-assisted minimally invasive McKewon esophagectomy using the daVinci Single-Port surgical system. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad026. [Google Scholar] [CrossRef]
- Giulianotti, P.C.; Coratti, A.; Angelini, M.; Sbrana, F.; Cecconi, S.; Balestracci, T.; Caravaglios, G. Robotics in general surgery: Personal experience in a large community hospital. Arch. Surg. 2003, 138, 777–784. [Google Scholar] [CrossRef] [PubMed]
- van Oosten, A.F.; Ding, D.; Habib, J.R.; Irfan, A.; Schmocker, R.K.; Sereni, E.; Kinny-Köster, B.; Wright, M.; Groot, V.P.; Molenaar, I.Q.; et al. Perioperative Outcomes of Robotic Pancreaticoduodenectomy: A Propensity-Matched Analysis to Open and Laparoscopic Pancreaticoduodenectomy. J. Gastrointest. Surg. 2021, 25, 1795–1804. [Google Scholar] [CrossRef]
- Tang, G.; Zhang, L.; Xia, L.; Zhang, J.; Chen, R.; Zhou, R. Comparison of short-term outcomes of robotic versus open Pancreaticoduodenectomy: A Meta-Analysis of randomized controlled trials and Propensity-Score-Matched studies. Int. J. Surg. 2024, 111, 1214–1230. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Zhang, J.; Zhang, L.; Xia, L.; Chen, R.; Zhou, R. Postoperative complications and surgical outcomes of robotic versus laparoscopic Pancreaticoduodenectomy: A meta-analysis of propensity-score-matched studies. Int. J. Surg. 2024, 111, 2257–2272. [Google Scholar] [CrossRef]
- Tang, G.; Zhang, J.; Zhang, L.; Xia, L.; Chen, R.; Zhou, R. Robotic versus laparoscopic pancreaticoduodenectomy for pancreatic and periampullary tumors: A meta-analysis. Front. Oncol. 2024, 14, 1486504. [Google Scholar] [CrossRef]
- Yu, Y.; Changyong, E.; Lin, C.; Wang, L.; Jiang, T. Safety and learning curve analysis of robotic-assisted pancreaticoduodenectomy: Experience of a single surgeon. J. Robot. Surg. 2024, 18, 92. [Google Scholar] [CrossRef]
- Stefanova, I.; Vescio, F.; Nickel, F.; Merali, N.; Ammendola, M.; Lahiri, R.P.; Pencavel, T.D.; Worthington, T.R.; Frampton, A.E. What are the true benefits of robotic pancreaticoduodenectomy for patients with pancreatic cancer? Expert. Rev. Gastroenterol. Hepatol. 2024, 18, 133–139. [Google Scholar] [CrossRef]
- Shyr, Y.; Wang, S.; Chen, S.; Shyr, B. Robotic pancreaticoduodenectomy for pancreatic head cancer and periampullary lesions. Ann. Gastroenterol. Surg. 2021, 5, 589–596. [Google Scholar] [CrossRef]
- Shyr, Y.-M.; Wang, S.-E.; Chen, S.-C.; Shyr, B.-U. Robotic pancreaticoduodenectomy in the era of minimally invasive surgery. . Chin. Med. Assoc. 2020, 83, 639–643. [Google Scholar] [CrossRef]
- Shyr, B.-S.; Shih, M.-S.; Chen, S.-C.; Wang, S.-E.; Shyr, Y.-M. Combined robotic/open pancreaticoduodenectomy in the young aged < 50 years. Updates Surg. 2025. [Google Scholar] [CrossRef]
- Shyr, B.; Chen, S.; Wang, S.; Shyr, Y. Impact of Obesity on Perioperative Outcomes in Robotic Pancreaticoduodenectomy: A Propensity Score-Matched Study. Int. J. Med. Robot. 2024, 20, e70034. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Sucandy, I.; Vasanthakumar, P.; Espeut, A.; Christodoulou, M.; Pattilachan, T.M.; Rosemurgy, A. Deconstructing the Operative Times of Robotic Pancreaticoduodenectomy. Am. Surg. 2024, 90, 1521–1530. [Google Scholar] [CrossRef]
- Rosemurgy, A.S.M.; Ross, S.B.M.; Espeut, A.; Nguyen, D.B.; Crespo, K.B.; Syblis, C.B.; Vasanthakumar, P.B.; Sucandy, I.M. Survival and Robotic Approach for Pancreaticoduodenectomy: A Propensity Score-Match Study. J. Am. Coll. Surg. 2022, 234, 677–684. [Google Scholar] [CrossRef]
- Chao, Y.-J.; Lu, W.-H.; Liao, T.-K.; Su, P.-J.; Wang, C.-J.; Lai, C.-H.; Hung, J.-Y.; Su, P.-F.; Shan, Y.-S. Feasibility of simultaneous development of laparoscopic and robotic pancreaticoduodenectomy. Sci. Rep. 2023, 13, 6190. [Google Scholar] [CrossRef] [PubMed]

| Surgeon 1 | Surgeon 2 | ||||||
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
| Age (years) | 64 | 82 | 69 | 55 | 46 | 45 | 60 |
| Sex | Female | Male | Male | Male | Female | Female | Female |
| BMI (kg/m2) | 24.1 | 21.9 | 22.5 | 28.6 | 17.6 | 18.6 | 23.4 |
| Medical hx | None | None | None | None | None | None | None |
| Surgical hx | None | None | None | None | None | None | None |
| Surgeon 1 | Surgeon 2 | ||||||
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
| Operation time (min) | 484 | 421 | 280 | 520 | 700 | 580 | 450 |
| Blood loss (mL) | 500 | 700 | 800 | 750 | 1200 | 700 | 800 |
| Complication | None | None | None | None | Drain site infection | None | None |
| POPF | None | Biochemical leak | None | Biochemical leak | None | Biochemical leak | Biochemical leak |
| Discharge (d) | 13 | 26 | 9 | 11 | 21 | 16 | 18 |
| Clinical diagnosis | SCN | AOV cancer(cTxN0) | Pancreatic cancer (cT2N+) | Pancreatic cancer (cT2N+) | CBD cancer (cTxN0) | SPN | Pancreatic cancer (cT3N0) |
| Pathology | SCN [2.2 × 1.3 cm, LN(0/12)] | AOV cancer [T1aN0, 0.2 × 0.2 cm,WD, LN(0/13)] RM(negative) | Pancreatic cancer [T2N2, 3.5 × 2.8 cm, MD,LN(4/24)] RM(negative) | Pancreaticancer [T3N2, 4.2 × 4.1 cm, MD, LN(5/19)] RM(negative) | CBD cancer [T2N1, 3.2 × 2.2 cm, MD, LN(1/42)] RM(negative) | SPN [3.2 × 2.2 cm, LN(0/16)] | Pancreatic cancer [T3N0, 4.1 × 2.3 cm, LN(0/48)] RM(negative) |
| Variable | Mean ±SD, or n (%) | p-Value | ||
| Multiport (n = 8) | Single Port (n = 7) | |||
| Age (years) | 54.1 ± 17.5 | 60.1 ± 13.1 | 0.46 | |
| Sex | Male | 4 (50%) | 3 (42.9%) | 1.00 |
| Female | 4 (50%) | 4 (58%) | ||
| BMI (kg/m2) | 22.9 ± 3.7 | 22.4 ± 3.7 | 0.78 | |
| Diganosis | Benign | 1 (12.5%) | 2 (28.6%) | 0.89 |
| Malignancy | 7 (87.5%) | 5 (71.4%) | ||
| Operation time (min) | 674.9 ± 133.4 | 490.7 ± 131.4 | 0.02 | |
| Estimated blood loss (mL) | 993.8 ± 865.4 | 778.6 ± 211.9 | 0.51 | |
| Hospital stay (days) | 13.5 ± 6.1 | 16.3 ± 5.9 | 0.38 | |
| POPF | None | 5 (62.5%) | 3 (42.9%) | 0.80 |
| Biochemical leak | 3 (37.5%) | 4 (57.1%) | ||
| B&C | 0 | 0 | ||
| Delayed gastric emptyng | None | 5 (62.5%) | 7 (100%) | 0.24 |
| DGE A | 3 (37.5%) | 0 (0%) | ||
| DGE B&C | 0 (0%) | 0 (0%) | ||
| Complication Grade ≥ 3 | 0 (0%) | 0 (0%) | 1.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.S.; Lim, J.H. Navigating the Intricacies of Robotic Pylorus-Preserving Pancreaticoduodenectomy Using the da Vinci SP (Single Port) System. J. Clin. Med. 2025, 14, 3193. https://doi.org/10.3390/jcm14093193
Kim HS, Lim JH. Navigating the Intricacies of Robotic Pylorus-Preserving Pancreaticoduodenectomy Using the da Vinci SP (Single Port) System. Journal of Clinical Medicine. 2025; 14(9):3193. https://doi.org/10.3390/jcm14093193
Chicago/Turabian StyleKim, Hyung Sun, and Jin Hong Lim. 2025. "Navigating the Intricacies of Robotic Pylorus-Preserving Pancreaticoduodenectomy Using the da Vinci SP (Single Port) System" Journal of Clinical Medicine 14, no. 9: 3193. https://doi.org/10.3390/jcm14093193
APA StyleKim, H. S., & Lim, J. H. (2025). Navigating the Intricacies of Robotic Pylorus-Preserving Pancreaticoduodenectomy Using the da Vinci SP (Single Port) System. Journal of Clinical Medicine, 14(9), 3193. https://doi.org/10.3390/jcm14093193





