The Prognostic Value of Tricuspid Annular Dimensions in TAVI Patients: A CT-Based Retrospective Analysis of Risk Stratification and Long-Term Outcomes
Abstract
1. Introduction
2. Material and Method
2.1. Study Population
2.2. Ethics Declaration
2.3. Data Extraction
2.4. Transthoracic Echocardiography
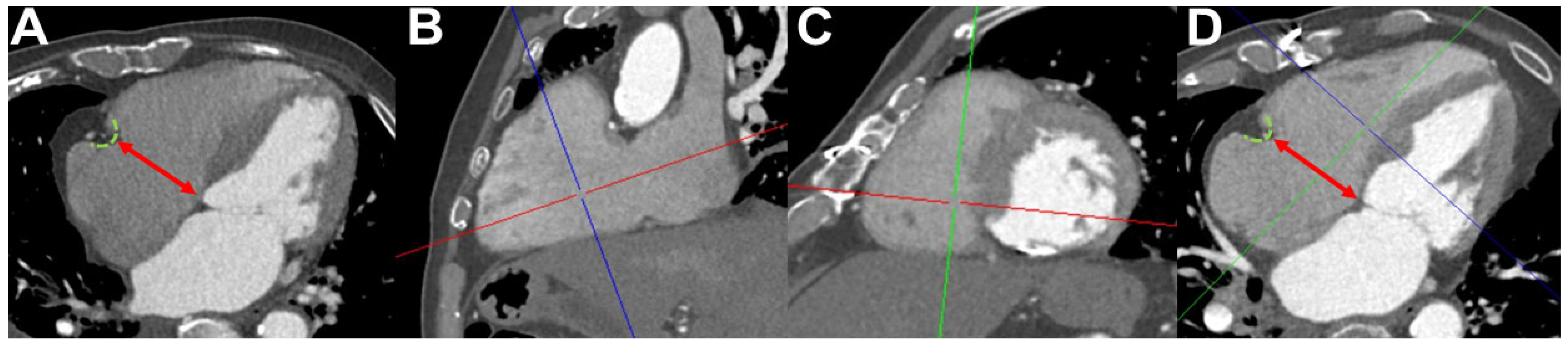
2.5. Computed Tomography Angiography Image Acquisition, Image Analysis, and Measurement of Tricuspid Annulus Diameters
2.6. TAVI Procedure
2.7. Outcomes
2.8. Statistical Analysis
3. Results
3.1. Study Cohort and Baseline Characteristic
3.2. ICC
3.3. Cut-Off Values of TA Dimensions for Right Ventricular Dysfunction
3.4. Comparison of Clinical Characteristics According to TA and TA/BSA Subgroups
3.5. Impact of TA Dimensions on Short-Term Outcomes
3.6. Impact of TA Dimensions on Long-Term Survival
3.7. Prognostic Value of TA Measurements for Mid- and Long-Term Mortality in the TAVI Cohort
3.8. Multivariate Cox Regression Analysis of Mortality Predictors
4. Discussion
4.1. Reassessing the Prognostic Value of TA Dimensions in TAVI Patients
4.2. Reassessment of Previous Findings
4.3. Alignment with Recent Literature
4.4. Shifting Focus: From Anatomy to Function
4.5. Methodological Considerations and Future Directions
4.6. Implications for Clinical Practice
4.7. Future Perspectives
- Developing integrative models that incorporate both anatomical and functional parameters of right heart function;
- Investigating the dynamic interplay between RV performance, TR severity, and pulmonary hemodynamics;
- Exploring novel imaging techniques and biomarkers that may offer superior prognostic value.
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srinivasan, A.; Wong, F.; Wang, B. Transcatheter aortic valve replacement: Past, present, and future. Clin. Cardiol. 2024, 47, e24209. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Chen, M.; Hayashida, K.; Latib, A.; Modine, T.; Piazza, N.; Redwood, S.; Søndergaard, L.; Prendergast, B.D. Transcatheter aortic valve implantation: Current status and future perspectives. Eur. Heart J. 2018, 39, 2625–2634. [Google Scholar] [CrossRef]
- Blankenberg, S.; Seiffert, M.; Vonthein, R.; Baumgartner, H.; Bleiziffer, S.; Borger, M.A.; Choi, Y.H.; Clemmensen, P.; Cremer, J.; Czerny, M.; et al. DEDICATE-DZHK6 Trial Investigators Transcatheter or Surgical Treatment of Aortic-Valve Stenosis. N. Engl. J. Med. 2024, 390, 1572–1583. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Evolut Low Risk Trial Investigators Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. PARTNER 3 Investigators Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Ihlemann, N.; Jørgensen, T.H.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Five-Year Clinical and Echocardiographic Outcomes from the NOTION Randomized Clinical Trial in Patients at Lower Surgical Risk. Circulation 2019, 139, 2714–2723. [Google Scholar] [CrossRef]
- Schewel, J.; Schmidt, T.; Kuck, K.H.; Frerker, C.; Schewel, D. Impact of Pulmonary Hypertension Hemodynamic Status on Long-Term Outcome After Transcatheter Aortic Valve Replacement. JACC. Cardiovasc. Interv. 2019, 12, 2155–2168. [Google Scholar] [CrossRef]
- Emami Meybodi, M.; Bamarinejad, A.; Bamarinejad, F.; Abhari, A.P.; Fakhrolmobasheri, M.; Khosravi Larijani, F.; Nasiri, S.; Shafie, D. Prognostic Implication of Preprocedural Pulmonary Hypertension in Patients with Severe Aortic Valve Stenosis Undergoing Transcatheter Aortic Valve Implantation: A Systematic Review and Meta-analysis. Cardiol. Rev. 2025, 33, 120–128. [Google Scholar] [CrossRef]
- Kammerlander, A.A.; Marzluf, B.A.; Graf, A.; Bachmann, A.; Kocher, A.; Bonderman, D.; Mascherbauer, J. Right ventricular dysfunction, but not tricuspid regurgitation, is associated with outcome late after left heart valve procedure. J. Am. Coll. Cardiol. 2014, 64, 2633–2642. [Google Scholar] [CrossRef]
- Bernardo, R.J.; Haddad, F.; Couture, E.J.; Hansmann, G.; de Jesus Perez, V.A.; Denault, A.Y.; de Man, F.S.; Amsallem, M. Mechanics of right ventricular dysfunction in pulmonary arterial hypertension and heart failure with preserved ejection fraction. Cardiovasc. Diagn. Ther. 2020, 10, 1580–1603. [Google Scholar] [CrossRef]
- Condello, F.; Gitto, M.; Stefanini, G.G. Etiology, epidemiology, pathophysiology and management of tricuspid regurgitation: An overview. Rev. Cardiovasc. Med. 2021, 22, 1115–1142. [Google Scholar] [CrossRef]
- Volpato, V.; Lang, R.M.; Yamat, M.; Veronesi, F.; Weinert, L.; Tamborini, G.; Muratori, M.; Fusini, L.; Pepi, M.; Genovese, D.; et al. Echocardiographic Assessment of the Tricuspid Annulus: The Effects of the Third Dimension and Measurement Methodology. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2019, 32, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Koo, H.J.; Kang, J.W.; Yang, D.H. Tricuspid Valve Imaging and Right Ventricular Function Analysis Using Cardiac CT and MRI. Korean J. Radiol. 2021, 22, 1946–1963. [Google Scholar] [CrossRef] [PubMed]
- Deseive, S.; Steffen, J.; Beckmann, M.; Jochheim, D.; Orban, M.; Zadrozny, M.; Gschwendtner, S.; Braun, D.; Rizas, K.; Curta, A.; et al. CT-Determined Tricuspid Annular Dilatation Is Associated with Increased 2-Year Mortality in TAVR Patients. JACC. Cardiovasc. Interv. 2020, 13, 2497–2507. [Google Scholar] [CrossRef]
- Khalique, O.K.; Cavalcante, J.L.; Shah, D.; Guta, A.C.; Zhan, Y.; Piazza, N.; Muraru, D. Multimodality Imaging of the Tricuspid Valve and Right Heart Anatomy. JACC. Cardiovasc. Imaging 2019, 12, 516–531. [Google Scholar] [CrossRef]
- Lee, G.; Chikwe, J.; Milojevic, M.; Wijeysundera, H.C.; Biondi-Zoccai, G.; Flather, M.; Gaudino, M.F.L.; Fremes, S.E.; Tam, D.Y. ESC/EACTS vs. ACC/AHA guidelines for the management of severe aortic stenosis. Eur. Heart J. 2023, 44, 796–812. [Google Scholar] [CrossRef]
- Boxhammer, E.; Kletzer, J.; Kellermair, J.; Scharinger, B.; Kaufmann, R.; Hammerer, M.; Blessberger, H.; Steinwender, C.; Lichtenauer, M.; Hergan, K.; et al. CT measured pulmonary artery to ascending aorta ratio stratified by echocardiographically obtained systolic pulmonary artery pressure values for noninvasive detection of pulmonary hypertension in patients with severe aortic valve stenosis. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2023, 112, 1394–1416. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. ESC/ERS Scientific Document Group 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. ESC/EACTS Scientific Document Group 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Hassani, C.; Ng, B.; Cen, S.; Tejura, T.K.; Saremi, F. Tricuspid Annular Diameter Measurement on Routine Chest CT Can Detect Significant Tricuspid Regurgitation. AJR. Am. J. Roentgenol. 2019, 213, W257–W263. [Google Scholar] [CrossRef]
- Hirasawa, K.; Fortuni, F.; van Rosendael, P.J.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. Association between computed tomography-derived tricuspid annular dimensions and prognosis: Insights from whole-beat computed tomography assessment. Eur. Heart journal. Cardiovasc. Imaging 2022, 23, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Fortuni, F.; Butcher, S.C.; Dietz, M.F.; van der Bijl, P.; Prihadi, E.A.; De Ferrari, G.M.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. Right Ventricular-Pulmonary Arterial Coupling in Secondary Tricuspid Regurgitation. Am. J. Cardiol. 2021, 148, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Y.; Li, Q.; Li, J.; Zhang, M.W.; Zhu, L.; Geng, J. Echocardiographic evaluation of the right atrial size and function: Relevance for clinical practice. Am. Heart J. Plus Cardiol. Res. Pract. 2023, 27, 100274. [Google Scholar] [CrossRef]


| Total Cohort | |
|---|---|
| No. (%) | |
| Total | 522 (100.0) |
| Sex (male) | 260 (49.8) |
| Age ≤80 >80 | 149 (28.5) 373 (71.5) |
| NYHA ≥ III | 210 (40.2) |
| Diabetes Mellitus | 141 (27.0) |
| Arterial Hypertension | 453 (86.8) |
| CHD | 264 (50.6) |
| AF | 189 (36.2) |
| PAOD | 44 (8.4) |
| COPD | 59 (11.3) |
| MI—Prehistory | 37 (7.1) |
| Stroke—Prehistory | 39 (7.5) |
| TRVmax ≥ 2.8 m/s | 238 (45.6) |
| sPAP ≥ 40 mmHg | 230 (44.1) |
| TAPSE/sPAP < 0.55 mmHg | 229 (43.9) |
| Mean ± SD | |
| Age (years) | 82.1 ± 5.2 |
| Height (cm) | 167.8 ± 8.8 |
| Weight (kg) | 72.9 ± 14.6 |
| BMI (kg/m2) | 25.8 ± 4.4 |
| BSA (m2) | 1.8 ± 0.2 |
| LVEF (%) | 53.0 ± 9.8 |
| LVEDD (mm) | 46.4 ± 6.8 |
| IVSd (mm) | 13.4 ± 2.1 |
| AV Vmax (m/s) | 4.4 ± 0.6 |
| AV MPG (mmHg) | 45.7 ± 12.2 |
| TRVmax (m/s) | 2.7 ± 0.8 |
| sPAP (mmHg) | 38.6 ± 17.0 |
| TAPSE (mm) | 21.9 ± 4.7 |
| TAPSE/sPAP (mm/mmHg) | 0.8 ± 0.8 |
| TA (mm) | 40.4 ± 6.7 |
| TA/BSA (mm/m2) | 22.4 ± 4.2 |
| MR ≥ moderate/severe | 178 (34.1) |
| TR ≥ moderate/severe | 138 (26.4) |
| Median ± IQR | |
| Creatinine (mg/dL) | 1.1 ± 0.5 |
| HK (%) | 38.0 ± 6.8 |
| HB (g/dL) | 12.8 ± 2.0 |
| CK (U/L) | 80.5 ± 60.3 |
| proBNP (pg/mL) | 2038.0 ± 3453.5 |
| TA < 44.00 mm | TA ≥ 44.0 mm | p | TA/BSA < 23.00 mm/m2 | TA/BSA ≥ 23.00 mm/m2 | p | |
|---|---|---|---|---|---|---|
| No. (%) | ||||||
| Total | 361 (69.2) | 161 (30.8) | - | 307 (58.8) | 213 (41.2) | - |
| Sex (male) | 154 (42.7) | 106 (65.8) | <0.001 | 161 (52.4) | 95 (44.6) | 0.041 |
| Age ≤80 >80 | 106 (29.4) 255 (70.6) | 43 (26.7) 118 (73.3) | 0.535 | 107 (34.9) 200 (65.1) | 41 (19.2) 172 (80.8) | <0.001 |
| NYHA ≥ III | 119 (33.0) | 59 (36.6) | 0.288 | 94 (30.6) | 80 (37.5) | 0.094 |
| Diabetes Mellitus | 109 (30.2) | 32 (19.9) | 0.014 | 90 (29.3) | 45 (21.1) | 0.024 |
| Arterial Hypertension | 316 (87.5) | 137 (85.1) | 0.551 | 259 (84.4) | 183 (85.9) | 0.983 |
| CHD | 181 (50.1) | 83 (51.6) | 0.765 | 149 (48.5) | 109 (51.2) | 0.741 |
| AF | 122 (33.8) | 67 (41.6) | 0.086 | 110 (35.8) | 76 (35.7) | 0.814 |
| PAOD | 32 (8.9) | 12 (7.5) | 0.592 | 31 (10.1) | 13 (6.1) | 0.091 |
| COPD | 44 (12.2) | 15 (9.3) | 0.339 | 38 (12.4) | 18 (8.5) | 0.131 |
| MI—Prehistory | 29 (8.0) | 8 (5.0) | 0.208 | 24 (7.8) | 11 (5.2) | 0.209 |
| Stroke—Prehistory | 28 (7.8) | 11 (6.8) | 0.711 | 24 (7.8) | 14 (6.6) | 0.542 |
| TRVmax ≥ 2.8 m/s | 147 (40.7) | 91 (56.5) | 0.001 | 122 (39.7) | 115 (54.0) | 0.003 |
| sPAP ≥ 40 mmHg | 138 (38.2) | 92 (57.1) | <0.001 | 112 (36.5) | 115 (54.0) | <0.001 |
| TAPSE/sPAP < 0.55 mmHg | 143 (39.6) | 86 (53.4) | 0.004 | 109 (35.5) | 118 (55.4) | <0.001 |
| Mean ± SD | ||||||
| Age (years) | 81.8 ± 5.4 | 82.6 ± 4.8 | 0.090 | 81.2 ± 5.6 | 83.2 ± 4.5 | <0.001 |
| Height (cm) | 166.9 ± 8.7 | 170.0 ± 8.6 | <0.001 | 169.3 ± 8.4 | 165.7 ± 8.9 | <0.001 |
| Weight (kg) | 72.5 ± 15.0 | 73.9 ± 13.9 | 0.320 | 77.3 ± 14.8 | 66.6 ± 11.9 | <0.001 |
| BMI (kg/m2) | 25.9 ± 4.6 | 25.4 ± 4.0 | 0.221 | 26.9 ± 4.6 | 24.2 ± 3.6 | <0.001 |
| BSA (m2) | 1.8 ± 0.2 | 1.8 ± 0.2 | 0.037 | 1.9 ± 0.2 | 1.7 ± 0.2 | <0.001 |
| LVEF (%) | 53.6 ± 9.3 | 51.8 ± 10.8 | 0.069 | 53.6 ± 9.6 | 52.2 ± 10.1 | 0.125 |
| LVEDD (mm) | 45.8 ± 6.7 | 47.6 ± 6.7 | 0.012 | 46.5 ± 6.8 | 46.2 ± 0.7 | 0.641 |
| IVSd (mm) | 13.4 ± 2.1 | 13.4 ± 2.0 | 0.952 | 13.5 ± 2.1 | 13.3 ± 2.0 | 0.421 |
| AV Vmax (m/s) | 4.4 ± 0.6 | 4.4 ± 0.5 | 0.793 | 4.4 ± 0.6 | 4.3 ± 0.5 | 0.436 |
| AV MPG (mmHg) | 45.7 ± 12.3 | 45.6 ± 12.0 | 0.932 | 46.0 ± 12.6 | 45.0 ± 11.5 | 0.372 |
| TRVmax (m/s) | 2.6 ± 0.9 | 2.9 ± 0.7 | <0.001 | 2.6 ± 0.9 | 2.8 ± 0.7 | <0.001 |
| sPAP (mmHg) | 36.4 ± 16.7 | 43.3 ± 16.8 | <0.001 | 36.1 ± 17.1 | 42.7 ± 16.1 | <0.001 |
| TAPSE (mm) | 21.8 ± 4.6 | 22.1 ± 5.0 | 0.534 | 22.0 ± 4.4 | 21.5 ± 5.1 | 0.220 |
| TAPSE/sPAP (mm/mmHg) | 0.9 ± 0.8 | 0.7 ± 0.6 | 0.003 | 0.9 ± 0.8 | 0.6 ± 0.5 | <0.001 |
| TA (mm) | 36.9 ± 4.4 | 48.2 ± 3.7 | <0.001 | 36.7 ± 4.9 | 45.6 ± 5.3 | <0.001 |
| TA/BSA (mm/m2) | 20.6 ± 3.2 | 26.4 ± 3.3 | <0.001 | 19.6 ± 2.3 | 26.4 ± 2.7 | <0.001 |
| Median ± IQR | ||||||
| Creatinine (mg/dL) | 1.1 ± 0.5 | 1.0 ± 0.5 | 0.816 | 1.1 ± 0.5 | 1.0 ± 0.5 | 0.955 |
| HK (%) | 37.4 ± 7.0 | 38.4 ± 7.3 | 0.480 | 38.2 ± 6.9 | 37.8 ± 7.0 | 0.954 |
| HB (g/dL) | 12.7 ± 2.0 | 13.1 ± 2.1 | 0.280 | 12.7 ± 2.1 | 12.9 ± 2.2 | 0.741 |
| CK (U/l) | 84.3 ± 60.0 | 77.5 ± 63.3 | 0.600 | 77.5 ± 62.0 | 84.5 ± 62.8 | 0.603 |
| proBNP (pg/mL) | 1947.0 ± 3868.0 | 2170.0 ± 2903.8 | 0.571 | 2152.0 ± 3891.6 | 1807.0 ± 3024.3 | 0.202 |
| TA < 44.00 mm | TA ≥ 44.0 mm | p | TA/BSA < 23.00 mm/m2 | TA/BSA ≥ 23.00 mm/m2 | p | |
|---|---|---|---|---|---|---|
| No. (%) | ||||||
| Procedural death | 2 (0.6) | 1 (0.6) | 0.925 | 2 (0.7) | 1 (0.5) | 0.772 |
| 30-day death | 4 (1.1) | 3 (1.9) | 0.488 | 3 (1.0) | 4 (1.9) | 0.399 |
| 30-day stroke/TIA | 9 (2.5) | 3 (1.9) | 0.657 | 6 (2.0) | 6 (2.8) | 0.790 |
| 30-day pacemaker necessity | 46 (12.7) | 20 (12.4) | 0.919 | 40 (13.0) | 26 (12.2) | 0.590 |
| 30-day major vascular complications | 16 (4.4) | 6 (3.7) | 0.717 | 12 (3.9) | 10 (4.7) | 0.900 |
| AUC (95% CI) | TA | TA/BSA |
|---|---|---|
| 12-month-mortality | 0.503 (0.419–0.587) | 0.457 (0.373–0.542) |
| 24-month-mortality | 0.486 (0.420–0.553) | 0.465 (0.399–0.532) |
| 36-month-mortality | 0.478 (0.419–0.538) | 0.474 (0.413–0.534) |
| 48-month-mortality | 0.518 (0.461–0.574) | 0.501 (0.445–0.557) |
| 60-month-mortality | 0.520 (0.466–0.574) | 0.508 (0.454–0.562) |
| 72-month-mortality | 0.516 (0.463–0.569) | 0.504 (0.451–0.557) |
| 84-month-mortality | 0.515 (0.462–0.568) | 0.498 (0.446–0.551) |
| Cox Regression | HR (95% CI) | p |
|---|---|---|
| TA ≥ 44.00 mm | 0.907 (0.649–1.268) | 0.569 |
| Age (per 1 year) | 1.022 (0.990–1.055) | 0.178 |
| LVEF (per 1%) | 0.992 (0.976–1.009) | 0.352 |
| AF | 1.890 (1.383–2.584) | <0.001 |
| TR ≥ moderate/severe | 0.823 (0.561–1.208) | 0.319 |
| sPAP ≥ 40 mmHg | 1.311 (0.933–1.843) | 0.118 |
| Cox Regression | HR (95% CI) | p |
|---|---|---|
| TA/BSA ≥ 23.00 mm/m2 | 0.906 (0.649–1.266) | 0.564 |
| Age (per 1 year) | 1.025 (0.992–1.058) | 0.140 |
| LVEF (per 1%) | 0.993 (0.977–1.010) | 0.445 |
| AF | 1.877 (1.367–2.578) | <0.001 |
| TR ≥ moderate/severe | 0.840 (0.563–1.254) | 0.394 |
| sPAP ≥ 40 mmHg | 1.295 (0.915–1.831) | 0.145 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schörghofer, N.; Knapitsch, C.; Hecke, G.; Clodi, N.; Brandstetter, L.; Hammerer, M.; Hergan, K.; Hoppe, U.C.; Boxhammer, E.; Scharinger, B. The Prognostic Value of Tricuspid Annular Dimensions in TAVI Patients: A CT-Based Retrospective Analysis of Risk Stratification and Long-Term Outcomes. J. Clin. Med. 2025, 14, 3191. https://doi.org/10.3390/jcm14093191
Schörghofer N, Knapitsch C, Hecke G, Clodi N, Brandstetter L, Hammerer M, Hergan K, Hoppe UC, Boxhammer E, Scharinger B. The Prognostic Value of Tricuspid Annular Dimensions in TAVI Patients: A CT-Based Retrospective Analysis of Risk Stratification and Long-Term Outcomes. Journal of Clinical Medicine. 2025; 14(9):3191. https://doi.org/10.3390/jcm14093191
Chicago/Turabian StyleSchörghofer, Nikolaos, Christoph Knapitsch, Gretha Hecke, Nikolaus Clodi, Lucas Brandstetter, Matthias Hammerer, Klaus Hergan, Uta C. Hoppe, Elke Boxhammer, and Bernhard Scharinger. 2025. "The Prognostic Value of Tricuspid Annular Dimensions in TAVI Patients: A CT-Based Retrospective Analysis of Risk Stratification and Long-Term Outcomes" Journal of Clinical Medicine 14, no. 9: 3191. https://doi.org/10.3390/jcm14093191
APA StyleSchörghofer, N., Knapitsch, C., Hecke, G., Clodi, N., Brandstetter, L., Hammerer, M., Hergan, K., Hoppe, U. C., Boxhammer, E., & Scharinger, B. (2025). The Prognostic Value of Tricuspid Annular Dimensions in TAVI Patients: A CT-Based Retrospective Analysis of Risk Stratification and Long-Term Outcomes. Journal of Clinical Medicine, 14(9), 3191. https://doi.org/10.3390/jcm14093191






