Trends in Shoulder Arthroplasty: A Narrative Review of Predominant Indications and the Most Commonly Employed Implant Designs
Abstract
1. Introduction
2. Materials and Methods
3. Discussion—Main Clinical Indications
3.1. Degenerative Changes in the Glenohumeral Joint—Primary Osteoarthritis
3.2. Proximal Humerus Fractures
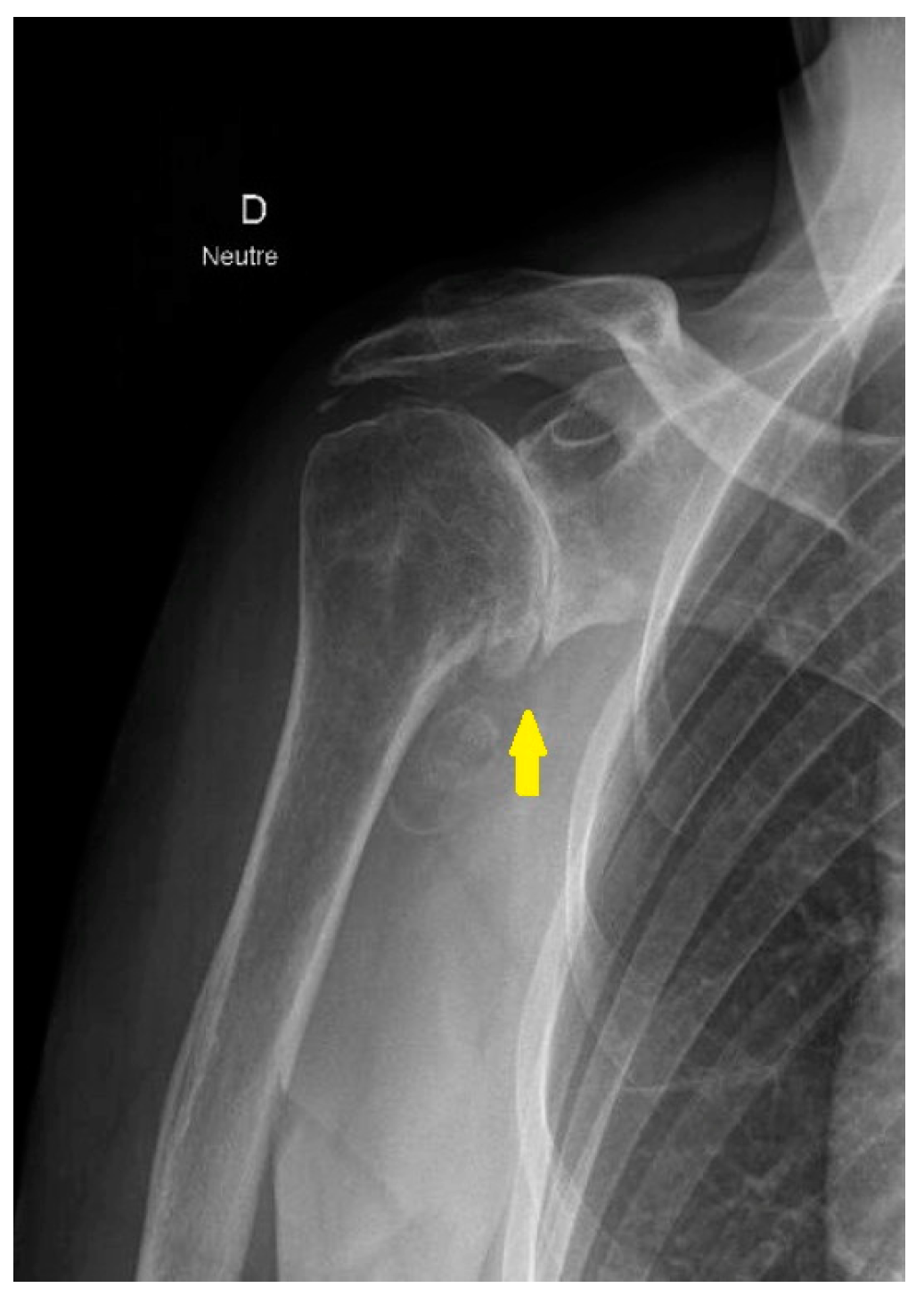
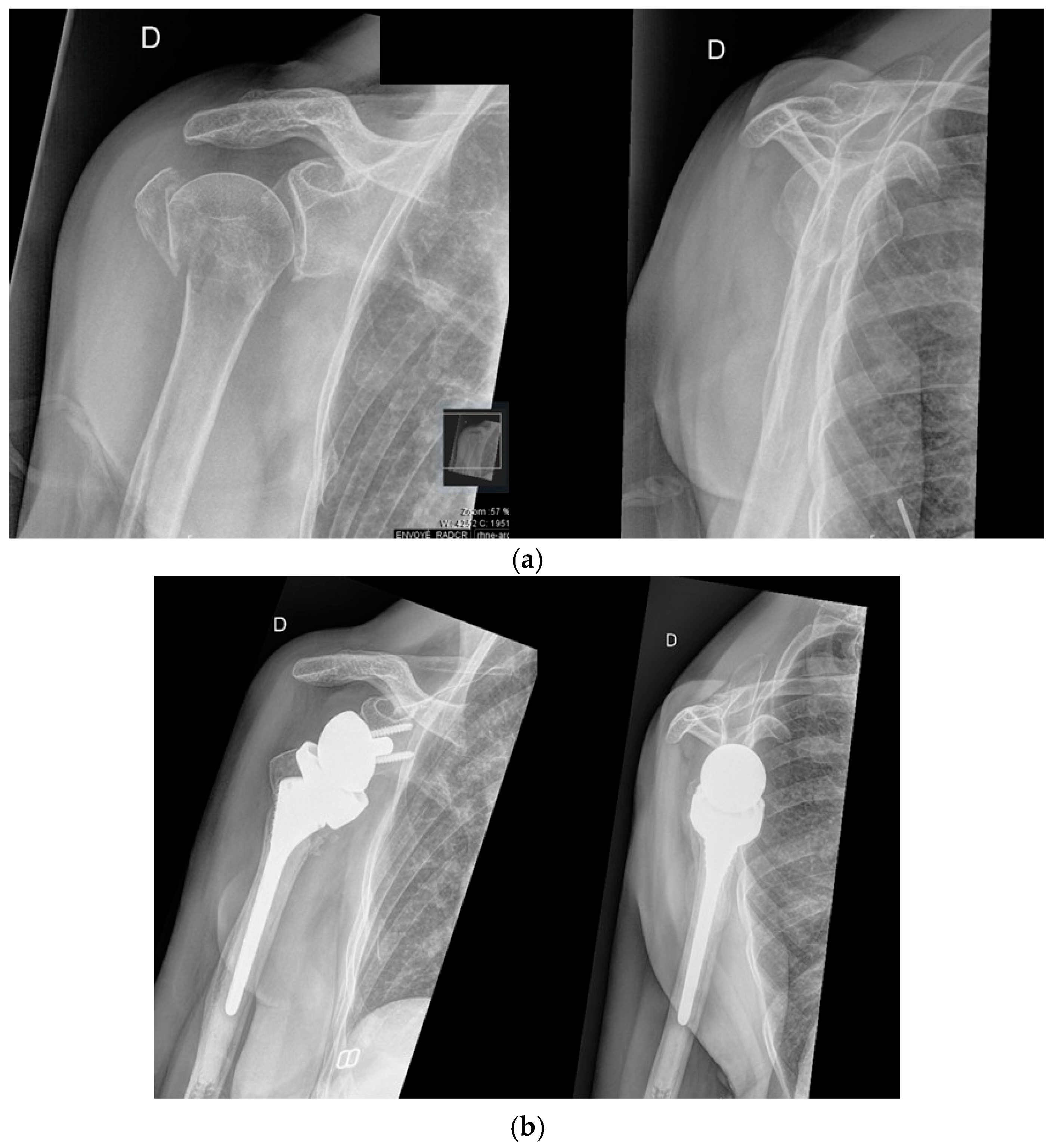
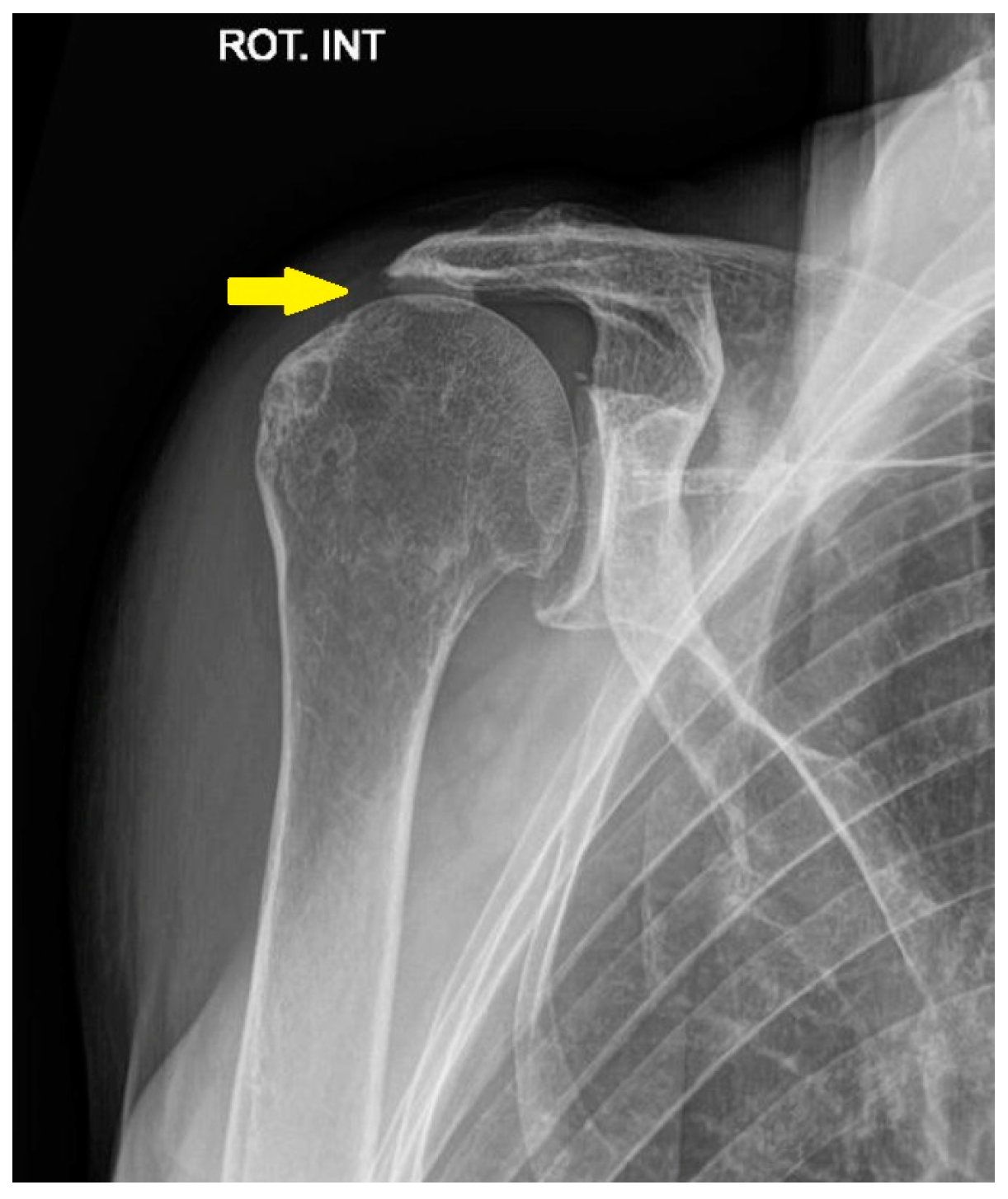
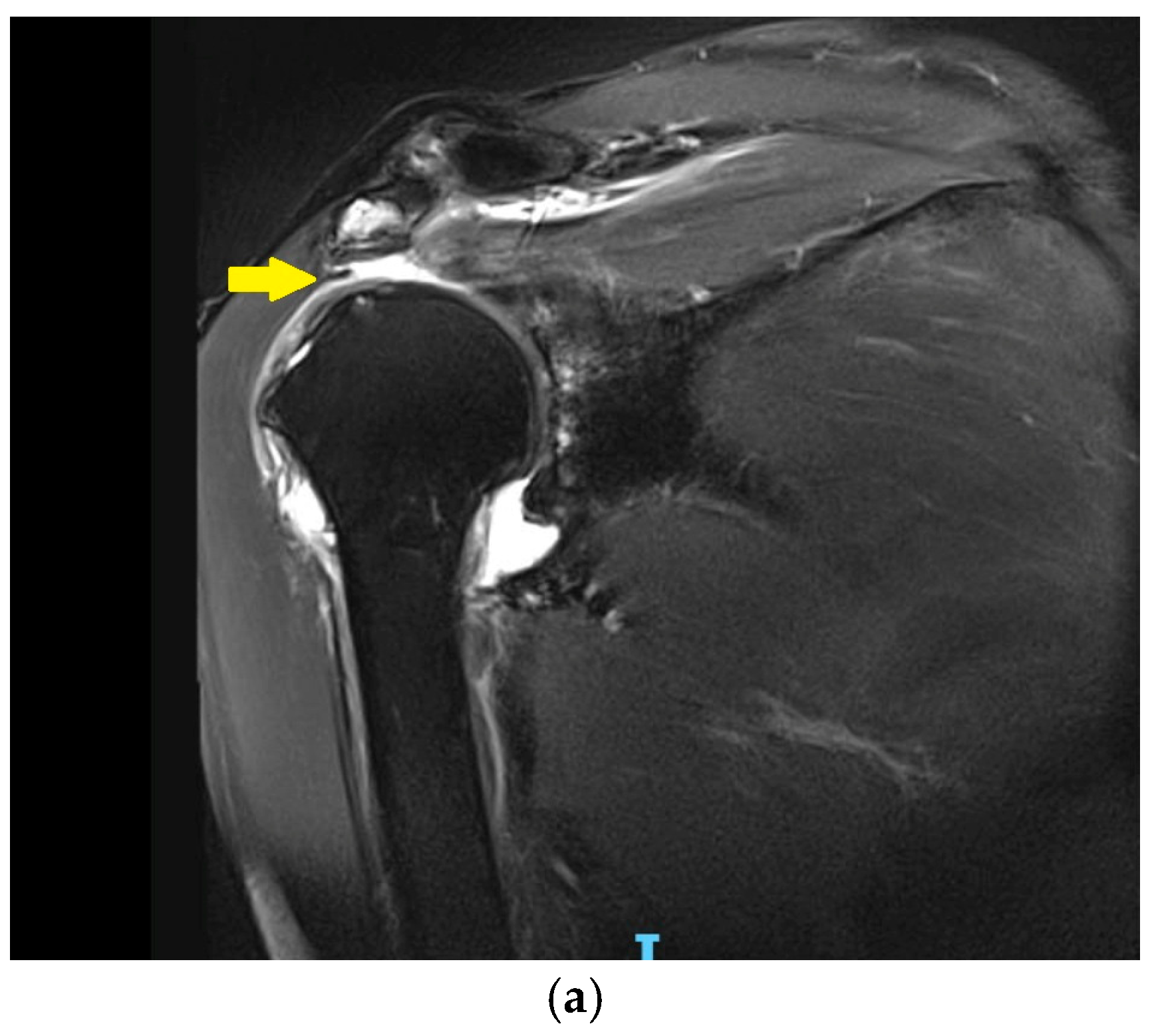
3.3. Irreparable Rotator Cuff Tears and Rotator Cuff Arthropathy
4. Discussion—Commonly Used Shoulder Arthroplasty Designs
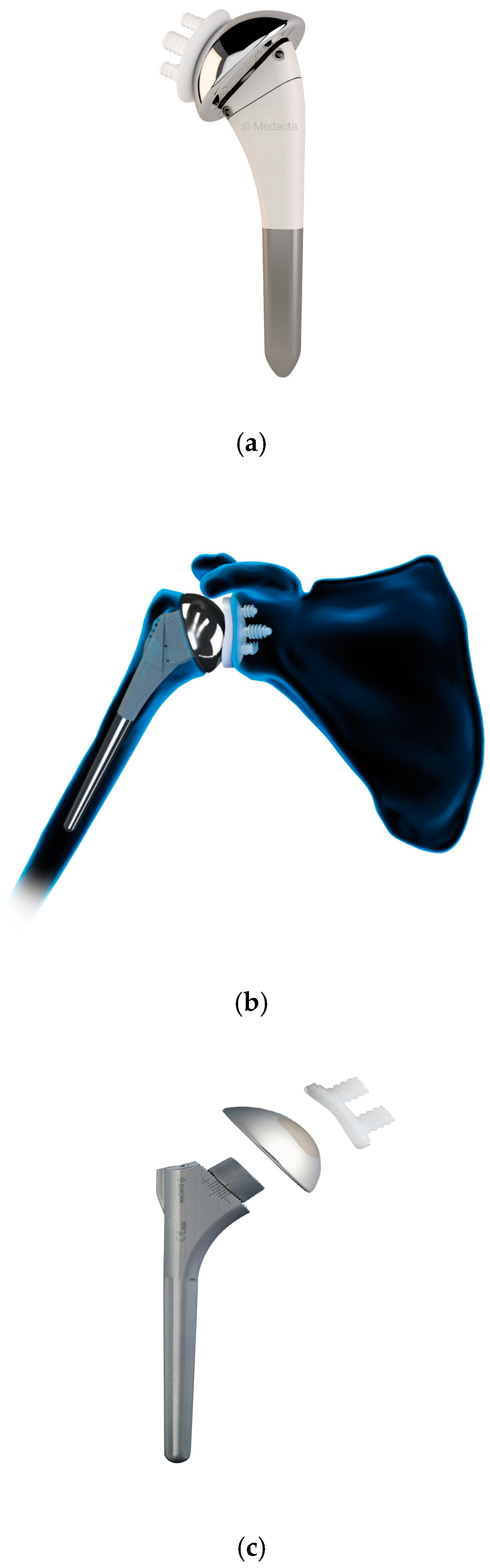
4.1. Anatomic Total Shoulder Arthroplasty
4.1.1. Patient Factors Influencing Outcomes of Anatomic Shoulder Arthroplasty
4.1.2. Bone and Implant Characteristics Influencing Outcomes in Anatomic Shoulder Arthroplasty
4.1.3. Outcomes of Total Anatomic Shoulder Arthroplasty Across Age Groups
4.1.4. Emerging Innovations and Future Directions in ATSA
4.2. Reverse Total Shoulder Arthroplasty
4.2.1. Range of Motion and Functional Results After RTSA
4.2.2. Revision Rate and Long-Term Survivorship of RTSA
4.2.3. Trends in the Use of Reverse Versus Anatomic Total Shoulder Arthroplasty: Clinical Outcomes and Cost Considerations
4.2.4. Emerging Innovations and Future Directions in RTSA
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NSAID | Non-Steroidal Anti-Inflammatory Drugs |
| ATSA | Anatomic Total Shoulder Arthroplasty |
| RTSA | Reverse Total Shoulder Arthroplasty |
| CT | Computer Tomography |
| ORIF | Open Reduction Internal Fixation |
| LPF | Locking Plate Fixation |
| ASES | American Shoulder And Elbow Surgeons |
| ROM | Range Of Motion |
| RCR | Rotator Cuff Repair |
| VAS | Visual Analog Scale |
| FF | Forward Flexion |
| ER | External Rotation |
References
- Grant, M.J.; Booth, A. A Typology of Reviews: An Analysis of 14 Review Types and Associated Methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Ibounig, T.; Simons, T.; Launonen, A.; Paavola, M. Glenohumeral Osteoarthritis: An Overview of Etiology and Diagnostics. Scand. J. Surg. 2021, 110, 441–451. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic Signaling Pathways and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Jensen, A.R.; Tangtiphaiboontana, J.; Marigi, E.; Mallett, K.E.; Sperling, J.W.; Sanchez-Sotelo, J. Anatomic Total Shoulder Arthroplasty for Primary Glenohumeral Osteoarthritis Is Associated with Excellent Outcomes and Low Revision Rates in the Elderly. J. Shoulder Elb. Surg. 2021, 30, S131–S139. [Google Scholar] [CrossRef]
- Roberson, T.A.; Bentley, J.C.; Griscom, J.T.; Kissenberth, M.J.; Tolan, S.J.; Hawkins, R.J.; Tokish, J.M. Outcomes of Total Shoulder Arthroplasty in Patients Younger than 65 Years: A Systematic Review. J. Shoulder Elb. Surg. 2017, 26, 1298–1306. [Google Scholar] [CrossRef]
- Neyton, L.; Kirsch, J.M.; Collotte, P.; Collin, P.; Gossing, L.; Chelli, M.; Walch, G. Mid- to Long-Term Follow-up of Shoulder Arthroplasty for Primary Glenohumeral Osteoarthritis in Patients Aged 60 or Under. J. Shoulder Elb. Surg. 2019, 28, 1666–1673. [Google Scholar] [CrossRef]
- Daher, M.; Boufadel, P.; Fares, M.Y.; Lopez, R.; Goltz, D.E.; Khan, A.Z.; Abboud, J.A. Reverse versus Anatomic Total Shoulder Arthroplasty for Glenohumeral Osteoarthritis with Intact Cuff: A Meta-Analysis of Clinical Outcomes. J. Shoulder Elb. Surg. 2025, 34, 190–202. [Google Scholar] [CrossRef]
- Park, C.N.; Zhang, G.X.; Chang, J.; Zeng, S.L.; Meyer, L.E.; Hurley, E.T.; Hatzidakis, A.M.; Anakwenze, O.; Klifto, C.S. Pyrocarbon Hemiarthroplasty of the Shoulder: A Systematic Review and Meta-Analysis of Clinical Results. J. Shoulder Elb. Surg. 2023, 32, 1323–1332. [Google Scholar] [CrossRef]
- McBride, A.P.; Ross, M.; Hoy, G.; Duke, P.; Page, R.; Peng, Y.; Taylor, F. Mid-Term Outcomes of Pyrolytic Carbon Humeral Resurfacing Hemiarthroplasty Compared with Metal Humeral Resurfacing and Metal Stemmed Hemiarthroplasty for Osteoarthritis in Young Patients: Analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J. Shoulder Elb. Surg. 2022, 31, 755–762. [Google Scholar] [CrossRef]
- Court-Brown, C.M.; Caesar, B. Epidemiology of Adult Fractures: A Review. Injury 2006, 37, 691–697. [Google Scholar] [CrossRef]
- Bell, J.-E.; Leung, B.C.; Spratt, K.F.; Koval, K.J.; Weinstein, J.D.; Goodman, D.C.; Tosteson, A.N. Trends and Variation in Incidence, Surgical Treatment, and Repeat Surgery of Proximal Humeral Fractures in the Elderly. J. Bone Jt. Surg.-Am. Vol. 2011, 93, 121–131. [Google Scholar] [CrossRef]
- Handoll, H.H.; Elliott, J.; Thillemann, T.M.; Aluko, P.; Brorson, S. Interventions for Treating Proximal Humeral Fractures in Adults. Cochrane Database Syst. Rev. 2022, 6, CD000434. [Google Scholar] [CrossRef]
- Spross, C.; Meester, J.; Mazzucchelli, R.A.; Puskás, G.J.; Zdravkovic, V.; Jost, B. Evidence-Based Algorithm to Treat Patients with Proximal Humerus Fractures—A Prospective Study with Early Clinical and Overall Performance Results. J. Shoulder Elb. Surg. 2019, 28, 1022–1032. [Google Scholar] [CrossRef]
- Hodgson, S.A.; Mawson, S.J.; Saxton, J.M.; Stanley, D. Rehabilitation of Two-Part Fractures of the Neck of the Humerus (Two-Year Follow-Up). J. Shoulder Elb. Surg. 2007, 16, 143–145. [Google Scholar] [CrossRef]
- Rangan, A.; Handoll, H.; Brealey, S.; Jefferson, L.; Keding, A.; Martin, B.C.; Goodchild, L.; Chuang, L.-H.; Hewitt, C.; Torgerson, D. Surgical vs Nonsurgical Treatment of Adults with Displaced Fractures of the Proximal Humerus: The PROFHER Randomized Clinical Trial. JAMA 2015, 313, 1037. [Google Scholar] [CrossRef]
- Corbacho, B.; Duarte, A.; Keding, A.; Handoll, H.; Chuang, L.H.; Torgerson, D.; Brealey, S.; Jefferson, L.; Hewitt, C.; Rangan, A. Cost Effectiveness of Surgical versus Non-Surgical Treatment of Adults with Displaced Fractures of the Proximal Humerus: Economic Evaluation alongside the PROFHER Trial. Bone Jt. J. 2016, 98-B, 152–159. [Google Scholar] [CrossRef]
- Handoll, H.; Brealey, S.; Rangan, A.; Keding, A.; Corbacho, B.; Jefferson, L.; Chuang, L.-H.; Goodchild, L.; Hewitt, C.; Torgerson, D. The ProFHER (PROximal Fracture of the Humerus: Evaluation by Randomisation) Trial—A Pragmatic Multicentre Randomised Controlled Trial Evaluating the Clinical Effectiveness and Cost-Effectiveness of Surgical Compared with Non-Surgical Treatment for Proximal Fracture of the Humerus in Adults. Health Technol. Assess. Winch. Engl. 2015, 19, 1–280. [Google Scholar] [CrossRef]
- Goudie, E.B.; Robinson, C.M. Prediction of Nonunion After Nonoperative Treatment of a Proximal Humeral Fracture. J. Bone Jt. Surg. Am. 2021, 103, 668–680. [Google Scholar] [CrossRef]
- Gallinet, D.; Ohl, X.; Decroocq, L.; Dib, C.; Valenti, P.; Boileau, P. Is Reverse Total Shoulder Arthroplasty More Effective than Hemiarthroplasty for Treating Displaced Proximal Humerus Fractures in Older Adults? A Systematic Review and Meta-Analysis. Orthop. Traumatol. Surg. Res. 2018, 104, 759–766. [Google Scholar] [CrossRef]
- Szerlip, B.W.; Morris, B.J.; Edwards, T.B. Reverse Shoulder Arthroplasty for Trauma: When, Where, and How. Instr. Course Lect. 2016, 65, 171–179. [Google Scholar]
- Suroto, H.; De Vega, B.; Deapsari, F.; Prajasari, T.; Wibowo, P.A.; Samijo, S.K. Reverse Total Shoulder Arthroplasty (RTSA) versus Open Reduction and Internal Fixation (ORIF) for Displaced Three-Part or Four-Part Proximal Humeral Fractures: A Systematic Review and Meta-Analysis. EFORT Open Rev. 2021, 6, 941–955. [Google Scholar] [CrossRef]
- Fraser, A.N.; Bjørdal, J.; Wagle, T.M.; Karlberg, A.C.; Lien, O.A.; Eilertsen, L.; Mader, K.; Apold, H.; Larsen, L.B.; Madsen, J.E.; et al. Reverse Shoulder Arthroplasty Is Superior to Plate Fixation at 2 Years for Displaced Proximal Humeral Fractures in the Elderly: A Multicenter Randomized Controlled Trial. J. Bone Jt. Surg. Am. 2020, 102, 477–485. [Google Scholar] [CrossRef]
- Chen, L.; Xing, F.; Xiang, Z. Effectiveness and Safety of Interventions for Treating Adults with Displaced Proximal Humeral Fracture: A Network Meta-Analysis and Systematic Review. PLoS ONE 2016, 11, e0166801. [Google Scholar] [CrossRef]
- Orman, S.; Mohamadi, A.; Serino, J.; Murphy, J.; Hanna, P.; Weaver, M.J.; Dyer, G.; Nazarian, A.; von Keudell, A. Comparison of Surgical and Non-Surgical Treatments for 3- and 4-Part Proximal Humerus Fractures: A Network Meta-Analysis. Shoulder Elb. 2020, 12, 99–108. [Google Scholar] [CrossRef]
- Davey, M.S.; Hurley, E.T.; Anil, U.; Condren, S.; Kearney, J.; O’Tuile, C.; Gaafar, M.; Mullett, H.; Pauzenberger, L. Management Options for Proximal Humerus Fractures—A Systematic Review & Network Meta-Analysis of Randomized Control Trials. Injury 2022, 53, 244–249. [Google Scholar] [CrossRef]
- Cole, P.A. A Word of Caution: Success May Be Limited to 2 Years and Highly Displaced OTA/AO B2 and C2 Injuries: Commentary on an Article by Alexander Nilsskog Fraser, MD; et al.: “Reverse Total Shoulder Arthroplasty Is Superior to Plate Fixation at 2 Years for Displaced Proximal Humeral Fractures in the Elderly. A Multicenter Randomized Controlled Trial”. J. Bone Jt. Surg. Am. 2020, 102, e30. [Google Scholar] [CrossRef]
- Alrabaa, R.G.; Ma, G.; Truong, N.M.; Lansdown, D.A.; Feeley, B.T.; Zhang, A.L.; Ma, C.B. Trends in Surgical Treatment of Proximal Humeral Fractures and Analysis of Postoperative Complications over a Decade in 384,158 Patients. JBJS Open Access 2022, 7, e22.00008. [Google Scholar] [CrossRef]
- Cooke, H.L.; Gabig, A.M.; Karzon, A.L.; Hussain, Z.B.; Ojemakinde, A.A.; Wagner, E.R.; Gottschalk, M.B. The Surgical Treatment of Proximal Humerus Fractures 2010-2019: United States National Case Volume and Incidence Trends. JSES Rev. Rep. Tech. 2024, 4, 146–152. [Google Scholar] [CrossRef]
- Seidel, H.D.; Bhattacharjee, S.; Koh, J.L.; Strelzow, J.A.; Shi, L.L. Acute Versus Delayed Reverse Shoulder Arthroplasty for the Primary Treatment of Proximal Humeral Fractures. J. Am. Acad. Orthop. Surg. 2021, 29, 832–839. [Google Scholar] [CrossRef]
- Lädermann, A.; Denard, P.J.; Collin, P. Massive Rotator Cuff Tears: Definition and Treatment. Int. Orthop. 2015, 39, 2403–2414. [Google Scholar] [CrossRef]
- Meyer, D.C.; Wieser, K.; Farshad, M.; Gerber, C. Retraction of Supraspinatus Muscle and Tendon as Predictors of Success of Rotator Cuff Repair. Am. J. Sports Med. 2012, 40, 2242–2247. [Google Scholar] [CrossRef]
- Kovacevic, D.; Suriani, R.J.; Grawe, B.M.; Yian, E.H.; Gilotra, M.N.; Hasan, S.A.; Srikumaran, U.; Hasan, S.S.; Cuomo, F.; Burks, R.T.; et al. Management of Irreparable Massive Rotator Cuff Tears: A Systematic Review and Meta-Analysis of Patient-Reported Outcomes, Reoperation Rates, and Treatment Response. J. Shoulder Elb. Surg. 2020, 29, 2459–2475. [Google Scholar] [CrossRef]
- Zingg, P.O.; Jost, B.; Sukthankar, A.; Buhler, M.; Pfirrmann, C.W.A.; Gerber, C. Clinical and Structural Outcomes of Nonoperative Management of Massive Rotator Cuff Tears. J. Bone Jt. Surg. Am. 2007, 89, 1928–1934. [Google Scholar] [CrossRef]
- Boin, M.A.; Ben-Ari, E.; Roche, C.P.; Zuckerman, J.D. Reverse Shoulder Arthroplasty for Massive Irreparable Rotator Cuff Tears: A Reliable Treatment Method. Semin. Arthroplast. JSES 2021, 31, 822–830. [Google Scholar] [CrossRef]
- Welch, J.M.; Hurley, E.T.; Lorentz, S.; Bethell, M.A.; Crook, B.S.; Dickens, J.F.; Anakwenze, O.; Klifto, C.S. Reverse Shoulder Arthroplasty Following Failed Rotator Cuff Repair: A Systematic Review and Meta-Analysis. Shoulder Elb. 2024, 16, 474–480. [Google Scholar] [CrossRef]
- Tantone, R.P.; Al-Humadi, S.; VanHelmond, T.; Kim, M.; Komatsu, D.E.; Wang, E.D. Outcomes of Reverse Shoulder Arthroplasty in Patients with Previous Rotator Cuff Repair: A Systematic Review and Meta-Analysis. JSES Rev. Rep. Tech. 2023, 3, 267–273. [Google Scholar] [CrossRef]
- Boileau, P.; Walch, G. Prothèses d’épaule: État Actuel; Sauramps Médical: Montpellier, France, 2008. [Google Scholar]
- Australian Orthopaedic Association; National Joint Replacement Registry. Hip, Knee & Shoulder Arthroplasty: 2023 Annual Report; Australian Orthopaedic Association: Adelaide, Australia, 2023. [Google Scholar]
- Reahl, G.B.; Abdul-Rassoul, H.; Kim, R.L.; Ardavanis, K.S.; Novikov, D.; Curry, E.J.; Galvin, J.W.; Eichinger, J.K.; Li, X. Anatomic vs. Reverse Shoulder Arthroplasty for the Treatment of Walch B2 Glenoid Morphology: A Systematic Review and Meta-Analysis. JSES Rev. Rep. Tech. 2021, 1, 317–328. [Google Scholar] [CrossRef]
- Italia, K.; Jomaa, M.; Pareyon, R.; Hollman, F.; Cutbush, K.; Gupta, A. Outcomes and Survivorship of Anatomic Total Shoulder Arthroplasty: Current Concepts. J. ISAKOS 2023, 8, 284–288. [Google Scholar] [CrossRef]
- Goetti, P.; Denard, P.J.; Collin, P.; Ibrahim, M.; Mazzolari, A.; Lädermann, A. Biomechanics of Anatomic and Reverse Shoulder Arthroplasty. EFORT Open Rev. 2021, 6, 918–931. [Google Scholar] [CrossRef]
- Schrumpf, M.; Maak, T.; Hammoud, S.; Craig, E.V. The Glenoid in Total Shoulder Arthroplasty. Curr. Rev. Musculoskelet. Med. 2011, 4, 191–199. [Google Scholar] [CrossRef]
- Gates, S.; Sager, B.; Khazzam, M. Preoperative Glenoid Considerations for Shoulder Arthroplasty: A Review. EFORT Open Rev. 2020, 5, 126–137. [Google Scholar] [CrossRef]
- Strauss, E.J.; Roche, C.; Flurin, P.-H.; Wright, T.; Zuckerman, J.D. The Glenoid in Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2009, 18, 819–833. [Google Scholar] [CrossRef]
- Boileau, P.; Moineau, G.; Morin-Salvo, N.; Avidor, C.; Godenèche, A.; Lévigne, C.; Baba, M.; Walch, G. Metal-Backed Glenoid Implant with Polyethylene Insert Is Not a Viable Long-Term Therapeutic Option. J. Shoulder Elb. Surg. 2015, 24, 1534–1543. [Google Scholar] [CrossRef]
- Sanchez-Sotelo, J. Current Concepts in Humeral Component Design for Anatomic and Reverse Shoulder Arthroplasty. J. Clin. Med. 2021, 10, 5151. [Google Scholar] [CrossRef]
- Flurin, P.-H.; Roche, C.P.; Wright, T.W.; Zuckerman, J.D. Correlation Between Clinical Outcomes and Anatomic Reconstruction with Anatomic Total Shoulder Arthroplasty. Bull. Hosp. Jt. Dis. 2013 2015, 73 (Suppl. 1), S92–S98. [Google Scholar]
- Barry, L.W.; Katayama, E.S.; Barnett, J.S.; Henderson, B.L.; Patel, A.V.; Cvetanovich, G.L.; Bishop, J.Y.; Rauck, R.C. Functionality, Complications, and Survivorship of Total Shoulder Arthroplasty in Patients under 60 Years Old. J. Orthop. 2024, 55, 59–63. [Google Scholar] [CrossRef]
- Hawi, N.; Magosch, P.; Tauber, M.; Lichtenberg, S.; Habermeyer, P. Nine-Year Outcome after Anatomic Stemless Shoulder Prosthesis: Clinical and Radiologic Results. J. Shoulder Elb. Surg. 2017, 26, 1609–1615. [Google Scholar] [CrossRef]
- Wiater, J.M.; Levy, J.C.; Wright, S.A.; Brockmeier, S.F.; Duquin, T.R.; Wright, J.O.; Codd, T.P. Prospective, Blinded, Randomized Controlled Trial of Stemless Versus Stemmed Humeral Components in Anatomic Total Shoulder Arthroplasty: Results at Short-Term Follow-Up. J. Bone Jt. Surg. Am. 2020, 102, 1974–1984. [Google Scholar] [CrossRef]
- Churchill, R.S. Stemless Shoulder Arthroplasty: Current Status. J. Shoulder Elb. Surg. 2014, 23, 1409–1414. [Google Scholar] [CrossRef]
- Schönweger, F.; Oldrini, L.M.; Feltri, P.; Filardo, G.; Candrian, C. Stemmed VS Stemless Total Shoulder Arthroplasty: A Systematic Review and Meta-Analysis. Arch. Orthop. Trauma Surg. 2024, 145, 3. [Google Scholar] [CrossRef]
- Kadum, B.; Hassany, H.; Wadsten, M.; Sayed-Noor, A.; Sjödén, G. Geometrical Analysis of Stemless Shoulder Arthroplasty: A Radiological Study of Seventy TESS Total Shoulder Prostheses. Int. Orthop. 2016, 40, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Athwal, G.S. Spare the Canal: Stemless Shoulder Arthroplasty Is Finally Here: Commentary on an Article by R. Sean Churchill, MD; et al.: “Clinical and Radiographic Outcomes of the Simpliciti Canal-Sparing Shoulder Arthroplasty System. A Prospective Two-Year Multicenter Study”. JBJS 2016, 98, e28. [Google Scholar] [CrossRef]
- Brabston, E.W.; Fehringer, E.V.; Owen, M.T.; Ponce, B.A. Stemless Humeral Implants in Total Shoulder Arthroplasty. JAAOS—J. Am. Acad. Orthop. Surg. 2020, 28, e277. [Google Scholar] [CrossRef]
- Krukenberg, A.; McBirnie, J.; Bartsch, S.; Böhler, N.; Wiedemann, E.; Jost, B.; Mansat, P.; Bellon-Champel, P.; Angeloni, R.; Scheibel, M. Sidus Stem-Free Shoulder System for Primary Osteoarthritis: Short-Term Results of a Multicenter Study. J. Shoulder Elb. Surg. 2018, 27, 1483–1490. [Google Scholar] [CrossRef]
- Habermeyer, P.; Lichtenberg, S.; Tauber, M.; Magosch, P. Midterm Results of Stemless Shoulder Arthroplasty: A Prospective Study. J. Shoulder Elb. Surg. 2015, 24, 1463–1472. [Google Scholar] [CrossRef]
- Beck, S.; Beck, V.; Wegner, A.; Dudda, M.; Patsalis, T.; Jäger, M. Long-Term Survivorship of Stemless Anatomical Shoulder Replacement. Int. Orthop. 2018, 42, 1327–1330. [Google Scholar] [CrossRef]
- Aibinder, W.R.; Bartels, D.W.; Sperling, J.W.; Sanchez-Sotelo, J. Mid-Term Radiological Results of a Cementless Short Humeral Component in Anatomical and Reverse Shoulder Arthroplasty. Bone Jt. J. 2019, 101-B, 610–614. [Google Scholar] [CrossRef]
- Schnetzke, M.; Preis, A.; Coda, S.; Raiss, P.; Loew, M. Anatomical and Reverse Shoulder Replacement with a Convertible, Uncemented Short-Stem Shoulder Prosthesis: First Clinical and Radiological Results. Arch. Orthop. Trauma Surg. 2017, 137, 679–684. [Google Scholar] [CrossRef]
- Romeo, A.A.; Thorsness, R.J.; Sumner, S.A.; Gobezie, R.; Lederman, E.S.; Denard, P.J. Short-Term Clinical Outcome of an Anatomic Short-Stem Humeral Component in Total Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2018, 27, 70–74. [Google Scholar] [CrossRef]
- Klawitter, J.J.; Patton, J.; More, R.; Peter, N.; Podnos, E.; Ross, M. In Vitro Comparison of Wear Characteristics of PyroCarbon and Metal on Bone: Shoulder Hemiarthroplasty. Shoulder Elb. 2020, 12, 11–22. [Google Scholar] [CrossRef]
- Rasmussen, J.V.; Olsen, B.S.; Al-Hamdani, A.; Brorson, S. Outcome of Revision Shoulder Arthroplasty After Resurfacing Hemiarthroplasty in Patients with Glenohumeral Osteoarthritis. JBJS 2016, 98, 1631. [Google Scholar] [CrossRef] [PubMed]
- Priddy, M.; Zarezadeh, A.; Farmer, K.W.; Struk, A.M.; King, J.J.; Wright, T.W.; Schoch, B.S. Early Results of Augmented Anatomic Glenoid Components. J. Shoulder Elb. Surg. 2019, 28, S138–S145. [Google Scholar] [CrossRef] [PubMed]
- Cvetanovich, G.L.; Naylor, A.J.; O’Brien, M.C.; Waterman, B.R.; Garcia, G.H.; Nicholson, G.P. Anatomic Total Shoulder Arthroplasty with an Inlay Glenoid Component: Clinical Outcomes and Return to Activity. J. Shoulder Elb. Surg. 2020, 29, 1188–1196. [Google Scholar] [CrossRef]
- Crosby, L.A.; Wright, T.W.; Yu, S.; Zuckerman, J.D. Conversion to Reverse Total Shoulder Arthroplasty with and without Humeral Stem Retention: The Role of a Convertible-Platform Stem. JBJS 2017, 99, 736. [Google Scholar] [CrossRef]
- Dilisio, M.F.; Miller, L.R.; Siegel, E.J.; Higgins, L.D. Conversion to Reverse Shoulder Arthroplasty: Humeral Stem Retention Versus Revision. Orthopedics 2015, 38, e773–e779. [Google Scholar] [CrossRef]
- Wieser, K.; Borbas, P.; Ek, E.T.; Meyer, D.C.; Gerber, C. Conversion of Stemmed Hemi- or Total to Reverse Total Shoulder Arthroplasty: Advantages of a Modular Stem Design. Clin. Orthop. Relat. Res. 2015, 473, 651. [Google Scholar] [CrossRef]
- Magosch, P.; Lichtenberg, S.; Tauber, M.; Martetschläger, F.; Habermeyer, P. Prospective Midterm Results of a New Convertible Glenoid Component in Anatomic Shoulder Arthroplasty: A Cohort Study. Arch. Orthop. Trauma Surg. 2021, 141, 717–724. [Google Scholar] [CrossRef]
- Merolla, G.; Chin, P.; Sasyniuk, T.M.; Paladini, P.; Porcellini, G. Total Shoulder Arthroplasty with a Second-Generation Tantalum Trabecular Metal-Backed Glenoid Component: Clinical and Radiographic Outcomes at a Mean Follow-up of 38 Months. Bone Jt. J. 2016, 98-B, 75–80. [Google Scholar] [CrossRef]
- Watson, S.T.; Gudger, G.K.; Long, C.D.; Tokish, J.M.; Tolan, S.J. Outcomes of Trabecular Metal–Backed Glenoid Components in Anatomic Total Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2018, 27, 493–498. [Google Scholar] [CrossRef]
- Valenti, P.; Katz, D.; Kany, J.; Werthel, J.-D. Convertible Glenoid Components Facilitate Revisions to Reverse Shoulder Arthroplasty Easier: Retrospective Review of 13 Cases. Am. J. Orthop. Belle Mead N. J. 2018, 47. [Google Scholar] [CrossRef]
- Favard, L.; Levigne, C.; Nerot, C.; Gerber, C.; De Wilde, L.; Mole, D. Reverse Prostheses in Arthropathies with Cuff Tear: Are Survivorship and Function Maintained over Time? Clin. Orthop. 2011, 469, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Ernstbrunner, L.; Andronic, O.; Grubhofer, F.; Camenzind, R.S.; Wieser, K.; Gerber, C. Long-Term Results of Reverse Total Shoulder Arthroplasty for Rotator Cuff Dysfunction: A Systematic Review of Longitudinal Outcomes. J. Shoulder Elb. Surg. 2019, 28, 774–781. [Google Scholar] [CrossRef]
- Grubhofer, F.; Wieser, K.; Meyer, D.C.; Catanzaro, S.; Beeler, S.; Riede, U.; Gerber, C. Reverse Total Shoulder Arthroplasty for Acute Head-Splitting, 3- and 4-Part Fractures of the Proximal Humerus in the Elderly. J. Shoulder Elb. Surg. 2016, 25, 1690–1698. [Google Scholar] [CrossRef]
- Grubhofer, F.; Wieser, K.; Meyer, D.C.; Catanzaro, S.; Schürholz, K.; Gerber, C. Reverse Total Shoulder Arthroplasty for Failed Open Reduction and Internal Fixation of Fractures of the Proximal Humerus. J. Shoulder Elb. Surg. 2017, 26, 92–100. [Google Scholar] [CrossRef]
- Australian Orthopaedic Association; National Joint Replacement Registry. Hip, Knee and Shoulder Arthroplasty: 2024 Annual Report; Australian Orthopaedic Association: Adelaide, Australia, 2024. [Google Scholar]
- Roche, C.P. Reverse Shoulder Arthroplasty Biomechanics. J. Funct. Morphol. Kinesiol. 2022, 7, 13. [Google Scholar] [CrossRef]
- Boileau, P.; Watkinson, D.J.; Hatzidakis, A.M.; Balg, F. Grammont Reverse Prosthesis: Design, Rationale, and Biomechanics. J. Shoulder Elb. Surg. 2005, 14, 147S–161S. [Google Scholar] [CrossRef]
- Bois, A.J.; Knight, P.; Alhojailan, K.; Bohsali, K.I. Clinical Outcomes and Complications of Reverse Shoulder Arthroplasty Used for Failed Prior Shoulder Surgery: A Systematic Review and Meta-Analysis. JSES Int. 2020, 4, 156–168. [Google Scholar] [CrossRef]
- Nunes, J.; Andrade, R.; Azevedo, C.; Ferreira, N.V.; Oliveira, N.; Calvo, E.; Espregueira-Mendes, J.; Sevivas, N. Improved Clinical Outcomes After Lateralized Reverse Shoulder Arthroplasty: A Systematic Review. Clin. Orthop. 2022, 480, 949–957. [Google Scholar] [CrossRef]
- Oak, S.R.; Kobayashi, E.; Gagnier, J.; Denard, P.J.; Sears, B.W.; Gobezie, R.; Lederman, E.; Werner, B.C.; Bedi, A.; Miller, B.S. Patient Reported Outcomes and Ranges of Motion after Reverse Total Shoulder Arthroplasty with and without Subscapularis Repair. JSES Int. 2022, 6, 923–928. [Google Scholar] [CrossRef]
- Bacle, G.; Nové-Josserand, L.; Garaud, P.; Walch, G. Long-Term Outcomes of Reverse Total Shoulder Arthroplasty: A Follow-up of a Previous Study. J. Bone Jt. Surg. Am. 2017, 99, 454–461. [Google Scholar] [CrossRef]
- Chelli, M.; Boileau, P.; Domos, P.; Clavert, P.; Berhouet, J.; Collin, P.; Walch, G.; Favard, L. Survivorship of Reverse Shoulder Arthroplasty According to Indication, Age and Gender. J. Clin. Med. 2022, 11, 2677. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, B.T.; Samuelsen, B.T.; Spratt, J.D.; Dornan, G.J.; Millett, P.J. Complications and Implant Survivorship Following Primary Reverse Total Shoulder Arthroplasty in Patients Younger than 65 Years: A Systematic Review. J. Shoulder Elb. Surg. 2020, 29, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Favard, L. Revision of Total Shoulder Arthroplasty. Orthop. Traumatol. Surg. Res. OTSR 2013, 99, S12–S21. [Google Scholar] [CrossRef] [PubMed]
- Melis, B.; DeFranco, M.; Lädermann, A.; Molé, D.; Favard, L.; Nérot, C.; Maynou, C.; Walch, G. An Evaluation of the Radiological Changes around the Grammont Reverse Geometry Shoulder Arthroplasty after Eight to 12 Years. J. Bone Jt. Surg. Br. 2011, 93, 1240–1246. [Google Scholar] [CrossRef]
- Melis, B.; Bonnevialle, N.; Neyton, L.; Lévigne, C.; Favard, L.; Walch, G.; Boileau, P. Glenoid Loosening and Failure in Anatomical Total Shoulder Arthroplasty: Is Revision with a Reverse Shoulder Arthroplasty a Reliable Option? J. Shoulder Elb. Surg. 2012, 21, 342–349. [Google Scholar] [CrossRef]
- Patel, D.N.; Young, B.; Onyekwelu, I.; Zuckerman, J.D.; Kwon, Y.W. Reverse Total Shoulder Arthroplasty for Failed Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2012, 21, 1478–1483. [Google Scholar] [CrossRef]
- Valenti, P.; Kilinc, A.S.; Sauzières, P.; Katz, D. Results of 30 Reverse Shoulder Prostheses for Revision of Failed Hemi- or Total Shoulder Arthroplasty. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 1375–1382. [Google Scholar] [CrossRef]
- Merolla, G.; Wagner, E.; Sperling, J.W.; Paladini, P.; Fabbri, E.; Porcellini, G. Revision of Failed Shoulder Hemiarthroplasty to Reverse Total Arthroplasty: Analysis of 157 Revision Implants. J. Shoulder Elb. Surg. 2018, 27, 75–81. [Google Scholar] [CrossRef]
- Bartels, D.W.; Marigi, E.; Sperling, J.W.; Sanchez-Sotelo, J. Revision Reverse Shoulder Arthroplasty for Anatomical Glenoid Component Loosening Was Not Universally Successful: A Detailed Analysis of 127 Consecutive Shoulders. J. Bone Jt. Surg. Am. 2021, 103, 879–886. [Google Scholar] [CrossRef]
- Aurich, M.; Farkhondeh Fal, M.; Albers, S.; Krane, F.; Kircher, J. Reverse Total Shoulder Arthroplasty Policy in Germany—An Analysis of the Health Care Reality from 2010 to 2022. J. Shoulder Elbow Surg. 2025, 34, 294–320. [Google Scholar] [CrossRef]
- Valsamis, E.M.; Prats-Uribe, A.; Koblbauer, I.; Cole, S.; Sayers, A.; Whitehouse, M.R.; Coward, G.; Collins, G.S.; Pinedo-Villanueva, R.; Prieto-Alhambra, D.; et al. Reverse Total Shoulder Replacement versus Anatomical Total Shoulder Replacement for Osteoarthritis: Population Based Cohort Study Using Data from the National Joint Registry and Hospital Episode Statistics for England. BMJ 2024, 385, e077939. [Google Scholar] [CrossRef] [PubMed]
- Best, M.J.; Aziz, K.T.; Wilckens, J.H.; McFarland, E.G.; Srikumaran, U. Increasing Incidence of Primary Reverse and Anatomic Total Shoulder Arthroplasty in the United States. J. Shoulder Elb. Surg. 2021, 30, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.J.; Dhodapkar, M.M.; Kim, L.; Modrak, M.; Medvecky, M.J.; Donohue, K.W.; Grauer, J.N. Anatomic vs. Reverse Total Shoulder Arthroplasty: Usage Trends and Perioperative Outcomes. Semin. Arthroplast. JSES 2024, 34, 91–96. [Google Scholar] [CrossRef]
- Lädermann, A.; Denard, P.J.; Collin, P.; Zbinden, O.; Chiu, J.C.-H.; Boileau, P.; Olivier, F.; Walch, G. Effect of Humeral Stem and Glenosphere Designs on Range of Motion and Muscle Length in Reverse Shoulder Arthroplasty. Int. Orthop. 2020, 44, 519–530. [Google Scholar] [CrossRef]
- Haidamous, G.; Lädermann, A.; Frankle, M.A.; Gorman, R.A.; Denard, P.J. The Risk of Postoperative Scapular Spine Fracture Following Reverse Shoulder Arthroplasty Is Increased with an Onlay Humeral Stem. J. Shoulder Elb. Surg. 2020, 29, 2556–2563. [Google Scholar] [CrossRef]
- Beltrame, A.; Benedetto, P.D.; Cicuto, C.; Cainero, V.; Chisoni, R.; Causero, A. Onlay versus Inlay Humeral Steam in Reverse Shoulder Arthroplasty (RSA): Clinical and Biomechanical Study. Acta Biomed. Atenei Parm. 2019, 90, 54–63. [Google Scholar] [CrossRef]
- Ascione, F.; Kilian, C.M.; Laughlin, M.S.; Bugelli, G.; Domos, P.; Neyton, L.; Godeneche, A.; Edwards, T.B.; Walch, G. Increased Scapular Spine Fractures after Reverse Shoulder Arthroplasty with a Humeral Onlay Short Stem: An Analysis of 485 Consecutive Cases. J. Shoulder Elb. Surg. 2018, 27, 2183–2190. [Google Scholar] [CrossRef]
- Upfill-Brown, A.; Satariano, N.; Feeley, B. Stemless Shoulder Arthroplasty: Review of Short and Medium-Term Results. JSES Open Access 2019, 3, 154–161. [Google Scholar] [CrossRef]
- Levy, O.; Panagopoulos, G.N.; Leonidou, A.; Atoun, E. Stemless Reverse Shoulder Arthroplasty: Indications, Technique and European Experience. Ann. Jt. 2018, 3, 108. [Google Scholar] [CrossRef]
- Liu, E.Y.; Kord, D.; Yee, N.J.; Horner, N.S.; Al Mana, L.; Leroux, T.; Alolabi, B.; Khan, M. Stemless Reverse Total Shoulder Arthroplasty: A Systematic Review of Short- and Mid-Term Results. Shoulder Elb. 2021, 13, 482–491. [Google Scholar] [CrossRef]
- Ballas, R.; Béguin, L. Results of a Stemless Reverse Shoulder Prosthesis at More than 58 Months Mean without Loosening. J. Shoulder Elb. Surg. 2013, 22, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Cogan, C.J.; Ho, J.C.; Entezari, V.; Iannotti, J.P.; Ricchetti, E.T. The Influence of Reverse Total Shoulder Arthroplasty Implant Design on Biomechanics. Curr. Rev. Musculoskelet. Med. 2023, 16, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.J.; Duquin, T.R.; Ehrensberger, M.T. Reverse Total Shoulder Glenoid Baseplate Stability with Superior Glenoid Bone Loss. J. Shoulder Elb. Surg. 2017, 26, 1748–1755. [Google Scholar] [CrossRef]
- Boileau, P.; Morin-Salvo, N.; Bessière, C.; Chelli, M.; Gauci, M.-O.; Lemmex, D.B. Bony Increased-Offset-Reverse Shoulder Arthroplasty: 5 to 10 Years’ Follow-Up. J. Shoulder Elb. Surg. 2020, 29, 2111–2122. [Google Scholar] [CrossRef]
- Merolla, G.; Giorgini, A.; Bonfatti, R.; Micheloni, G.M.; Negri, A.; Catani, F.; Tarallo, L.; Paladini, P.; Porcellini, G. BIO-RSA vs. Metal-Augmented Baseplate in Shoulder Osteoarthritis with Multiplanar Glenoid Deformity: A Comparative Study of Radiographic Findings and Patient Outcomes. J. Shoulder Elb. Surg. 2023, 32, 2264–2275. [Google Scholar] [CrossRef]
- Terrier, A.; Goetti, P.; Becce, F.; Farron, A. Reduction of Scapulohumeral Subluxation with Posterior Augmented Glenoid Implants in Anatomic Total Shoulder Arthroplasty: Short-Term 3D Comparison between Pre- and Post-Operative CT. Orthop. Traumatol. Surg. Res. 2020, 106, 681–686. [Google Scholar] [CrossRef]
- Leafblad, N.; Asghar, E.; Tashjian, R.Z. Innovations in Shoulder Arthroplasty. J. Clin. Med. 2022, 11, 2799. [Google Scholar] [CrossRef]
- Sandow, M.; Schutz, C. Total Shoulder Arthroplasty Using Trabecular Metal Augments to Address Glenoid Retroversion: The Preliminary Result of 10 Patients with Minimum 2-Year Follow-Up. J. Shoulder Elb. Surg. 2016, 25, 598–607. [Google Scholar] [CrossRef]
- Porcellini, G.; Micheloni, G.M.; Tarallo, L.; Paladini, P.; Merolla, G.; Catani, F. Custom-Made Reverse Shoulder Arthroplasty for Severe Glenoid Bone Loss: Review of the Literature and Our Preliminary Results. J. Orthop. Traumatol. Off. J. Ital. Soc. Orthop. Traumatol. 2021, 22, 2. [Google Scholar] [CrossRef]


| Welch et al. (2024) [35] | Tantone et al. (2023) [36] | |
|---|---|---|
| Study type | Systematic review and meta-analysis | Systematic review and meta-analysis |
| Population | 2149 patients (760 with failed RCR) | 1590 patients (527 with failed RCR) |
| ASES | −8.31 (95% CI: −10.96, −5.66) | −6.12 (95% CI: −8.45, −3.79) |
| VAS Pain | +0.85 (95% CI: 0.47, 1.22) | +0.58 (95% CI: 0.23, 0.93) |
| ROM—FF | −6.71° (95% CI: −11.75°, −1.67°) | −9.45° (95% CI: −13.69°, −5.20°) |
| ROM—ER | No significant difference | −3.61° (95% CI: −5.73°, −1.48°) |
| Complications | No significant difference | Increased risk (OR = 1.57, 95% CI: 1.12, 2.19) |
| Revision rates | No significant difference | No significant difference |
| Author | Number of Studies | Total Number of Cases | Average Follow-up | Clinical Outcomes |
|---|---|---|---|---|
| Ernstbrunner et al. [74] 2019 | 8 | 365 shoulders | 9.5 years | Active forward elevation and abduction showed significant improvement (p = 0.004 and p = 0.009, respectively). No significant improvement was observed in active external rotation |
| Bois et al. [80] 2020 | 43 | 1041 implants | 43.8 months | Except for external rotation, range of motion showed improvement in all groups |
| Nunes et al. [81] 2021 | 9 | 1670 patients | 41.1 months | Improvement in forward flexion, abduction, and external rotation |
| Author | Number of Patients | Revision Rate | Comments |
|---|---|---|---|
| Favard et al. [73] 2011 | 506 patients (527 prostheses) | Revision rate of 5% | Twelve prostheses were removed due to infection within the first 3 years, with a marked increase in revisions occurring in the first 2 years. |
| Melis et al. [87] 2011 | 119 patients (122 prostheses) | Revision rate of 7% | Mainly caused by infections or glenoid loosening. |
| Bacle et al. [83] 2017 | 84 patients (87 prostheses) | Revision rate of 12% | Main causes: infection and glenoid loosening. |
| Author | Results |
|---|---|
| Favard et al. [73] 2011 | 89% implant survival at 10 years post-surgery. |
| Bacle et al. [83] 2017 | 93% implant survival at 10 years post-surgery. |
| Goldenberg et al. [85] 2020 | Systematic analysis of seven studies on 286 shoulders of patients under 65, showing implant survival rates of 99% at 2 years, 91–98% at 5 years, and 88% at 10 years. |
| Chelli et al. [84] 2022 | 91% survival rate after primary RTSA and 80.9% survival rate after revision RTSA. For tumors, survival is 53.1%. In patients under 60, the survival rate is 75.7%, compared to 94.3% in patients over 80. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fornaciari, P.; Jamei-Martel, O.; Vial, P. Trends in Shoulder Arthroplasty: A Narrative Review of Predominant Indications and the Most Commonly Employed Implant Designs. J. Clin. Med. 2025, 14, 3186. https://doi.org/10.3390/jcm14093186
Fornaciari P, Jamei-Martel O, Vial P. Trends in Shoulder Arthroplasty: A Narrative Review of Predominant Indications and the Most Commonly Employed Implant Designs. Journal of Clinical Medicine. 2025; 14(9):3186. https://doi.org/10.3390/jcm14093186
Chicago/Turabian StyleFornaciari, Paolo, Omid Jamei-Martel, and Philippe Vial. 2025. "Trends in Shoulder Arthroplasty: A Narrative Review of Predominant Indications and the Most Commonly Employed Implant Designs" Journal of Clinical Medicine 14, no. 9: 3186. https://doi.org/10.3390/jcm14093186
APA StyleFornaciari, P., Jamei-Martel, O., & Vial, P. (2025). Trends in Shoulder Arthroplasty: A Narrative Review of Predominant Indications and the Most Commonly Employed Implant Designs. Journal of Clinical Medicine, 14(9), 3186. https://doi.org/10.3390/jcm14093186







