The Influence of Anti-PAR 1 and Anti-ACE 2 Antibody Levels on the Course of Specific Glomerulonephritis Types
Abstract
:1. Introduction
2. Materials and Methods
3. Results
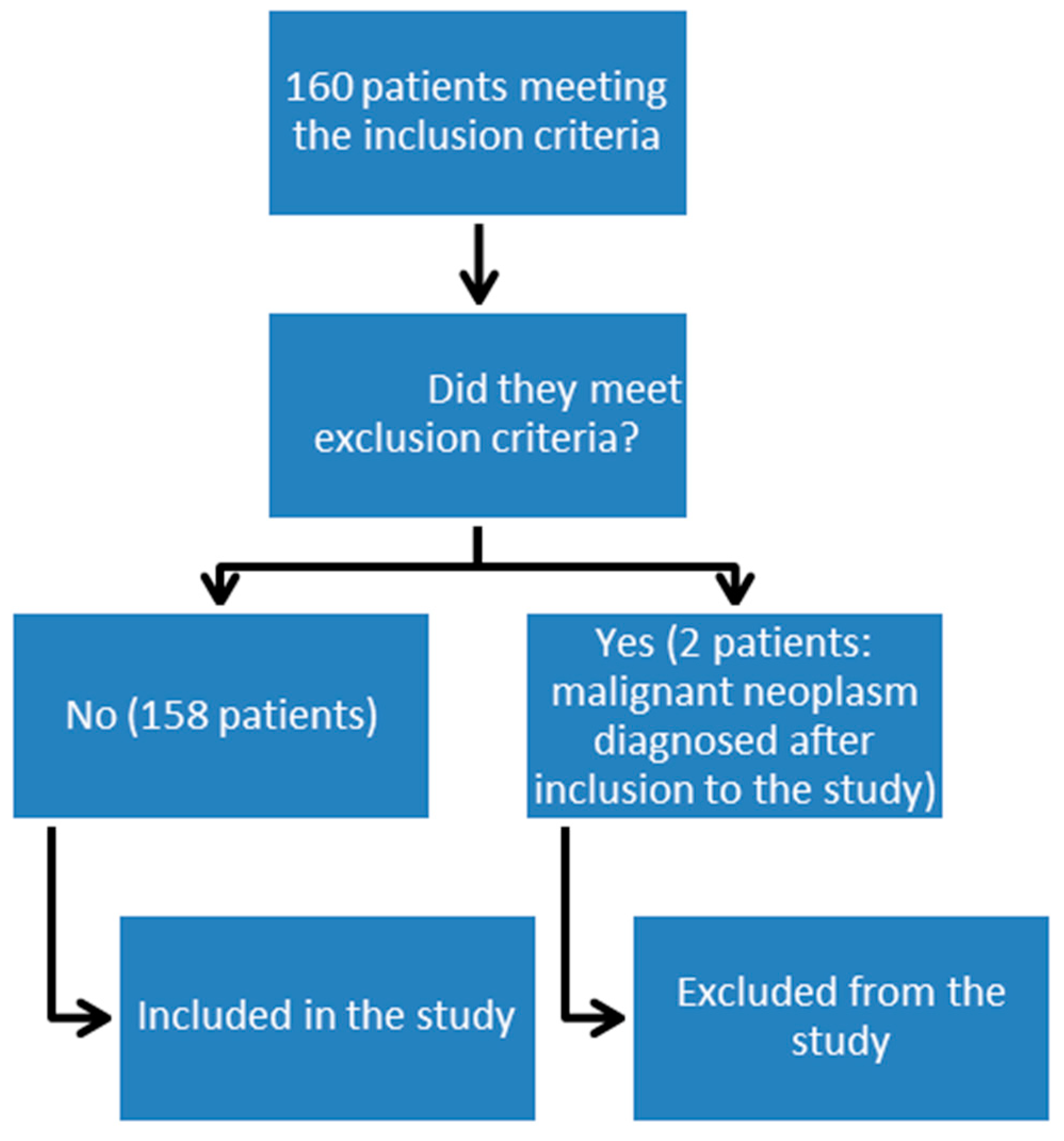
3.1. Characteristics of Patients
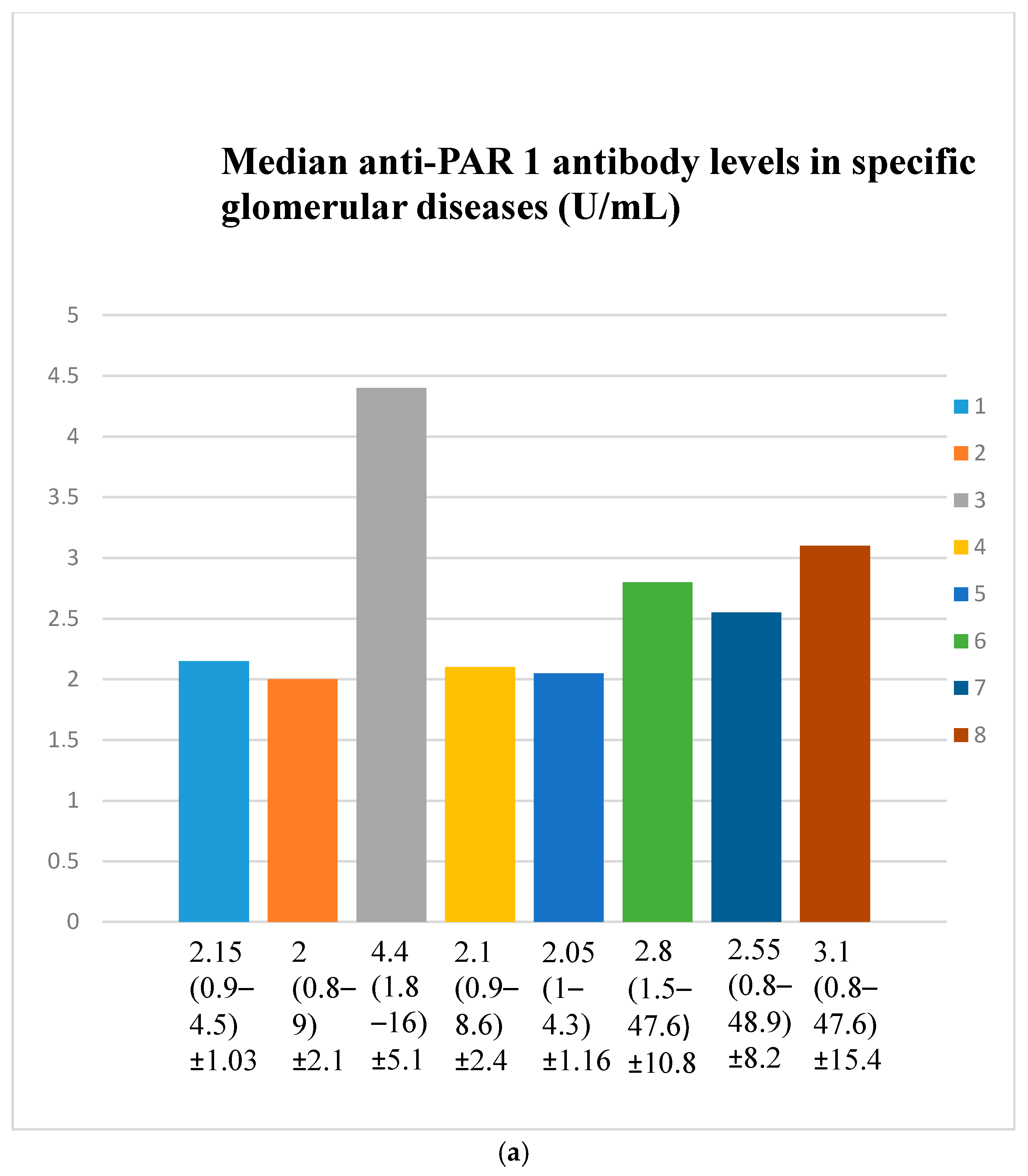
3.2. Anti-PAR 1 Antibody Evaluation Results
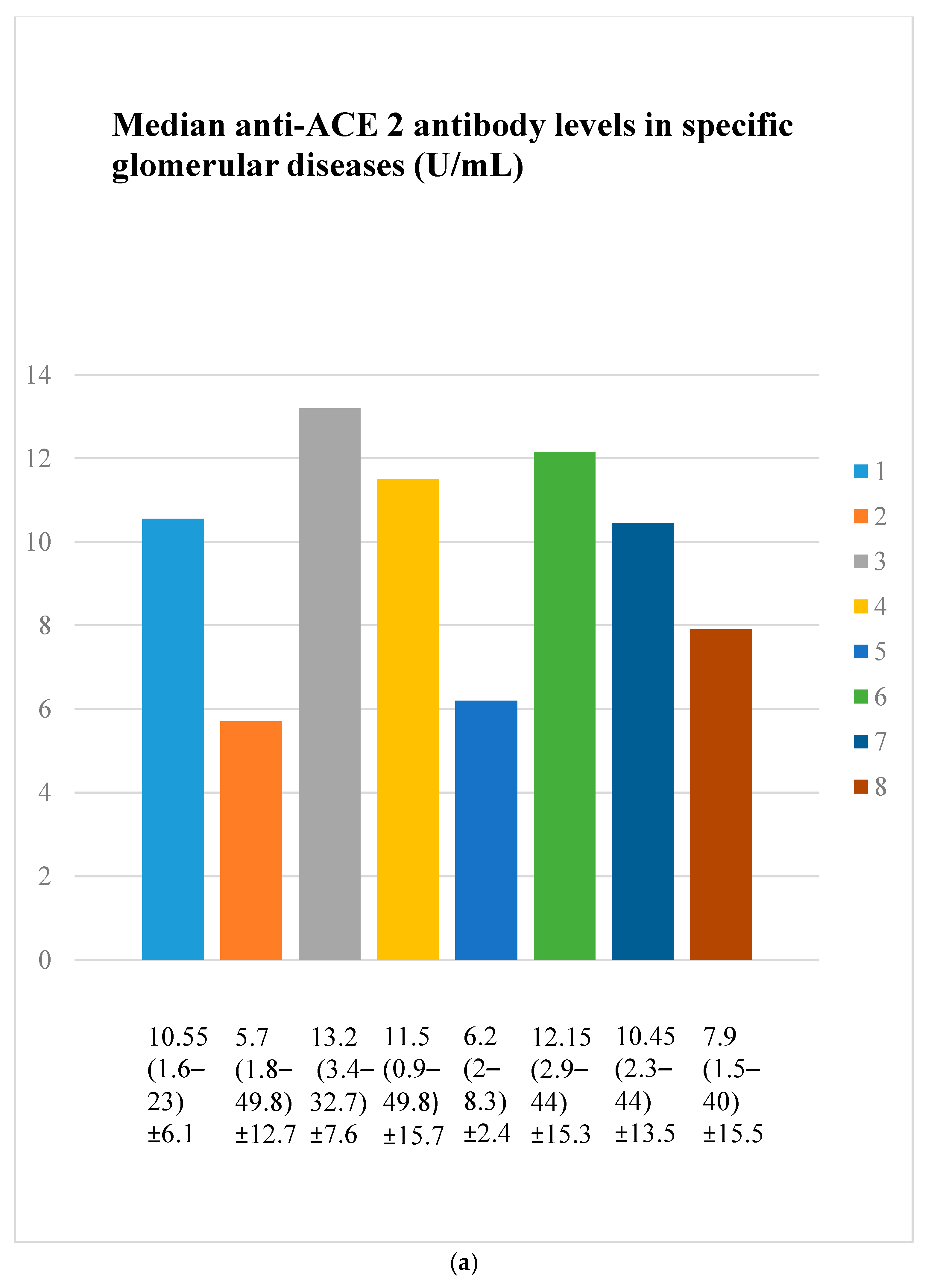
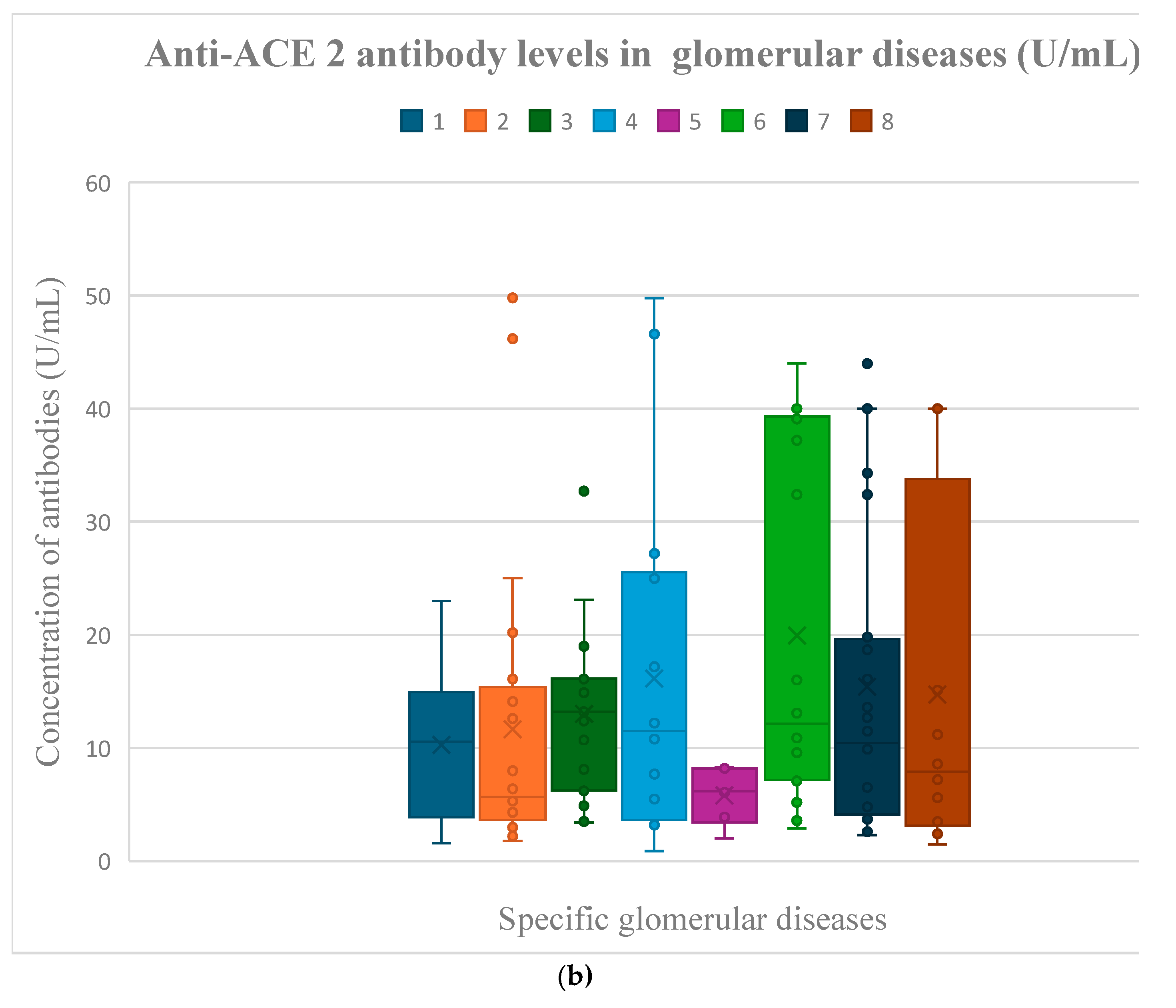
3.3. Anti-ACE 2 Antibody Evaluation Results
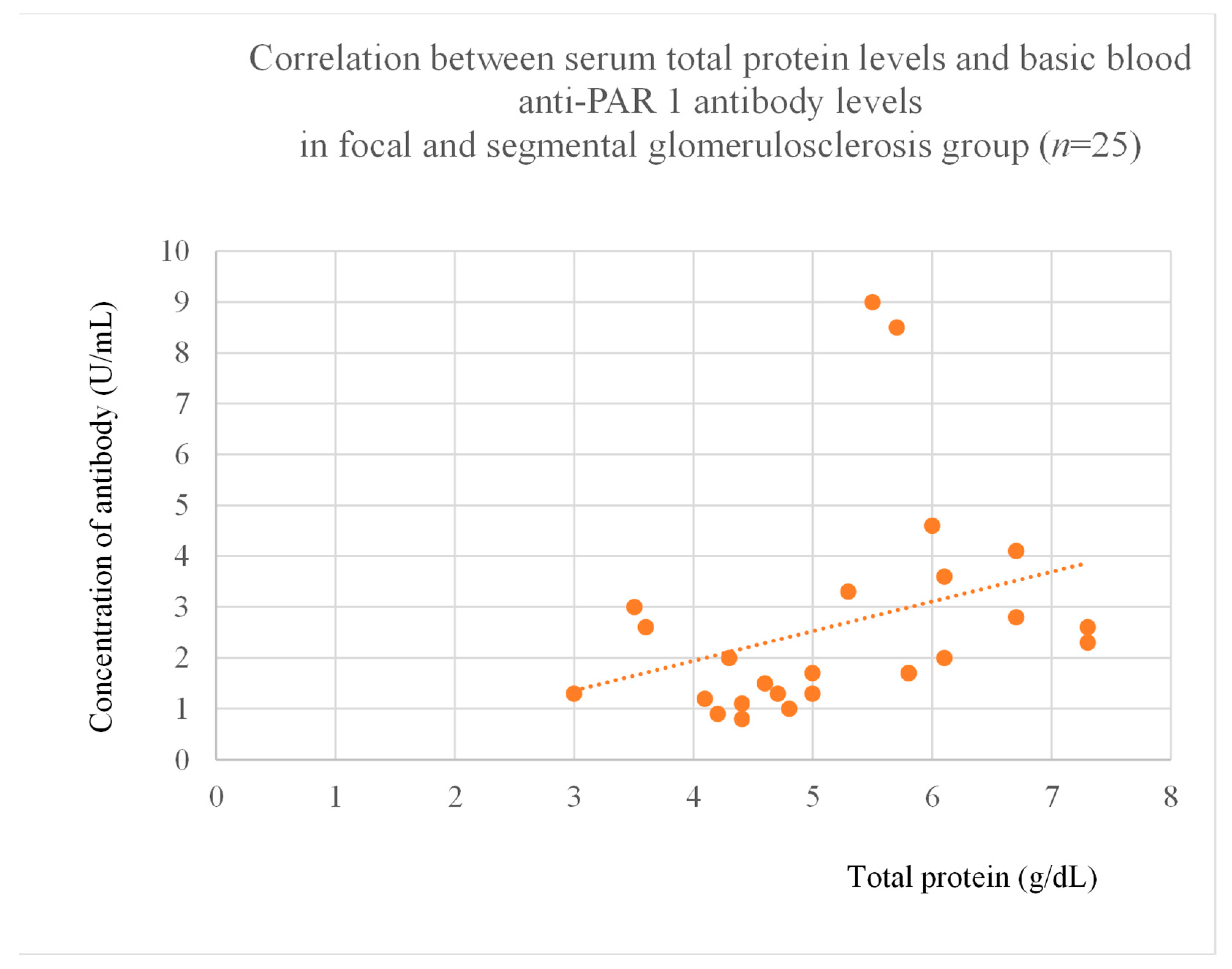
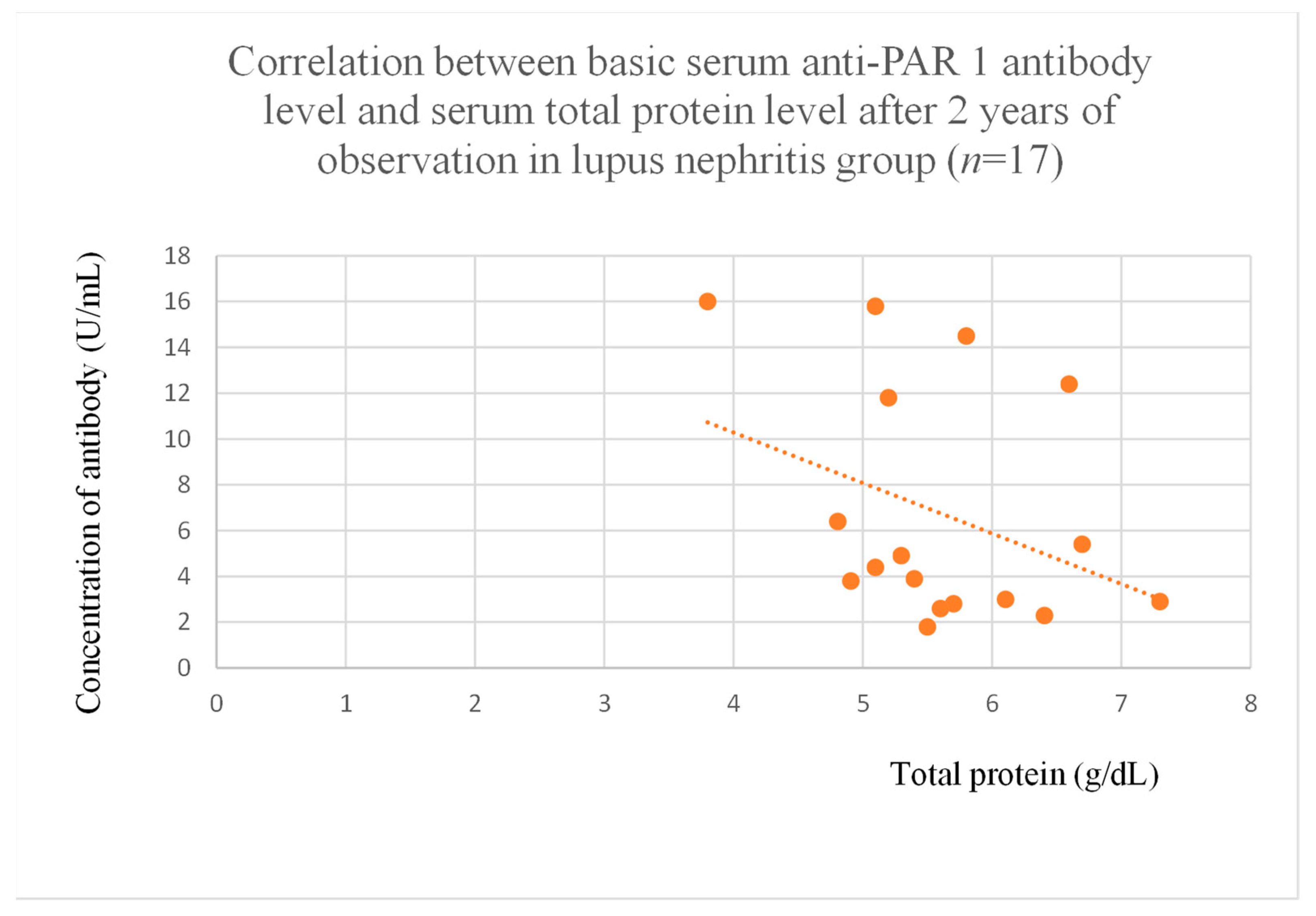
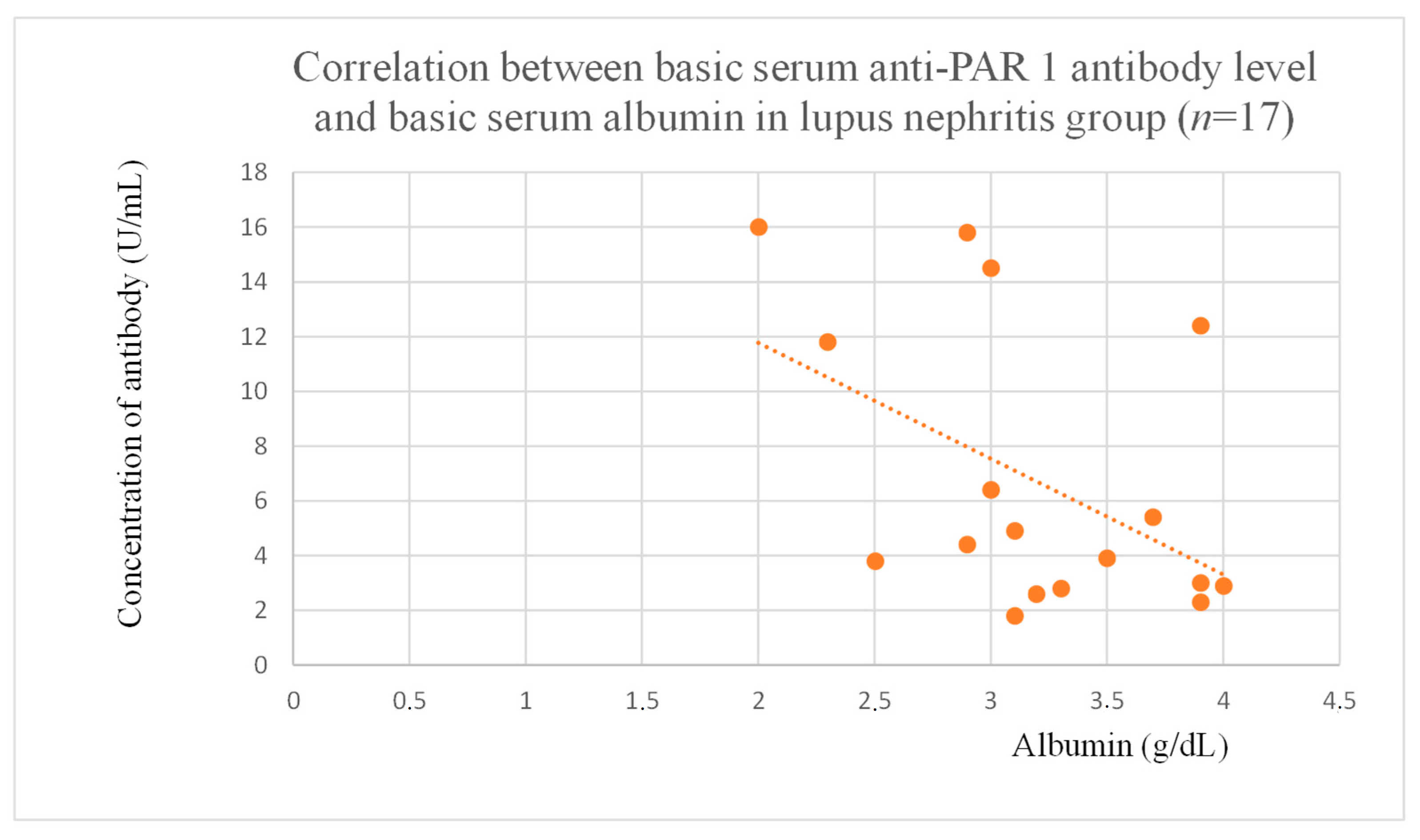
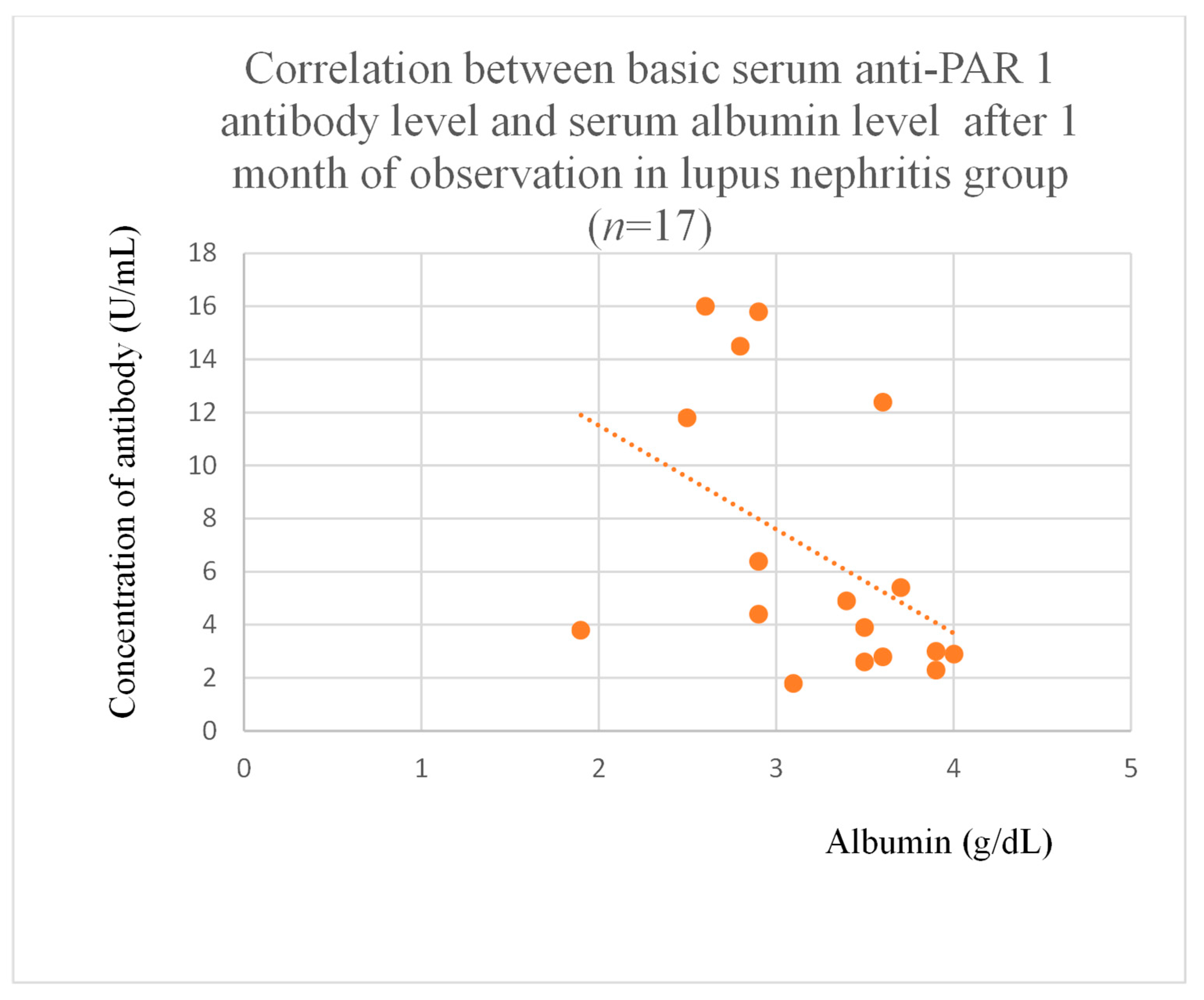
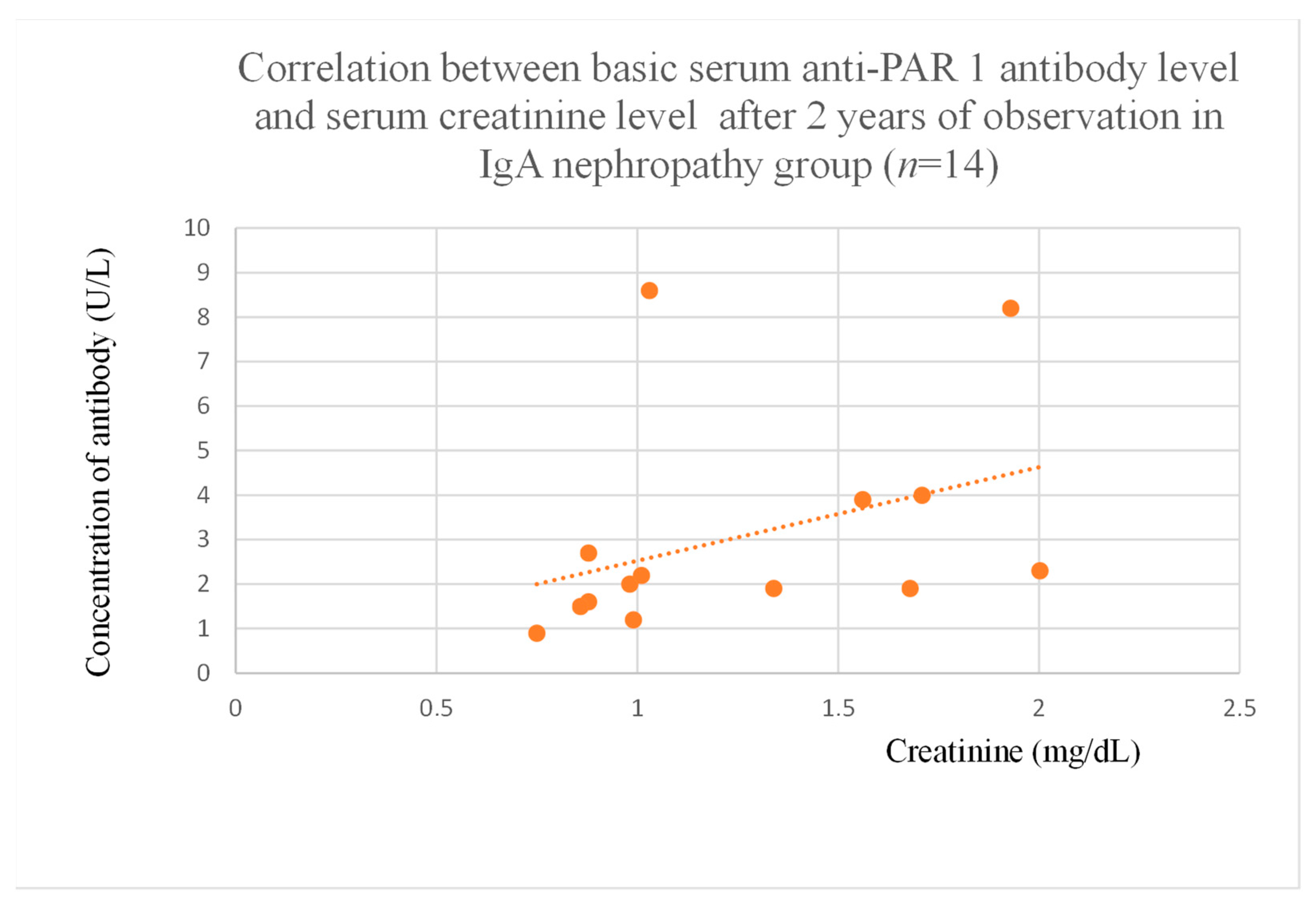
3.4. Correlations Between Anti-PAR 1 Antibody Levels and Clinical Data of Patients
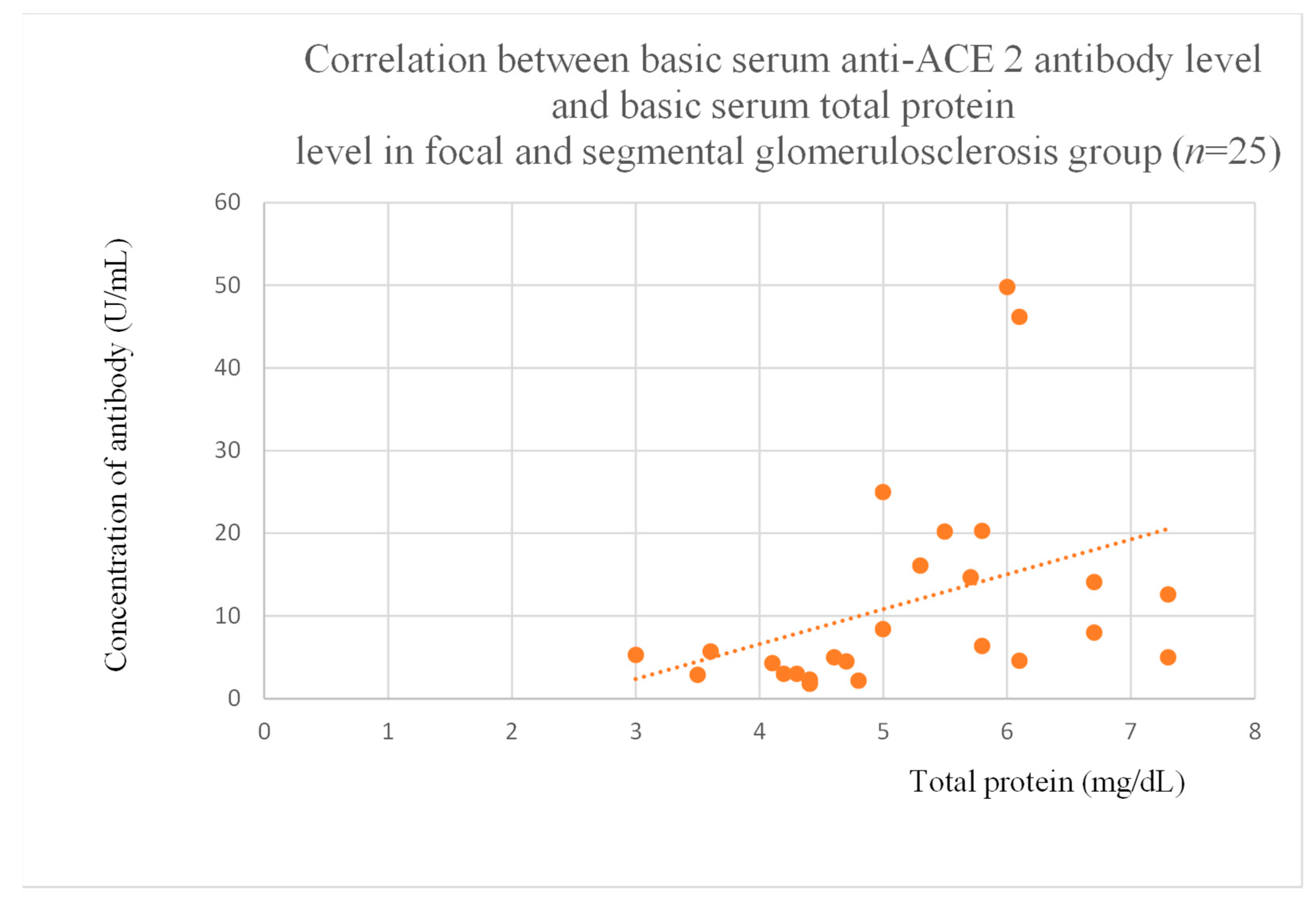
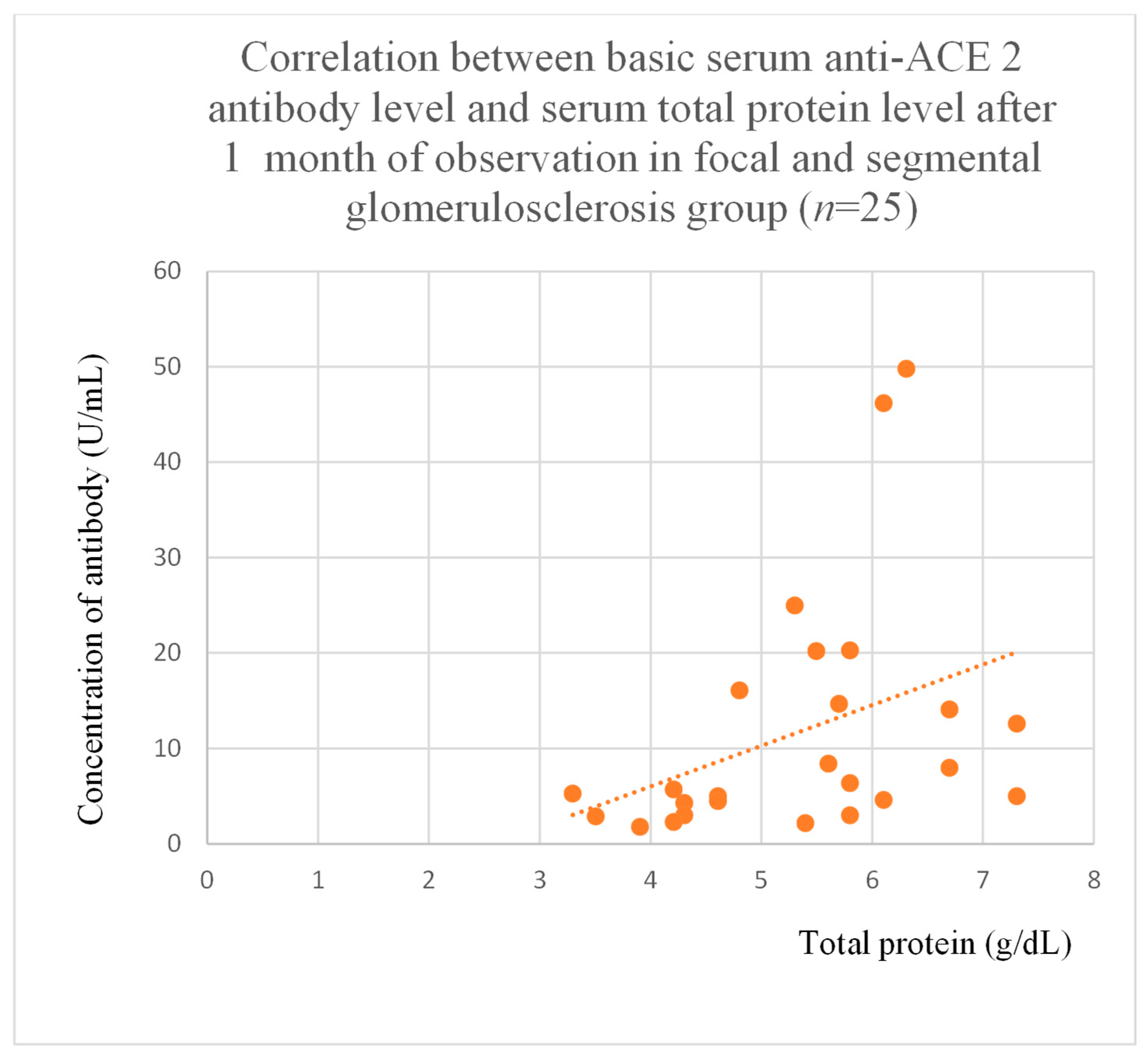
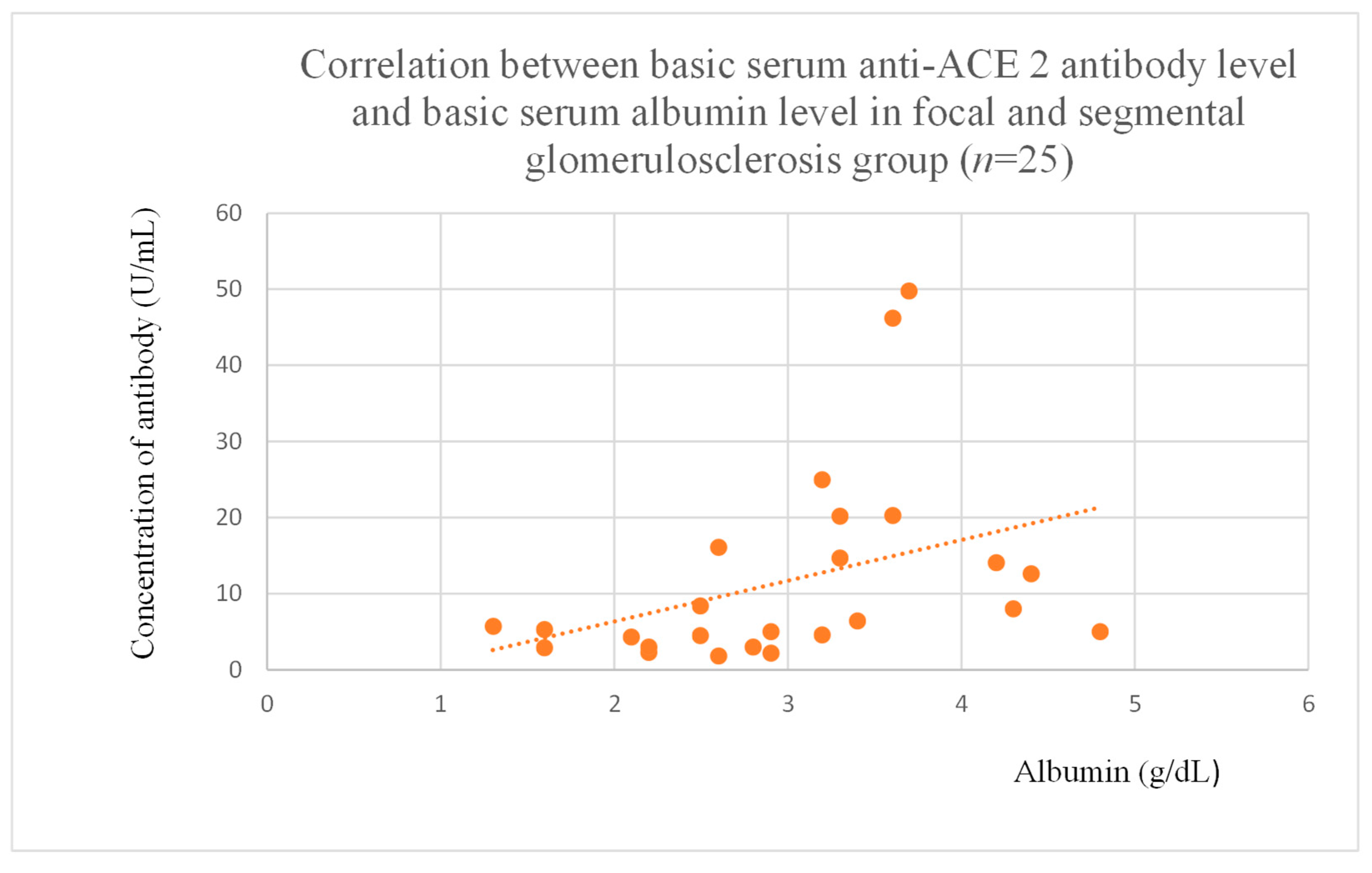
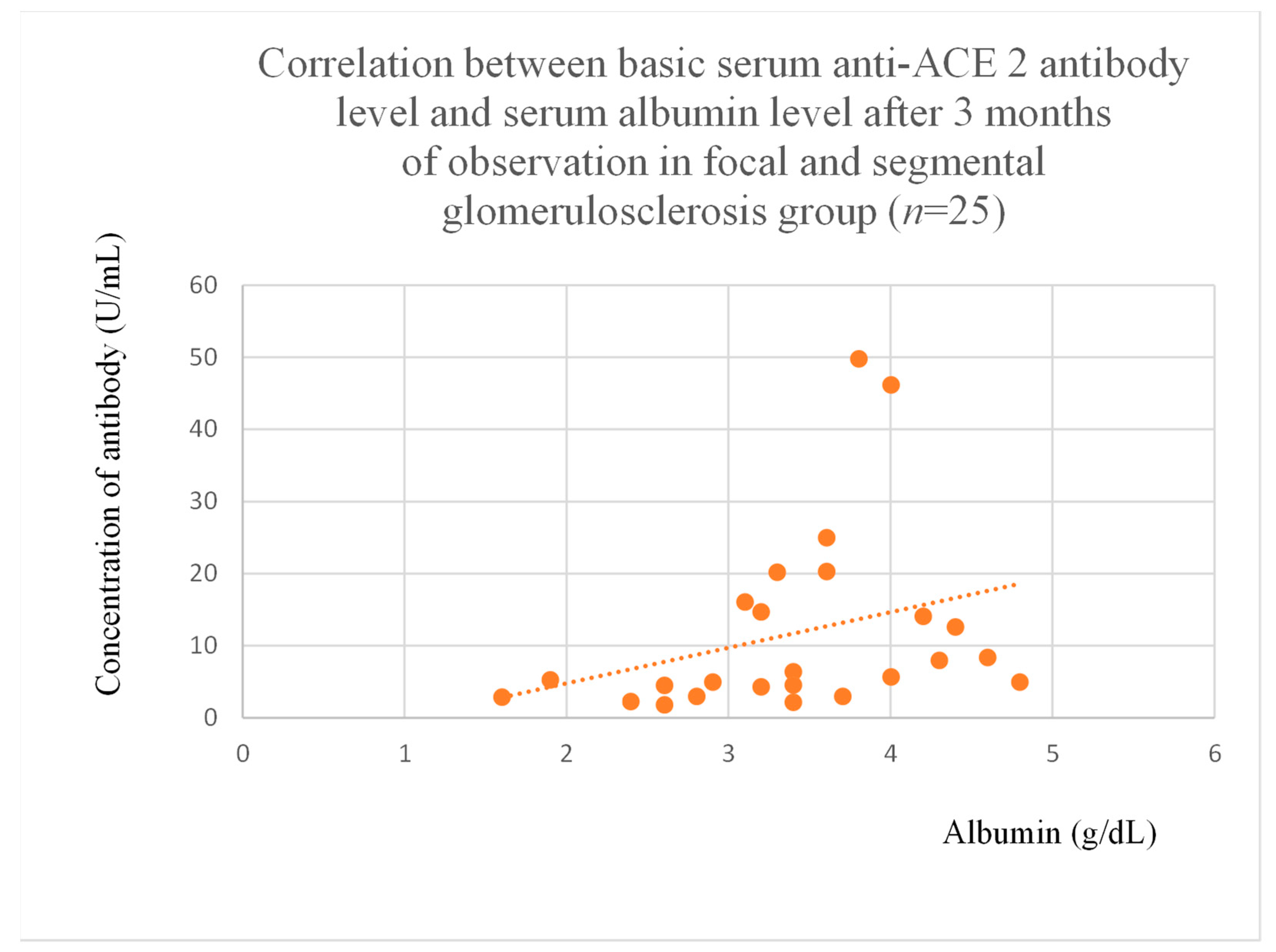
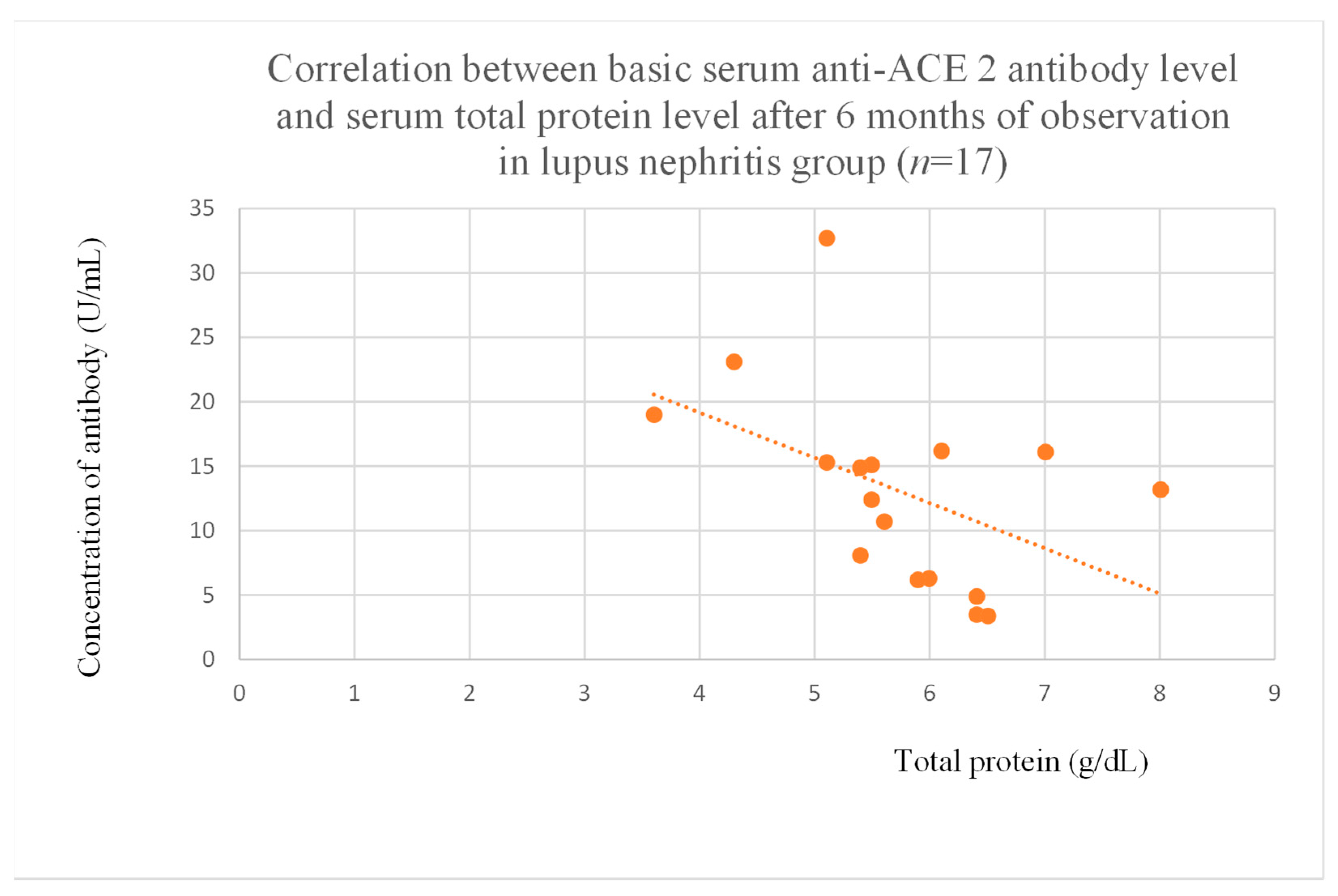
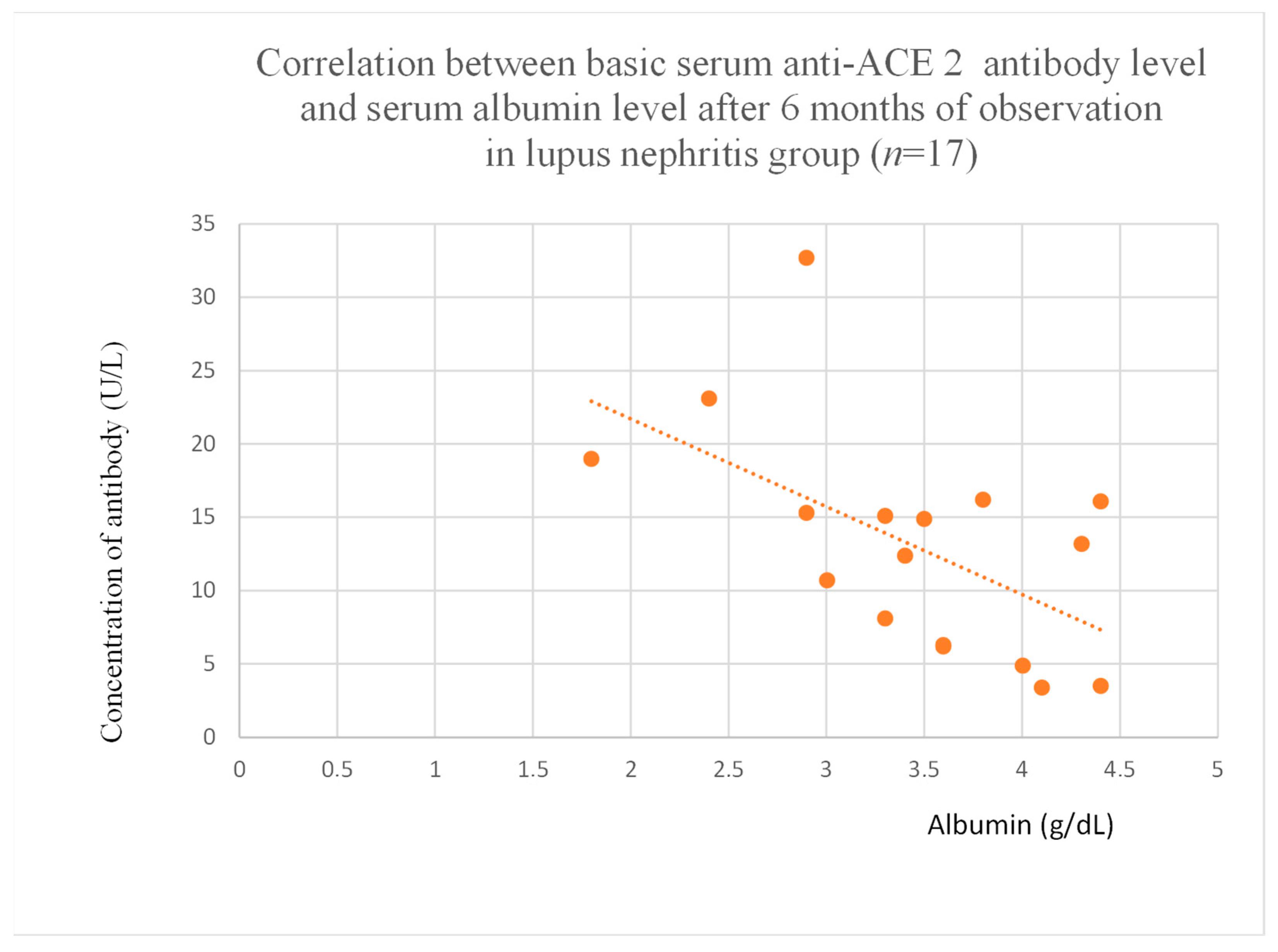
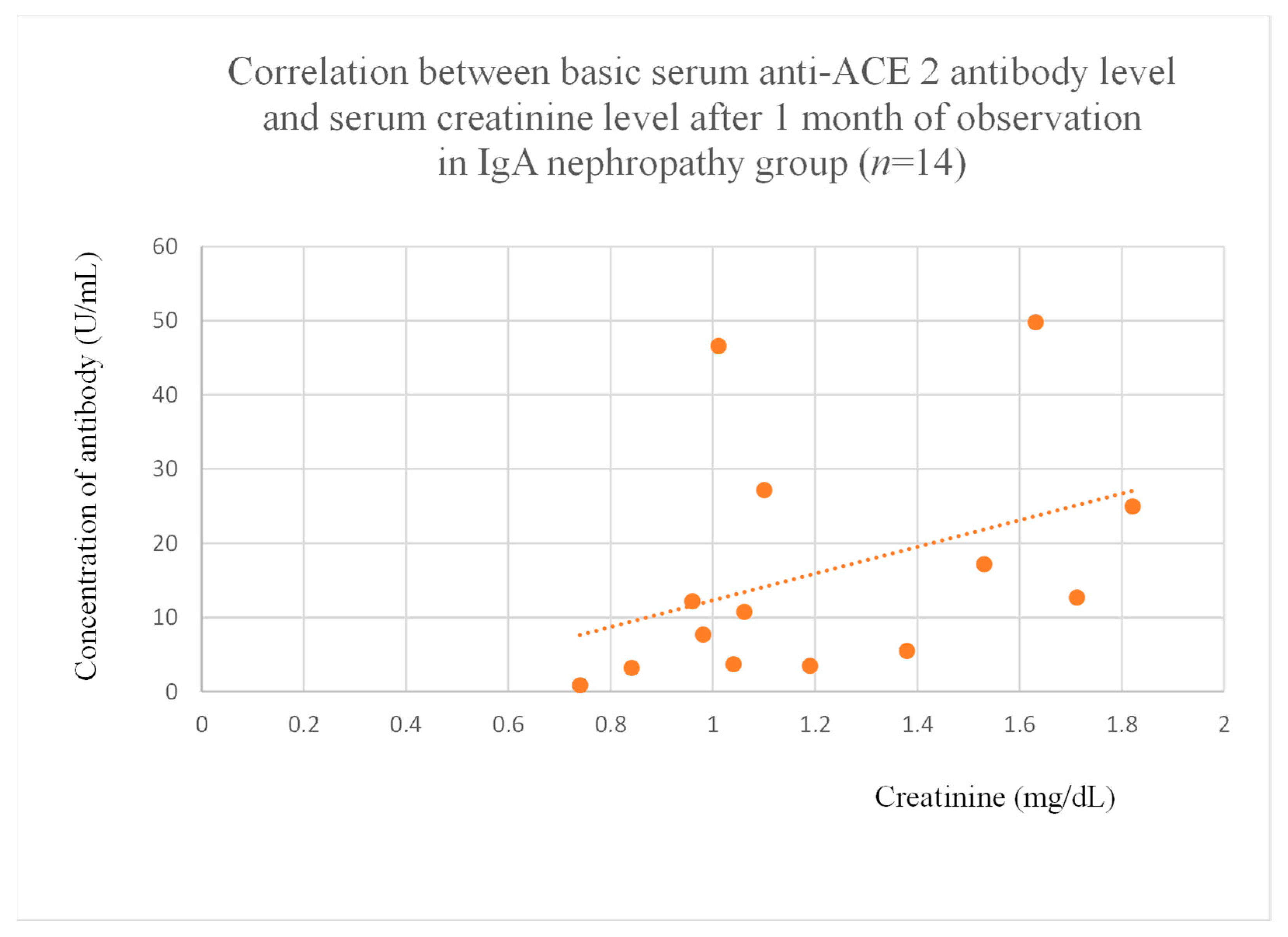
3.5. Correlations Between Anti-ACE 2 Antibody Levels and Clinical Data of Patients
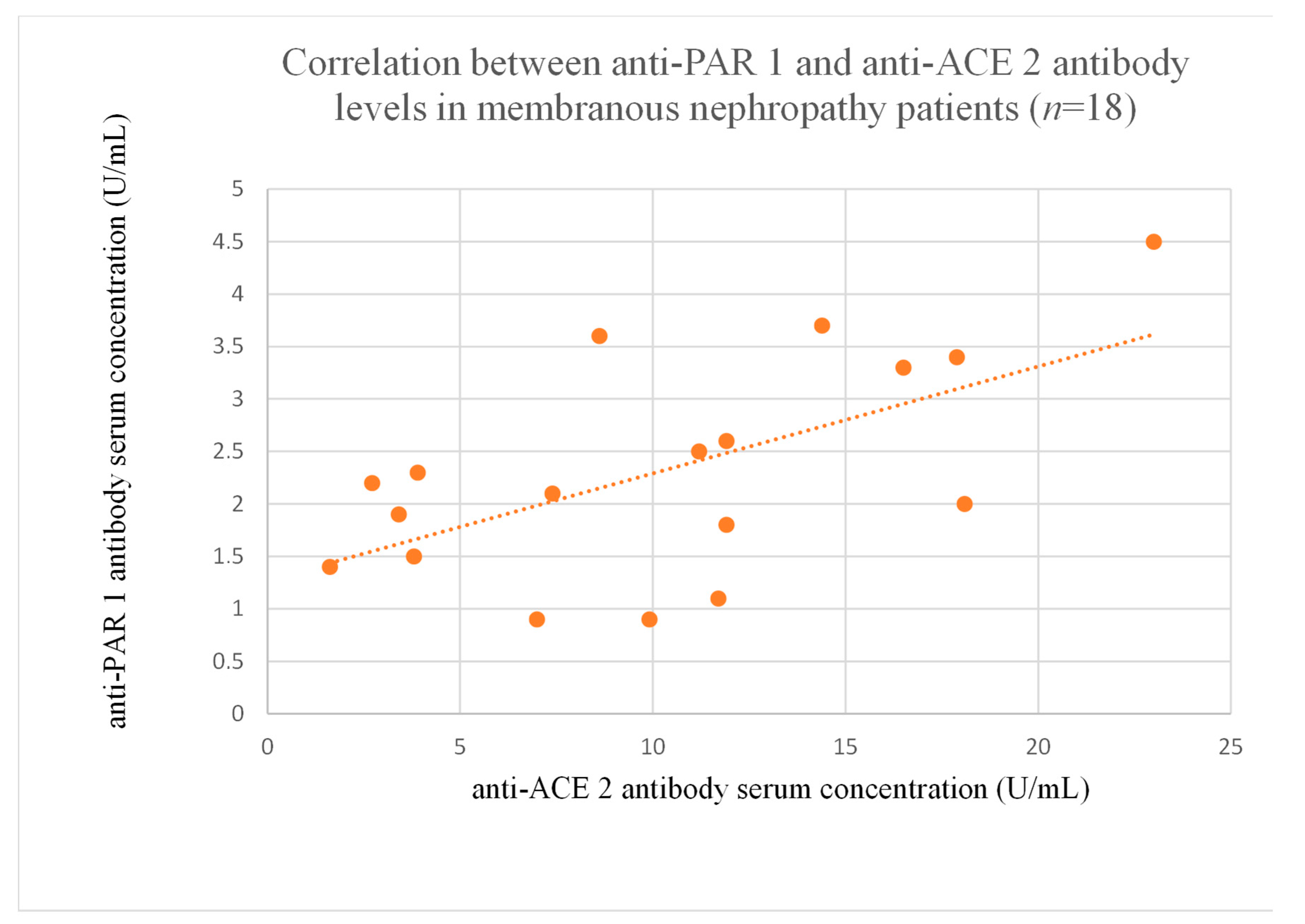
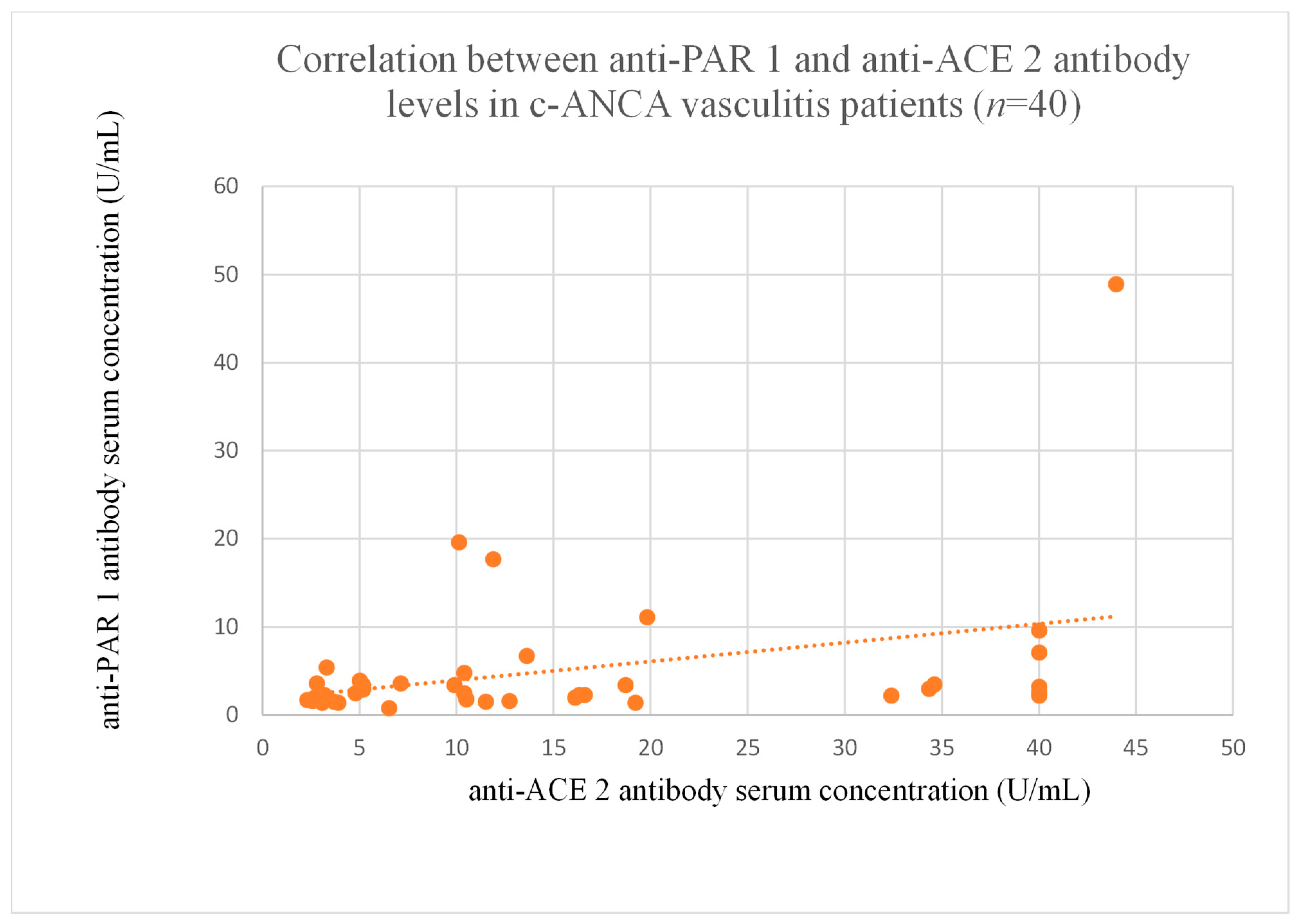
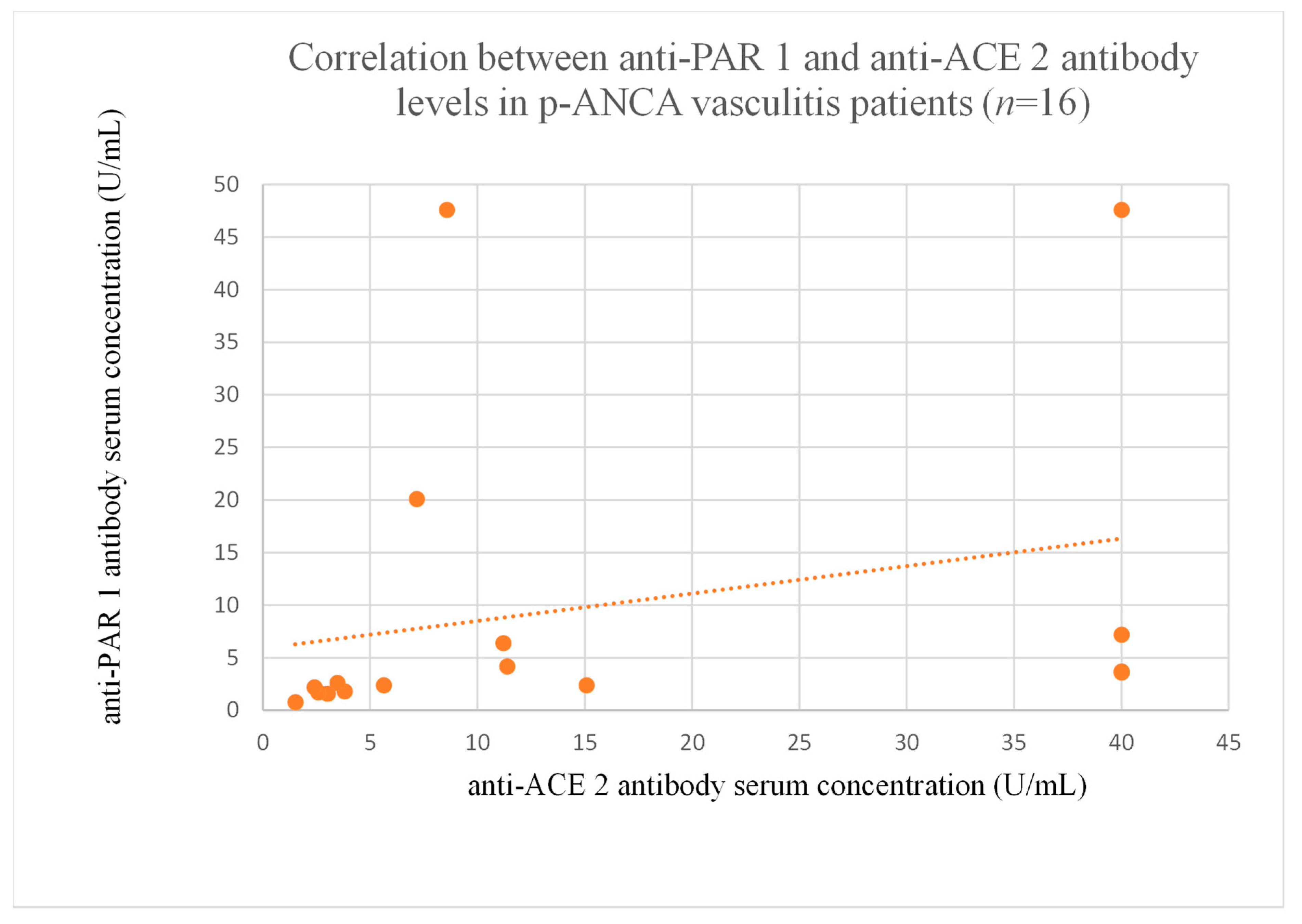
3.6. Correlations Between Anti-PAR 1 and Anti-ACE 2 Antibody Levels
4. Discussion
4.1. Study Limitations
4.2. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | angiotensin 2-converting enzyme type 1 |
| ACE 2 | angiotensin 2-converting enzyme type 2 |
| ANAs | antinuclear antibodies |
| ANOVA | analysis of variance |
| AT1R | angiotensin 2 type 1 receptor |
| AT2R | angiotensin 2 type 2 receptor |
| cANCA | anti-neutrophil cytoplasmic antibodies |
| ELISA | enzyme-linked immunosorbent assay |
| ETAR | endothelin A receptor |
| FSGS | focal and segmental glomerulosclerosis |
| HLA | human leukocyte antigens |
| LN | lupus nephritis |
| PAR 1 | protease-activated receptor type 1 |
| pANCA | perinuclear anti-neutrophil cytoplasmic antibodies |
| POD | horseradish peroxidase |
| TMB | tetramethylbenzidine |
References
- Szymczak, M.; Heidecke, H.; Żabińska, M.; Janek, Ł.; Wronowicz, J.; Kujawa, K.; Bukowiec-Marek, K.; Gołębiowski, T.; Skalec, K.; Schulze-Forster, K.; et al. The influence of anti-ETAR and anti-CXCR3 antibody levels on the course of specific glomerulonephritis types. J. Clin. Med. 2024, 13, 7752. [Google Scholar] [CrossRef]
- Sorohan, B.M.; Baston, C.; Tacu, D.; Bucșa, C.; Țincu, C.; Vizireanu, P.; Sinescu, I.; Constantinescu, I. Non-HLA Antibodies in Kidney Transplantation: Immunity and Genetic Insights. Biomedicines 2022, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Dragun, D.; Müller, D.N.; Bräsen, J.H.; Fritsche, L.; Nieminen-Kelhä, M.; Dechend, R.; Kintscher, U.; Rudolph, B.; Hoebeke, J.; Eckert, D.; et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N. Engl. J. Med. 2005, 352, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, M.; Heidecke, H.; Żabińska, M.; Rukasz, D.; Wiśnicki, K.; Kujawa, K.; Kościelska-Kasprzak, K.; Krajewska, M.; Banasik, M. Angiotensin II Type 2 Receptor Antibodies in Glomerular Diseases. Arch. Immunol. Ther. Exp. 2024, 72, 1. [Google Scholar] [CrossRef] [PubMed]
- Biswas, I.; Giri, H.; Panicker, S.R.; Rezaie, A.R. Thrombomodulin Switches Signaling and Protease-Activated Receptor 1 Cleavage Specificity of Thrombin. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 603–616. [Google Scholar] [CrossRef]
- Zheng, J.; Hao, H. Targeting renal damage: The ACE2/Ang-(1-7)/mas axis in chronic kidney disease. Cell Signal. 2024, 124, 111413. [Google Scholar] [CrossRef]
- Bagang, N.; Gupta, K.; Singh, G.; Kanuri, S.H.; Mehan, S. Protease-activated receptors in kidney diseases: A comprehensive review of pathological roles, therapeutic outcomes and challenges. Rev. Chem. Biol. Interact. 2023, 377, 110470. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Mora-Fernández, C.; Muros de Fuentes, M.; García-Pérez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 327–340. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Tanaka-Ishikawa, M.; Abe, K.; Hirano, M.; Hirooka, Y.; Tsutsui, H.; Sunagawa, K.; Hirano, K. Proteinase-activated receptor 1 antagonism ameliorates experimental pulmonary hypertension. Cardiovasc. Res. 2019, 115, 1357–1368. [Google Scholar] [CrossRef]
- Höcherl, K.; Gerl, M.; Schweda, F. Proteinase-activated receptors 1 and 2 exert opposite effects on renal renin release. Hypertension 2011, 58, 611–618. [Google Scholar] [CrossRef]
- Andersson, L.I.; Sjöström, D.J.; Quach, H.Q.; Hägerström, K.; Hurler, L.; Kajdácsi, E.; Cervenak, L.; Prohászka, Z.; Toonen, E.J.M.; Mohlin, C.; et al. Storage of Transfusion Platelet Concentrates Is Associated with Complement Activation and Reduced Ability of Platelets to Respond to Protease-Activated Receptor-1 and Thromboxane A2 Receptor. Int. J. Mol. Sci. 2024, 25, 1091. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Ithychanda, S.S.; Plow, E.F. Histone 2B Facilitates Plasminogen-Enhanced Endothelial Migration through Protease-Activated Receptor 1 (PAR1) and Protease-Activated Receptor 2 (PAR2). Biomolecules 2022, 12, 211. [Google Scholar] [CrossRef] [PubMed]
- Dólleman, S.C.; Agten, S.M.; Spronk, H.M.H.; Hackeng, T.M.; Bos, M.H.A.; Versteeg, H.H.; van Zonneveld, A.J.; de Boer, H.C. Thrombin in complex with dabigatran can still interact with PAR-1 via exosite-I and instigate loss of vascular integrity. J. Thromb. Haemost. 2022, 20, 996–1007. [Google Scholar] [CrossRef]
- Waasdorp, M.; Duitman, J.; Florquin, S.; Spek, C.A. Protease-activated receptor-1 deficiency protects against streptozotocin-induced diabetic nephropathy in mice. Sci. Rep. 2016, 6, 33030. [Google Scholar] [CrossRef]
- Guan, Y.; Nakano, D.; Zhang, Y.; Li, L.; Liu, W.; Nishida, M.; Kuwabara, T.; Morishita, A.; Hitomi, H.; Mori, K.; et al. A protease-activated receptor-1 antagonist protects against podocyte injury in a mouse model of nephropathy. J. Pharmacol. Sci. 2017, 135, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Kim, I.S.; Rezaie, A.R. Thrombin down-regulates the TGF-beta-mediated synthesis of collagen and fibronectin by human proximal tubule epithelial cells through the EPCR-dependent activation of PAR-1. J. Cell. Physiol. 2010, 225, 233–239. [Google Scholar] [CrossRef]
- Palygin, O.; Ilatovskaya, D.V.; Staruschenko, A. Protease-activated receptors in kidney disease progression. Am. J. Physiol. Renal. Physiol. 2016, 311, F1140–F1144. [Google Scholar] [CrossRef]
- May, C.J.; Chesor, M.; Hunter, S.E.; Hayes, B.; Barr, R.; Roberts, T.; Barrington, F.A.; Farmer, L.; Ni, L.; Jackson, M.; et al. Podocyte protease activated receptor 1 stimulation in mice produces focal segmental glomerulosclerosis mirroring human disease signaling events. Kidney Int. 2023, 104, 265–278. [Google Scholar] [CrossRef]
- Medipally, A.; Xiao, M.; Biederman, L.; Satoskar, A.A.; Ivanov, I.; Rovin, B.; Parikh, S.; Kerlin, B.A.; Brodsky, S.V. Role of protease-activated receptor-1 (PAR-1) in the glomerular filtration barrier integrity. Physiol. Rep. 2022, 10, e15343. [Google Scholar] [CrossRef]
- Sharma, R.; Waller, A.P.; Agrawal, S.; Wolfgang, K.J.; Luu, H.; Shahzad, K.; Isermann, B.; Smoyer, W.E.; Nieman, M.T.; Kerlin, B.A. Thrombin-Induced Podocyte Injury Is Protease-Activated Receptor Dependent. J. Am. Soc. Nephrol. 2017, 28, 2618–2630. [Google Scholar] [CrossRef]
- Stefanenko, M.; Fedoriuk, M.; Mamenko, M.; Semenikhina, M.; Nowling, T.K.; Lipschutz, J.H.; Maximyuk, O.; Staruschenko, A.; Palygin, O. PAR1-mediated Non-periodical Synchronized Calcium Oscillations in Human Mesangial Cells. Function 2024, 5, zqae030. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Lücht, C.; Hosp, I.; Zhao, H.; Wu, D.; Heidecke, H.; Witowski, J.; Budde, K.; Riemekasten, G.; Catar, R. Autoantibodies from Patients with Scleroderma Renal Crisis Promote PAR-1 Receptor Activation and IL-6 Production in Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 11793. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, D.; Kamińska, D.; Catar, R.; Banasik, M.; Heidecke, H.; Schulze-Forster, K.; Korybalska, K.; Rutkowski, R.; Łuczak, J.; Jabłecki, J.; et al. Non-HLA Antibodies in Hand Transplant Recipients Are Connected to Multiple Acute Rejection Episodes and Endothelial Activation. J. Clin. Med. 2022, 11, 833. [Google Scholar] [CrossRef]
- Reinshagen, L.; Nageswaran, V.; Heidecke, H.; Schulze-Forster, K.; Wilde, A.B.; Ramezani Rad, P.; Poller, W.; Asmus, E.; Simmons, S.; Kuebler, W.M.; et al. Protease-Activated Receptor-1 IgG Autoantibodies in Patients with COVID-19. Thromb. Haemost. 2024, 124, 1164–1166. [Google Scholar] [CrossRef]
- Ferrario, C.M. Angiotensin-converting enzyme 2 and angiotensin-(1-7): An evolving story in cardiovascular regulation. Hypertension. 2006, 47, 515–521. [Google Scholar] [CrossRef]
- Sandoval, J.; Del Valle-Mondragón, L.; Masso, F.; Zayas, N.; Pulido, T.; Teijeiro, R.; Gonzalez-Pacheco, H.; Olmedo-Ocampo, R.; Sisniega, C.; Paez-Arenas, A.; et al. Angiotensin converting enzyme 2 and angiotensin (1-7) axis in pulmonary arterial hypertension. Eur. Respir. J. 2020, 56, 1902416. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.P.; Zhang, X.L.; Li, J. New perspectives on angiotensin-converting enzyme 2 and its related diseases. World J. Diabetes. 2021, 12, 839–854. [Google Scholar] [CrossRef]
- Mizuiri, S.; Ohashi, Y. ACE and ACE2 in kidney disease. World J. Nephrol. 2015, 4, 74–82. [Google Scholar] [CrossRef]
- Anguiano, L.; Riera, M.; Pascual, J.; Soler, M.J. Circulating ACE2 in Cardiovascular and Kidney Diseases. Curr. Med. Chem. 2017, 24, 3231–3241. [Google Scholar] [CrossRef]
- Liao, K.; Sikkema, D.; Wang, C.; Lee, T.N. Development of an enzymatic assay for the detection of neutralizing antibodies against therapeutic angiotensin-converting enzyme 2 (ACE2). J. Immunol. Methods 2013, 389, 52–60. [Google Scholar] [CrossRef]
- Chen, X.; Li, R.; Pan, Z.; Qian, C.; Yang, Y.; You, R.; Zhao, J.; Liu, P.; Gao, L.; Li, Z.; et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell. Mol. Immunol. 2020, 17, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Mecoli, C.A.; Yoshida, A.; Paik, J.J.; Lin, C.T.; Danoff, S.; Hanaoka, H.; Rosen, A.; Christopher-Stine, L.; Kuwana, M.; Casciola-Rosen, L. Presence and Implications of Anti-Angiotensin Converting Enzyme-2 Immunoglobulin M Antibodies in Anti-Melanoma-Differentiation-Associated 5 Dermatomyositis. ACR. Open Rheumatol. 2022, 4, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Stillman, I.E.; Campbell, K.N.; Meliambro, K. Focal Segmental Glomerulosclerosis. Adv. Kidney Dis. Health 2024, 31, 275–289. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, S.; Wu, L.; Xing, C.; Mao, H.; Liang, H.; Yuan, Y. Animal models of membranous nephropathy: More choices and higher similarity. Front. Immunol. 2024, 15, 1412826. [Google Scholar] [CrossRef]
- Lee, H.J.; Shin, K.H.; Kim, I.Y.; Choi, B.H.; Kim, H.H. Association between anti-endothelial antigen antibodies and allograft rejection in kidney transplantation. J. Clin. Lab. Anal. 2023, 37, e24961. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.; Candiano, G.; Petretto, A.; Angeletti, A.; Meroni, P.L.; Prunotto, M.; Ghiggeri, G.M. Technology Innovation for Discovering Renal Autoantibodies in Autoimmune Conditions. Int. J. Mol. Sci. 2024, 25, 12659. [Google Scholar] [CrossRef]
- Wang, P.; Wu, D.; Gong, Z.; Adu-Gyamfi, M.; Kamhieh-Milz, J.; da Fonseca, D.L.M.; Sürücü, G.; Ashraf, M.I.; Heidecke, H.; Sikorska, D.; et al. Stimulation of endothelin-1 production by autoantibodies present in patients with scleroderma renal crisis. Clin. Immunol. 2025, 273, 110454. [Google Scholar] [CrossRef]
- Abdel-Fattah, M.M.; Elgendy, A.N.A.M.; Mohamed, W.R. Xanthenone, ACE2 activator, counteracted gentamicin-induced nephrotoxicity in rats: Impact on oxidative stress and ACE2/Ang-(1-7) signaling. Life Sci. 2021, 275, 119387. [Google Scholar] [CrossRef]
- Waasdorp, M.; Duitman, J.; Florquin, S.; Spek, C.A. Vorapaxar treatment reduces mesangial expansion in streptozotocin-induced diabetic nephropathy in mice. Oncotarget 2018, 9, 21655–21662. [Google Scholar] [CrossRef]






| Diagnosis | Serum Creatinine (mg/dL) | eGFR (mL/min./1.73 m2) MDRD | BUN (mg/dL) | Proteinuria (mg/per day) | Albumin to Creatinine Ratio | Total Serum Protein (g/dL) | Serum Albumin (g/dL) |
|---|---|---|---|---|---|---|---|
| membranous nephropathy (n = 18) | 1.25 (0.8–3.3) | 66 (15–106) | 12 (8–32) | 2640 (100–1580) | 1.6 (0.3–7.1) | 4.8 (3.7–5.9) | 2.8 (1.7–3.9) |
| focal and segmental glomerulosclerosis (n = 25) | 1.21 (0.73–3.19) | 68 (26–126) | 12 (8–30) | 2300 (70–13,990) | 1.3 (0.3–7.5) | 5 (3–7.3) | 2.9 (1.3–4.8) |
| lupus nephritis (n = 17) | 1.06 (0.77–2.19) | 68 (31–116) | 9 (7–23) | 1590 (180–5950) | 0.8 (0.3–3.1) | 5.5 (3.8–7.3) | 3.1 (2–4) |
| IgA nephropathy (n = 14) | 1.06 (0.71–1.82) | 70 (35–131) | 9.5 (7–20) | 940 (90–4540) | 0.6 (0.3–2.2) | 5.65 (4.4–6.5) | 3.4 (2.2–4) |
| mesangial proliferative (non-IgA) glomerulonephritis (n = 6) | 0.93 (0.59–1.55) | 105 (40–131) | 8.5 (6–16) | 2580 (620–7130) | 0.8 (0.4–2.9) | 4.8 (3.9–5.2) | 2.8 (1.6–3.2) |
| control group (n = 22) | 1.2 (0.9–1.3) | 63 (60–78) | 12 (9–16) | 0 (0–0) | 0 (0–0) | 7.4 (6.6–8.2) | 4.4 (3.5–5.2) |
| c-ANCA vasculitis (n = 40) | 1.81 (0.69–7.78) | 28 (7–126) | 19.8 (7–75) | 640 (60–19,000) | 0.4 (0.3–10.9) | 6.3 (5.3–7.1) | 3.6 (2.4–4.6) |
| p-ANCA vasculitis (n = 16) | 3.13 (0.79–9.04) | 19 (5–93)) | 30 (8–81) | 1730 (140–12,300) | 0.8 (0.3–5) | 5.95 (4.8–8.3) | 3.5 (2.8–4.3) |
| Diagnosis | Age (Years) | Sex (Percent of Females) | Hb (g/dL) | HCT (%) | Leukocytes (Number/Microlitre) |
|---|---|---|---|---|---|
| membranous nephropathy (n = 18) | 51.5 (28–69) | 45% | 13.5 (11.3–16) | 44.5 (38–52.6) | 6715 (2500–10,780) |
| focal and segmental glomerulosclerosis (n = 25) | 48 (19–74) | 44% | 13.9 (9.3–18) | 45.7 (36.8–55.2) | 7690 (4130–10,500) |
| lupus nephritis (n = 17) | 34 (19–66) | 53% | 13.2 (10.5–17.3) | 43 (34.5–56.9) | 6600 (4090–10,900) |
| IgA nephropathy (n = 14) | 45.5 (20–60) | 50% | 14.8 (12.2–16.5) | 48.6 (46–54.3) | 8420 (5360–10,900) |
| mesangial proliferative (non-IgA) glomerulonephritis (n = 6) | 28 (20–52) | 50% | 14.8 (10.1–18) | 45.2 (33.2–55) | 10,200 (6200–10,490) |
| control group (n = 22) | 44 (26–80) | 50% | 14.6 (12.3–16.9) | 45.7 (37.9–53) | 6270 (4280–7780) |
| c-ANCA vasculitis (n = 40) | 58 (21–81) | 55% | 12 (7.7–16.1) | 37.3 (27–49.7) | 6555 (3740–10,450) |
| p-ANCA vasculitis (n = 16) | 62 (37–87) | 44% | 9.6 (8.3–13.2) | 30.4 (28.4–42) | 4905 (3240–9620) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczak, M.; Heidecke, H.; Żabińska, M.; Janek, Ł.; Wronowicz, J.; Kujawa, K.; Schulze-Forster, K.; Marek-Bukowiec, K.; Gołębiowski, T.; Banasik, M. The Influence of Anti-PAR 1 and Anti-ACE 2 Antibody Levels on the Course of Specific Glomerulonephritis Types. J. Clin. Med. 2025, 14, 3178. https://doi.org/10.3390/jcm14093178
Szymczak M, Heidecke H, Żabińska M, Janek Ł, Wronowicz J, Kujawa K, Schulze-Forster K, Marek-Bukowiec K, Gołębiowski T, Banasik M. The Influence of Anti-PAR 1 and Anti-ACE 2 Antibody Levels on the Course of Specific Glomerulonephritis Types. Journal of Clinical Medicine. 2025; 14(9):3178. https://doi.org/10.3390/jcm14093178
Chicago/Turabian StyleSzymczak, Maciej, Harald Heidecke, Marcelina Żabińska, Łucja Janek, Jakub Wronowicz, Krzysztof Kujawa, Kai Schulze-Forster, Karolina Marek-Bukowiec, Tomasz Gołębiowski, and Mirosław Banasik. 2025. "The Influence of Anti-PAR 1 and Anti-ACE 2 Antibody Levels on the Course of Specific Glomerulonephritis Types" Journal of Clinical Medicine 14, no. 9: 3178. https://doi.org/10.3390/jcm14093178
APA StyleSzymczak, M., Heidecke, H., Żabińska, M., Janek, Ł., Wronowicz, J., Kujawa, K., Schulze-Forster, K., Marek-Bukowiec, K., Gołębiowski, T., & Banasik, M. (2025). The Influence of Anti-PAR 1 and Anti-ACE 2 Antibody Levels on the Course of Specific Glomerulonephritis Types. Journal of Clinical Medicine, 14(9), 3178. https://doi.org/10.3390/jcm14093178









