Pelvic and Perineal Reconstruction After Bowel, Gynecological or Sacral Tumor Resection: A Case Series
Abstract
:1. Introduction
2. Patients and Methods
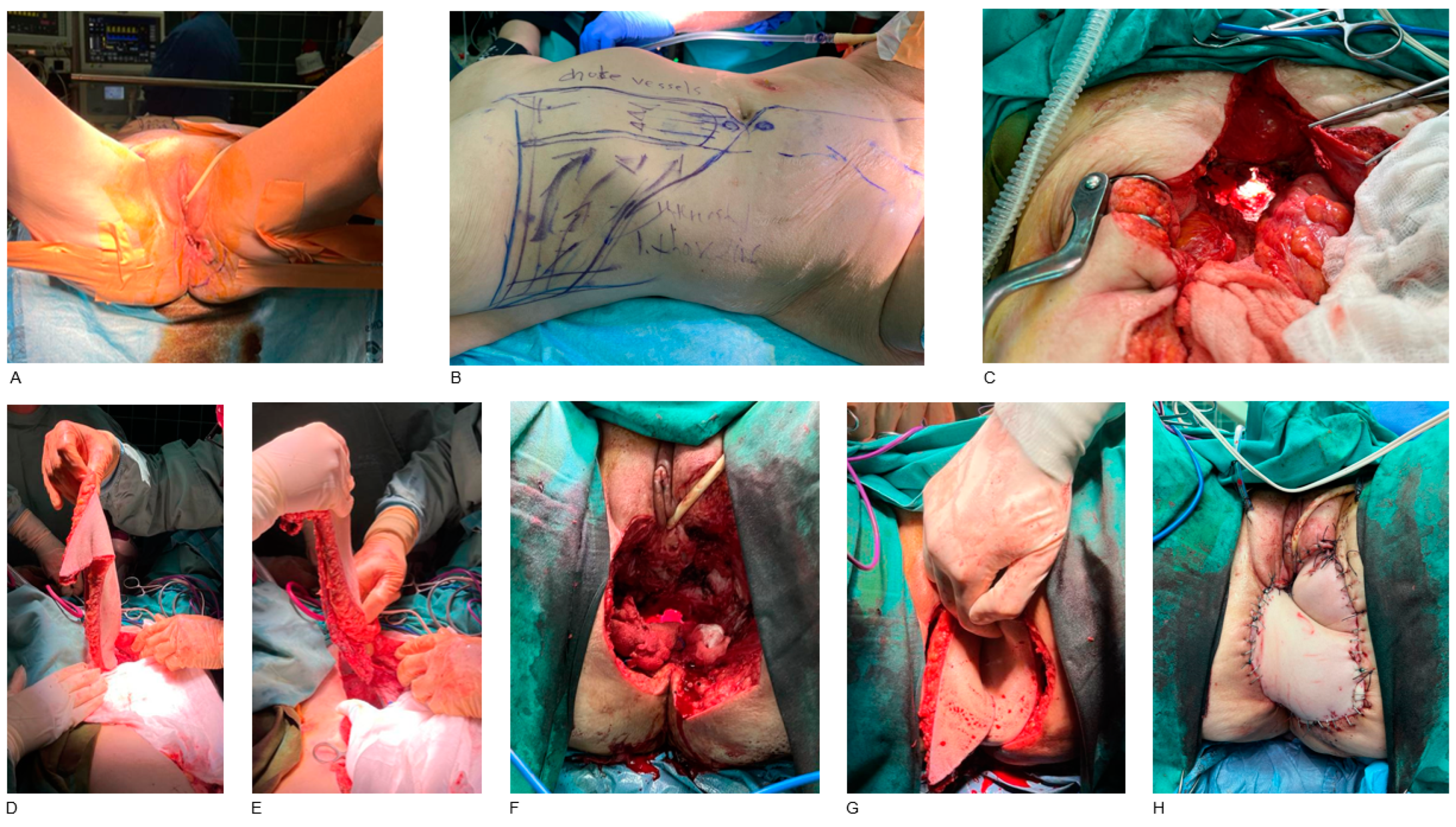
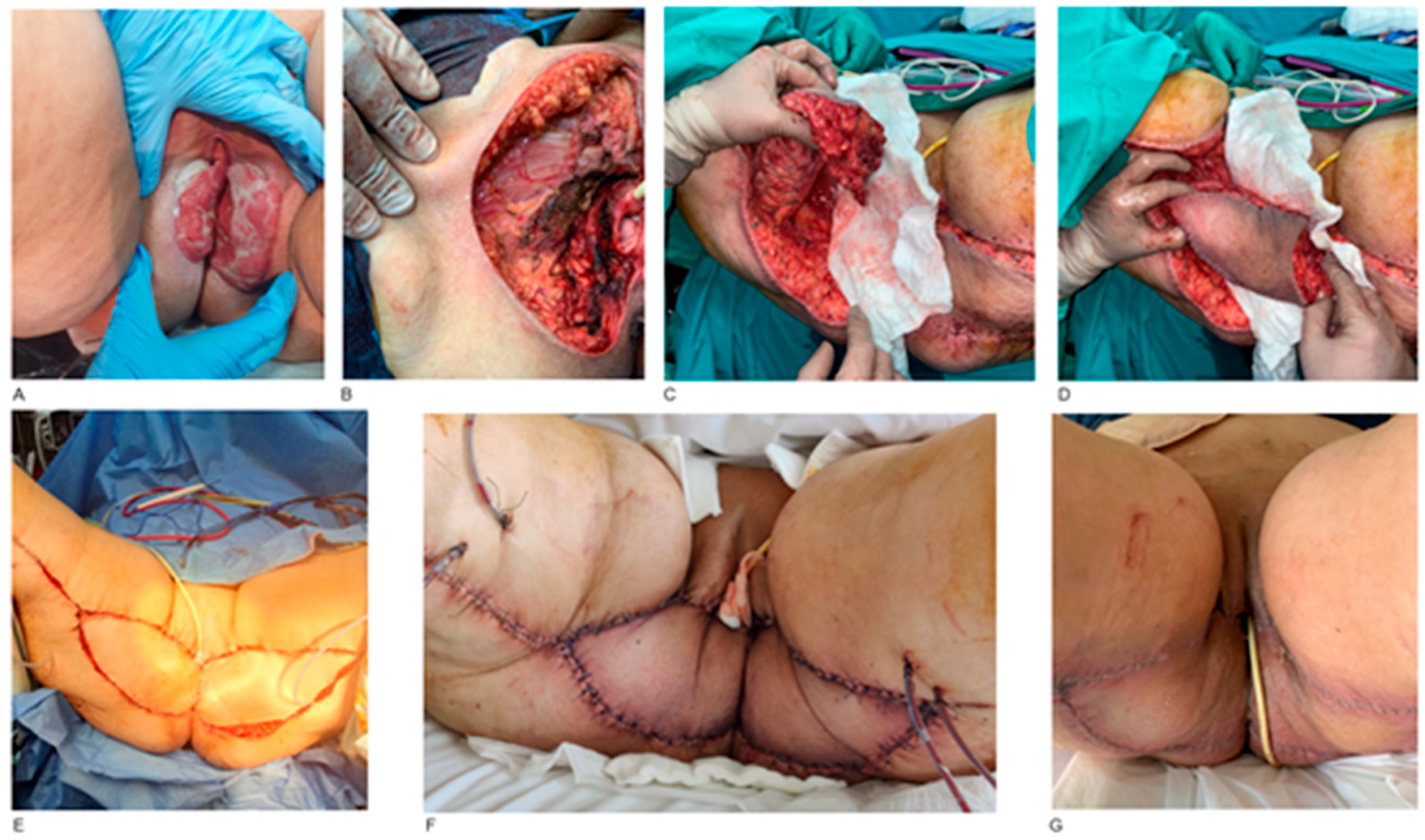
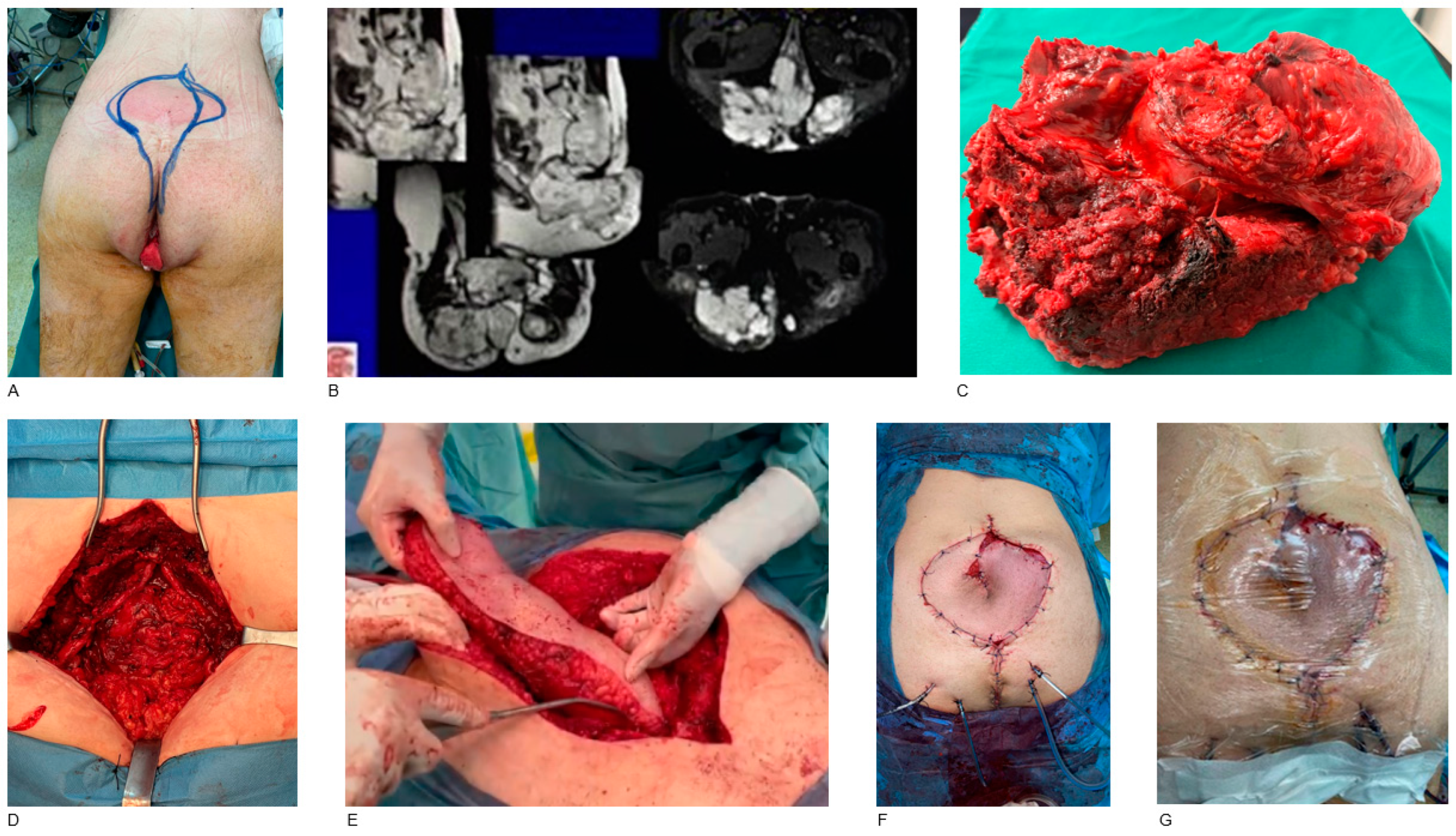
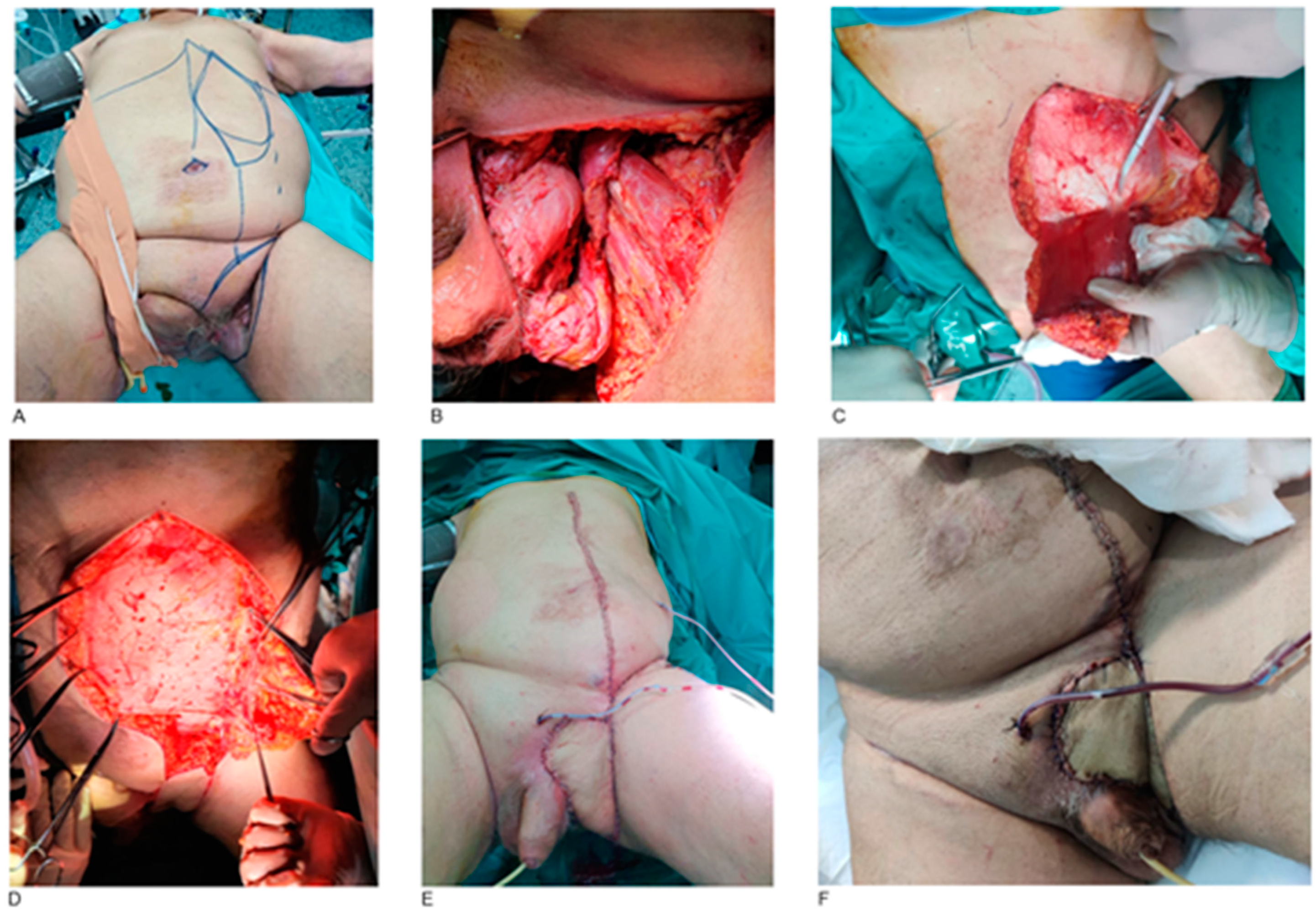
3. Results
4. Discussion
4.1. Anatomical Site and Histological Subtypes
- Rectum, anus and perianal area
- Vulva and vagina
- Sacrum
4.2. Reconstruction Methods
- External pelvis and perineum
- Vulva and vagina
- Internal pelvis and perineum
- Sacrum
- Free flaps
- Biological meshes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, D.S. Reconstruction of the perineum. Ann. Plast. Surg. 2014, 73 (Suppl. S1), S74–S81. [Google Scholar] [CrossRef] [PubMed]
- Papas, Y.; Laurent, R.; Efanov, I.J.; Paek, L.; Danino, M.A. Oversized lotus petal flap for reconstruction of extensive perineal defects following abdomino perineal resection. Ann. Chir. Plast. Esthet. 2022, 67, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Nassif, M.O.; Trabulsi, N.H.; Bullard Dunn, K.M.; Nahal, A.; Meguerditchian, A.N. Soft tissue tumors of the anorectum: Rare, complex and misunderstood. J. Gastrointest. Oncol. 2013, 4, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Sand, F.L.; Thomsen, S.F. Clinician’s Update on the Benign, Premalignant, and Malignant Skin Tumours of the Vulva: The Dermatologist’s View. Int. Sch. Res. Not. 2017, 2017, 2414569. [Google Scholar] [CrossRef] [PubMed]
- Deskoulidi, P.; Stavrianos, S.D.; Mastorakos, D.; Kontogeorgakos, V.A.; Savvidou, O.; Chrysikos, D.; Samolis, A.; Pappas, N.; Troupis, T.; Papagelopoulos, P.J. Anatomical Considerations and Plastic Surgery Reconstruction Options of Sacral Chordoma Resection. Cureus 2023, 15, e37965. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Govender, S. Sacral chordoma: A review of literature. J. Orthop. 2018, 15, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Dinh, T.; Potochny, J. Reconstruction of the perineum. Semin. Surg. Oncol. 2000, 19, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Schive, Ø.; Frich, L. Reconstruction of wound defects in the perineum. Tidsskr. Nor. Laegeforen. 2021, 141, 1451–1456. [Google Scholar] [CrossRef]
- Yii, N.W.; Niranjan, N.S. Lotus petal flaps in vulvo-vaginal reconstruction. Br. J. Plast. Surg. 1996, 49, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Kolehmainen, M.; Suominen, S.; Tukiainen, E. Pelvic, perineal and genital reconstructions. Scand. J. Surg. 2013, 102, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kinsley, S.; Huang, A.; Ricci, J.A.; Clancy, T.E.; Irani, J.; Goldberg, J.; Breen, E.; Bleday, R.; Talbot, S.G. Gracilis Flap Reconstruction of the Perineum: An Outcomes Analysis. J. Am. Coll. Surg. 2016, 223, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Pantelides, N.M.; Davies, R.J.; Fearnhead, N.S.; Malata, C.M. The gluteal fold flap: A versatile option for perineal reconstruction following anorectal cancer resection. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.R.; Weber, J.; Neeff, H.P.; Manegold, P.; Fichtner-Feigl, S.; Stark, G.B.; Eisenhardt, S.U. Reconstruction of Perineal Defects: A Comparison of the Myocutaneous Gracilis and the Gluteal Fold Flap in Interdisciplinary Anorectal Tumor Resection. Front. Oncol. 2020, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Jakubietz, R.G.; Jakubietz, M.G.; Meffert, R.H.; Holzapfel, B.; Schmidt, K. Pedicled anterolateral thigh flap reconstruction in the region of the lower abdomen, groin, perineum, and hip. Oper. Orthop. Traumatol. 2022, 34, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, I.; Abe, Y.; Nakanishi, H. The internal pudendal artery perforator flap: Free-style pedicle perforator flaps for vulva, vagina, and buttock reconstruction. Plast. Reconstr. Surg. 2014, 133, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.F.; Choi, J.Y.; Han, H.H.; Oh, D.H.; Rhie, J.W.; Ahn, S.T.; Moon, S.H. Perforators as recipients for free flap reconstruction of the inguinal and perineal region. Microsurgery 2015, 35, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Han, J.G.; Wang, Z.J.; Gao, Z.G.; Yang, Y.; Du, Y.F. Human acellular dermal matrix for pelvic floor reconstruction after cylindrical abdominoperineal resection. Hepatogastroenterology 2011, 58, 1205–1207. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.D.; Pathak, S.; Smart, N.J.; Branagan, G.; Longman, R.J.; Thomas, M.G.; Francis, N. Reconstruction of the perineum following extralevator abdominoperineal excision for carcinoma of the lower rectum: A systematic review. Colorectal. Dis. 2012, 14, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
| Anatomical Site and Gender | Histopathology | Reconstruction | Recurrence |
|---|---|---|---|
| Rectum-anus (M = 4, F = 12) | SCC (n = 16) | ORAM/VRAM (n = 16) | n = 13 |
| Perianal region (F = 1) | EMPD (n = 1) | IPAP (n = 1) | n = 0 |
| External genitalia (F = 5) | Buschke-Lowenstein (n = 5) | Gracilis (n = 3) IPAP (n = 1) Lotus petal (n = 1) | n = 1 |
| Vulva-Vagina (F = 4) | SCC (n = 4) | Gracilis (n = 4) | n = 2 |
| Sacrum (M = 4) | Chordomas (n = 4) | ORAM/VRAM (n = 4) | n = 3 |
| Inguinal fold (M = 2) | SCC (n = 2) | VRAM (n = 2) | n = 0 |
| Gluteal region (M = 1, F = 1) | mGCT (n = 1, male) | IGAP (n = 1) | n = 0 |
| Liposarcoma (n = 1, female) | IGAP (n = 1) | n = 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bini, A.; Stavrianos, S. Pelvic and Perineal Reconstruction After Bowel, Gynecological or Sacral Tumor Resection: A Case Series. J. Clin. Med. 2025, 14, 3172. https://doi.org/10.3390/jcm14093172
Bini A, Stavrianos S. Pelvic and Perineal Reconstruction After Bowel, Gynecological or Sacral Tumor Resection: A Case Series. Journal of Clinical Medicine. 2025; 14(9):3172. https://doi.org/10.3390/jcm14093172
Chicago/Turabian StyleBini, Aikaterini, and Spyridon Stavrianos. 2025. "Pelvic and Perineal Reconstruction After Bowel, Gynecological or Sacral Tumor Resection: A Case Series" Journal of Clinical Medicine 14, no. 9: 3172. https://doi.org/10.3390/jcm14093172
APA StyleBini, A., & Stavrianos, S. (2025). Pelvic and Perineal Reconstruction After Bowel, Gynecological or Sacral Tumor Resection: A Case Series. Journal of Clinical Medicine, 14(9), 3172. https://doi.org/10.3390/jcm14093172






