Outcomes of Post-Keratoplasty Microbial Keratitis: A 16-Year Analysis
Abstract
1. Introduction
2. Methods
- Full-thickness corneal transplant: Penetrating keratoplasty (PK);
- Posterior lamellar keratoplasty: Descemet stripping automated endothelial keratoplasty (DSAEK);
- Anterior lamellar keratoplasty: Deep anterior lamellar keratoplasty (DALK).
2.1. Data Collection
- (a)
- Topical therapy: antibacterial treatment included either monotherapy (using a fluoroquinolone, aminoglycoside, or chloramphenicol) or combination therapy (gentamicin plus vancomycin, or a fluoroquinolone combined with an aminoglycoside). Additional topical therapies comprised antifungal, antiviral, anti-amoebic agents, and corticosteroids.
- (b)
- Systemic therapy: immunosuppression.
2.2. Statistical Methods
3. Results
3.1. Demographics
3.2. Risk Factors and Clinical Characteristic
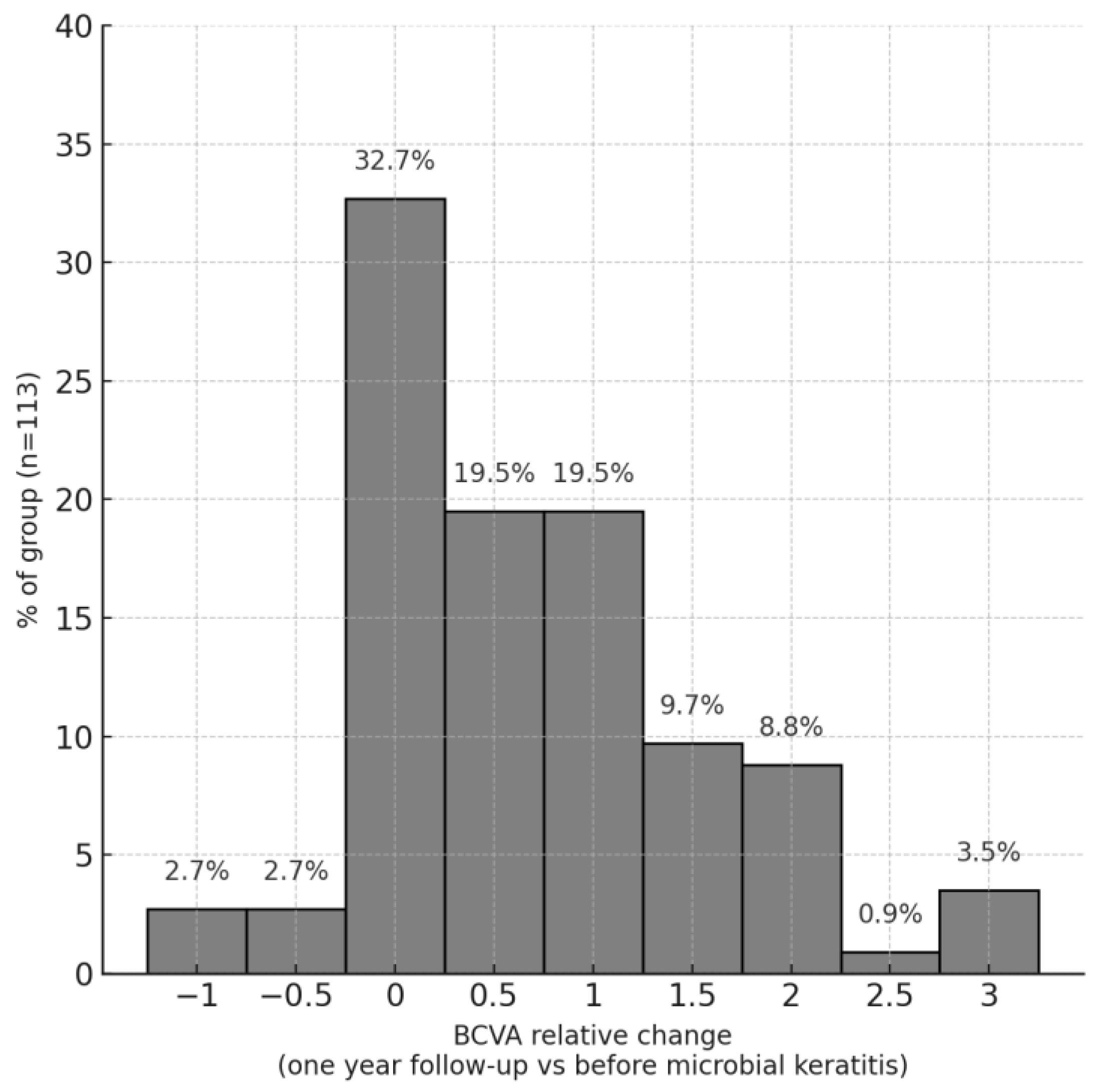
3.3. Visual Acuity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drzyzga, K.; Krupka-Matuszczyk, I.; Drzyzga, Ł.; Mrukwa-Kominek, E.; Kucia, K. Quality of Life and Mental State After Sight Restoration by Corneal Transplantation. Psychosomatics 2016, 57, 414–422. [Google Scholar] [CrossRef]
- Gómez-Benlloch, A.; Montesel, A.; Pareja-Aricò, L.; Mingo-Botín, D.; Michael, R.; Barraquer, R.I.; Alió, J. Causes of corneal transplant failure: A multicentric study. Acta Ophthalmol. 2021, 99, E922–E928. [Google Scholar] [CrossRef]
- Dave, T.V.; Dave, V.P.; Sharma, S.; Karolia, R.; Joseph, J.; Pathengay, A.; Pappuru, R.R.; Das, T. Infectious endophthalmitis leading to evisceration: Spectrum of bacterial and fungal pathogens and antibacterial susceptibility profile. J. Ophthalmic Inflamm. Infect. 2019, 9, 9. [Google Scholar] [CrossRef]
- Sun, J.-P.; Chen, W.-L.; Huang, J.-Y.; Hou, Y.-C.; Wang, I.-J.; Hu, F.-R. Microbial Keratitis After Penetrating Keratoplasty. Arch. Ophthalmol. 2017, 178, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Vreijsen, E.M.; Elsman, E.B.M.M.; van Nispen, R.M.A.; Nuijts, R.M.M.A.; van Rens, G.H.M.B. Effect of Corneal Transplantation on Patient-Reported Outcomes and Potential Predictors: A Systematic Review. Cornea 2020, 39, 1463–1472. [Google Scholar] [CrossRef]
- Quilendrino, R.; de Mora, M.R.-C.; Baydoun, L.; Ham, L.; van Dijk, K.; Dapena, I.; Oellerich, S.; Melles, G.R.J. Prevention and Management of Descemet Membrane Endothelial Keratoplasty Complications. Cornea 2017, 36, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Song, P.; Echegaray, J.J.; Jia, Y.; Li, S.; Du, M.; Perez, V.L.; Shi, W. Big Bubble Deep Anterior Lamellar Keratoplasty for Management of Deep Fungal Keratitis. J. Ophthalmol. 2014, 2014, 209759. [Google Scholar] [CrossRef] [PubMed]
- Davila, J.R.; Mian, S.I. Infectious keratitis after keratoplasty. Curr. Opin. Ophthalmol. 2016, 27, 358–366. [Google Scholar] [CrossRef]
- Przybek-Skrzypecka, J.; Ryk-Adamska, M.; Szewczuk, A.; Skrzypecki, J.; Izdebska, J.; Udziela, M.; Rypniewska, A.; Suh, L.H.; Szaflik, J.P. Severe Microbial Keratitis in Virgin and Transplanted Cornea—Probability of Visual Acuity Improvement. J. Clin. Med. 2024, 14, 124. [Google Scholar] [CrossRef]
- Dohse, N.; Wibbelsman, T.D.; Rapuano, S.B.; Hammersmith, K.M.; Nagra, P.K.; Rapuano, C.J.; Syed, Z.A. Microbial keratitis and clinical outcomes following penetrating and endothelial keratoplasty. Acta Ophthalmol. 2020, 98, E895–E900. [Google Scholar] [CrossRef]
- Constantinou, M.; Jhanji, V.; Vajpayee, R.B. Clinical and Microbiological Profile of Post-Penetrating Keratoplasty Infectious Keratitis in Failed and Clear Grafts. Arch. Ophthalmol. 2013, 155, 233–237.e2. [Google Scholar] [CrossRef]
- Zafar, S.; Wang, P.; Woreta, F.A.; Aziz, K.; Makary, M.; Ghous, Z.; Srikumaran, D. Postoperative Complications in Medicare Beneficiaries Following Endothelial Keratoplasty Surgery. Arch. Ophthalmol. 2020, 219, 1–11. [Google Scholar] [CrossRef]
- Wagoner, M.D.; Al-Swailem, S.A.; Sutphin, J.E.; Zimmerman, M.B. Bacterial Keratitis after Penetrating Keratoplasty. Ophthalmology 2007, 114, 1073–1079.e2. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.; Walkden, A.; Okonkwo, A.; Au, L.; Brahma, A.; Carley, F. Microbial Keratitis in Corneal Transplants: A 12-Year Analysis. Clin. Ophthalmol. 2020, 14, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Deshmukh, R.; Lin, H.; Ang, M.; Mehta, J.S.; Chodosh, J.; Said, D.G.; Dua, H.S.; Ting, D.S.J. Post-keratoplasty Infectious Keratitis: Epidemiology, Risk Factors, Management, and Outcomes. Front. Med. 2021, 8, 707242. [Google Scholar] [CrossRef] [PubMed]
- Ittah-Cohen, I.; Knoeri, M.J.; Bourcier, T.; Merabet, L.; Bouheraoua, N.; Borderie, V.M. Infectious keratitis following corneal transplantation: A long-term cohort study. Clin. Exp. Ophthalmol. 2024, 52, 402–415. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, S.Y.; Jeong, H.J.; Kim, H.J.; Kim, M.K.; Oh, J.Y. Glucocorticoids induce corneal allograft tolerance through expansion of monocytic myeloid-derived suppressor cells. Am. J. Transplant. 2018, 18, 3029–3037. [Google Scholar] [CrossRef]
- Williams, K.A.; Lowe, M.; Bartlett, C.; Kelly, T.-L.; Coster, D.J. Risk factors for human corneal graft failure within the Australian corneal graft registry. Transplantation 2008, 86, 1720–1724. [Google Scholar] [CrossRef]
- Major, J.; Foroncewicz, B.; Szaflik, J.P.; Mucha, K. Immunology and Donor-Specific Antibodies in Corneal Transplantation. Arch. Immunol. Ther. Exp. 2021, 69, 32. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Lee, S.-M.; Sung, J.; Niutta, M.; Coassin, M.; Mashaghi, A.; Inomata, T. Variable Responses to Corneal Grafts: Insights from Immunology and Systems Biology. J. Clin. Med. 2020, 9, 586. [Google Scholar] [CrossRef]
- Sharma, N.; Sachdev, R.; Jhanji, V.; Titiyal, J.S.; Vajpayee, R.B. Therapeutic keratoplasty for microbial keratitis. Curr. Opin. Ophthalmol. 2010, 21, 293–300. [Google Scholar] [CrossRef]
- Ong, Z.Z.; Wong, T.L.; Suresh, L.; Hammoudeh, Y.; Lister, M.; Said, D.G.; Dua, H.S.; Ting, D.S.J. A 7-year review of clinical characteristics, predisposing factors and outcomes of post-keratoplasty infectious keratitis: The Nottingham infectious keratitis study. Front. Cell. Infect. Microbiol. 2023, 13, 1250599. [Google Scholar] [CrossRef]
- Veugen, J.M.J.; Dunker, S.L.; Wolffs, P.F.G.; Savelkoul, P.H.M.; Winkens, B.; Biggelaar, F.J.H.M.v.D.; Nuijts, R.M.M.A.; Dickman, M.M.; Netherlands Cornea Transplant Network (NCTN). Corneal Transplantation for Infectious Keratitis: A Prospective Dutch Registry Study. Cornea 2023, 42, 1414–1421. [Google Scholar] [CrossRef]
- Sati, A.; Wagh, S.; Mishra, S.K.; Kumar, S.V.; Kumar, P. Post-corneal transplant Candida keratitis—Incidence and outcome. Indian J. Ophthalmol. 2022, 70, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Agrawal, D. Recurrence of Infection in Corneal Grafts After Therapeutic Penetrating Keratoplasty for Microbial Keratitis. Cornea 2019, 39, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Yoon, C.H.; Kim, M.K.; Oh, J.Y. The Incidence and Outcomes of Recurrence of Infection after Therapeutic Penetrating Keratoplasty for Medically-Uncontrolled Infectious Keratitis. J. Clin. Med. 2020, 9, 3696. [Google Scholar] [CrossRef]
- Tixier, J.; Bourcier, T.; Borderie, V.; Laroche, L. Infectious keratitis after penetrating keratoplasty. J. Fr. Ophtalmol. 2001, 24, 597–602. [Google Scholar] [PubMed]
- Chen, H.-C.; Lee, C.-Y.; Lin, H.-Y.; Ma, D.H.-K.; Chen, P.Y.-F.; Hsiao, C.-H.; Lin, H.-C.; Yeh, L.-K.; Tan, H.-Y. Shifting trends in microbial keratitis following penetrating keratoplasty in Taiwan. Medicine 2017, 96, e5864. [Google Scholar] [CrossRef]
- Harris, D.J.; Stulting, R.D.; Waring, G.O.; Wilson, L.A. Late Bacterial and Fungal Keratitis after Corneal Transplantation. Ophthalmology 1988, 95, 1450–1457. [Google Scholar] [CrossRef]
- Moorthy, S.; Graue, E.; Jhanji, V.; Constantinou, M.; Vajpayee, R.B. Microbial Keratitis After Penetrating Keratoplasty: Impact of Sutures. Arch. Ophthalmol. 2011, 152, 189–194.e2. [Google Scholar] [CrossRef]
- Ti, S.-E.; Scott, J.A.; Janardhanan, P.; Tan, D.T. Therapeutic Keratoplasty for Advanced Suppurative Keratitis. Arch. Ophthalmol. 2007, 143, 755–762.e2. [Google Scholar] [CrossRef] [PubMed]
- Anitha, V.; Ravindran, M.; Jacob, A.D.; Ghorpade, A.; Uduman, M.S. Outcomes of optical penetrating keratoplasty with the large sized donor for failed therapeutic grafts for infectious keratitis from the tertiary eye care center, South India. Eur. J. Ophthalmol. 2022, 32, 3411–3419. [Google Scholar] [CrossRef] [PubMed]
- Vajpayee, R.B.; Boral, S.K.; Dada, T.; Murthy, G.V.S.; Pandey, R.M.; Satpathy, G. Risk factors for graft infection in India: A case-control study. Br. J. Ophthalmol. 2002, 86, 261–265. [Google Scholar] [CrossRef]
- Lamas-Francis, D.; Navarro, D.; Mansilla, R.; De-Rojas, V.; Moreno, C.; Dios, E.; Rigueiro, J.; Álvarez, D.; Crego, P.; Rodríguez-Ares, T.; et al. Evaluating Medical Therapy Failure in Microbial Keratitis: Risk Factors and Management Alternatives. Ocul. Immunol. Inflamm. 2024, 33, 641–648. [Google Scholar] [CrossRef] [PubMed]

| Variable | Whole Cohort | Penetrating Keratoplasty (PKP) | Anterior Lamellar Keratoplasty (DALK) | Posterior Lamellar Keratoplasty (DSAEK) | p * |
|---|---|---|---|---|---|
| Number of transplants performed in our centre in 2008–2023, n (%) | 2869 & | 1897 (66.3) | 114 (4) | 821 (28.6) | - |
| Number of microbial keratitis cases, n (%) | 125 (100.0) | 112 (89.6) | 10 (8) | 3 (2.4) | - |
| Age at microbial keratitis, mean ± SD | 62.73 ± 17.45 | 63.21 ± 17.17 | 53.30 ± 19.73 | 76.00 ± 5.29 | 0.086 1 |
| Sex, female, n (%) | 64 (51.2) | 58 (51.8) | 4 (40.0) | 2 (66.7) | 0.701 3 |
| Eye, right, n (%) | 62 (50.4) | 58 (52.7) | 3 (30.0) | 1 (33.3) | 0.296 3 |
| Systemic autoimmunological disease, n (%) | 41 (32.8) | 39 (34.8) | 1 (10.0) | 1 (33.3) | 0.163 |
| Treatment mode, n (%) | |||||
| Intpatient | 114 (93.4) | 102 (93.6) | 9 (90.0) | 3 (100.0) | 0.516 |
| Outpatient | 8 (6.6) | 7 (6.4) | 1 (10.0) | 0 (0.0) | |
| Hospitalization, days, median (IQR) | 9.00 (7.00; 12.00) | 9.00 (7.00; 12.00) | 3.00 (2.00; 12.00) | 13.00 (10.50; 17.00) | 0.200 2 |
| Variable | Whole Cohort | Penetrating Keratoplasty (PK) | Anterior Lamellar Keratoplasty (DALK) | Posterior Lamellar Keratoplasty (DSAEK) | p |
|---|---|---|---|---|---|
| Number of microbial keratitis cases, n (%) | 125 (100.0) | 112 (100.0) | 10 (100.0) | 3 (100.0) | - |
| Recurrent infections, n (%) | 72 (57.6) | 67 (59.8) | 4 (40.0) | 1 (33.3) | 0.317 |
| Time from the last transplant to microbial keratitis, months, median (IQR) | 17.00 (6.00; 40.00) | 17.00 (5.75; 39.25) | 18.50 (11.25; 57.50) | 7.00 (6.00; 45.00) | 0.611 |
| Time from the first transplant to microbial keratitis, months, median (IQR) | 76.00 (34.25; 143.75) | 81.00 (35.00; 144.00) | 12.00 # (12.00; 12.00) | - | 0.169 |
| Indication for transplantation, n (%) | |||||
| Active infection | 65 (52.0) | 59 (52.7) | 6 (60.0) | 0 (0.0) | 0.196 |
| Post-infection corneal scar | 13 (10.4) | 11 (9.8) | 2 (20.0) | 0 (0.0) | |
| Keratoconus | 12 (9.6) | 12 (10.7) | 0 (0.0) | 0 (0.0) | |
| Bullous keratopathy | 22 (17.6) | 19 (17.0) | 0 (0.0) | 3 (100.0) | |
| Immunological | 3 (2.4) | 2 (1.8) | 1 (10.0) | 0 (0.0) | |
| Chemical burn | 5 (4.0) | 5 (4.5) | 0 (0.0) | 0 (0.0) | |
| Corneal dystrophy | 1 (0.8) | 1 (0.9) | 0 (0.0) | 0 (0.0) | |
| Other | 4 (3.2) | 3 (2.7) | 1 (10.0) | 0 (0.0) |
| Variable | Whole Cohort | Penetrating Keratoplasty (PKP) | Anterior Lamellar Keratoplasty (DALK) | Posterior Lamellar Keratoplasty (DSAEK) | p |
|---|---|---|---|---|---|
| Contact lens use, n (%) | 2 (1.8) | 2 (2.0) | 0 (0.0) | 0 (0.0) | >0.999 |
| Trauma, n (%) | 3 (2.7) | 3 (3.0) | 0 (0.0) | 0 (0.0) | >0.999 |
| Glaucoma topical treatment, n (%) | 62 (52.5) | 55 (51.4) | 4 (50.0) | 3 (100.0) | >0.999 |
| Glaucoma surgical history, n (%) | 28 (23.9) | 23 (21.7) | 3 (37.5) | 2 (66.7) | 0.380 |
| Broken/loose suture, n (%) | 5 (4.0) | 4 (3.6) | 1 (10.0) | 0 (0.0) | 0.353 |
| Dry eye syndrome, n (%) | 41 (32.8) | 37 (33.0) | 3 (30.0) | 1 (33.3) | >0.999 |
| Recurrent erosions, n (%) | 46 (36.8) | 42 (37.5) | 2 (20.0) | 2 (66.7) | 0.327 |
| Local immunosuppression at the time of infection, n (%) | 66 (56.4) | 62 (58.5) | 2 (25.0) | 2 (66.7) | 0.135 |
| Systemic immunosuppression, n (%) | 59 (50.4) | 58 (54.7) | 1 (12.5) | 0 (0.0) | 0.028 |
| Systemic immunosuppression—type, n (%) | |||||
| MFM & | 18 (30.5) | 18 (31.0) | 0 (0.0) | 0 (0.0) | 0.305 |
| Steroids | 14 (23.7) | 13 (22.4) | 1 (100.0) | 0 (0.0) | |
| MFM + steroids | 23 (39.0) | 23 (39.7) | 0 (0.0) | 0 (0.0) | |
| Others | 4 (6.8) | 4 (6.9) | 0 (0.0) | 0 (0.0) | |
| Previous immunosuppression, n (%) | 59 (74.7) | 52 (74.3) | 4 (66.7) | 3 (100.0) | 0.651 |
| Previous immunosuppression—type, n (%) | |||||
| Steroids | 20 (45.5) | 15 (39.5) | 2 (66.7) | 3 (100.0) | 0.756 |
| MFM | 6 (13.6) | 6 (15.8) | 0 (0.0) | 0 (0.0) | |
| MFM + steroids | 18 (40.9) | 17 (44.7) | 1 (33.3) | 0 (0.0) | |
| Time from ceased immunosuppression, months, median (IQR) | 22.50 (8.50; 43.50) | 22.50 (10.75; 36.00) | 46.00 (23.50; 109.00) | 4.00 (2.50; 37.00) | 0.616 |
| Active uveitis, n (%) | 29 (23.2) | 28 (25.0) | 0 (0.0) | 1 (33.3) | 0.114 |
| Therapeutic transplant, n (%) | 19 (17.3) | 18 (18.4) | 0 (0.0) | 1 (50.0) | 0.208 |
| Variable | Whole Cohort | Penetrating on IMT | No IMT | p |
|---|---|---|---|---|
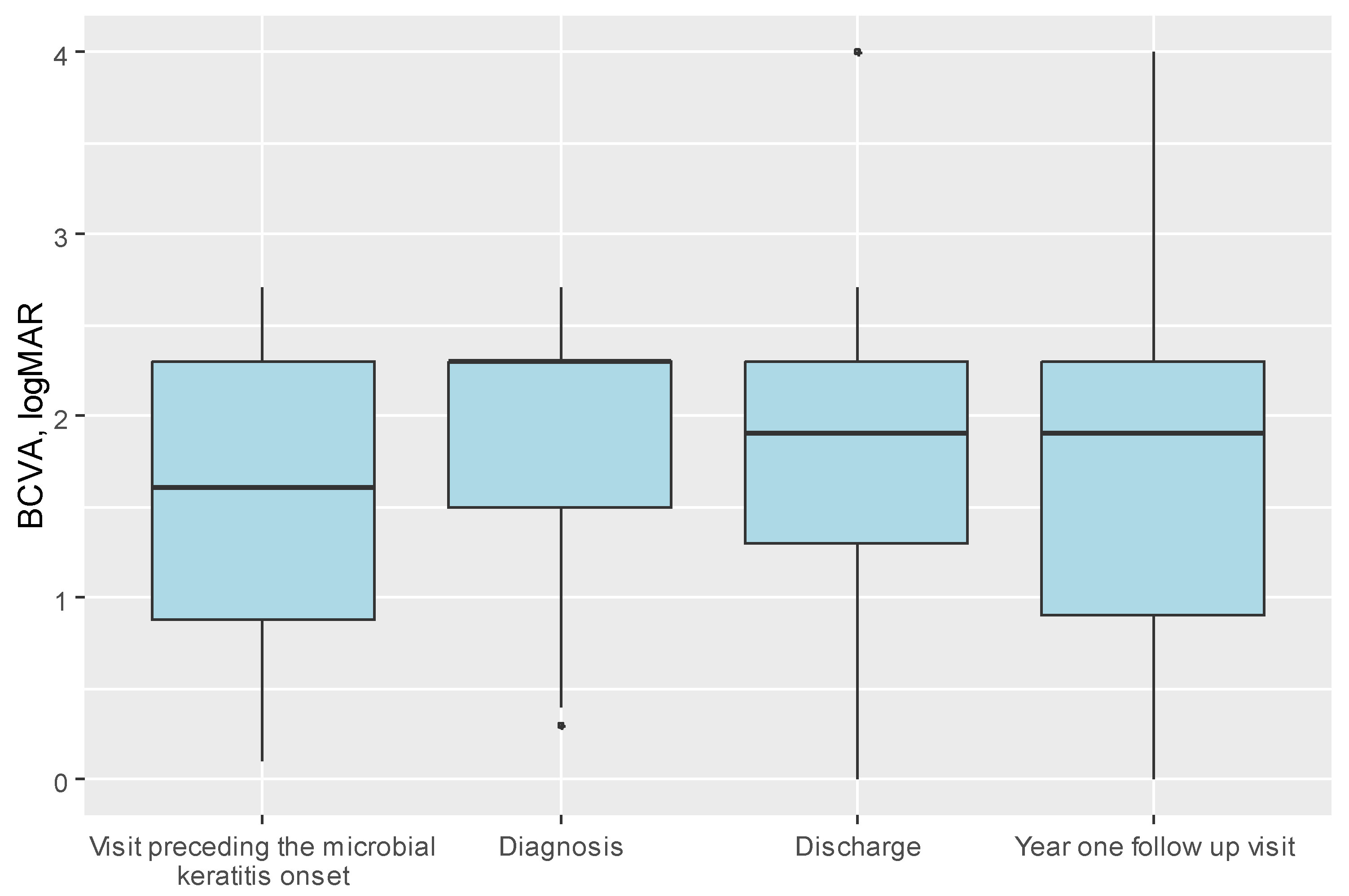
| BCVA—diagnosis, median (IQR) | 2.30 (1.50; 2.30) | 1.90 (1.23; 2.30) | 2.30 (1.60; 2.70) | 0.006 1 |
| BCVA—discharge, median (IQR) | 1.90 (1.30; 2.30) | 1.90 (1.30; 2.30) | 2.30 (1.50; 2.40) | 0.052 1 |
| BCVA—year one follow up visit, median (IQR) | 1.90 (0.90; 2.30) | 1.90 (0.70; 2.30) | 1.90 (0.90; 2.40) | 0.639 1 |
| BCVA—visit preceding the microbial keratitis onset, median (IQR) | 1.60 (0.88; 2.30) | 1.30 (0.80; 1.90) | 1.60 (1.00; 2.30) | 0.022 1 |
| Lens status, n (%) | ||||
| Phacic, transparent PCIOL ACIOL Cataract Aphacic | 26 (22.6) | 15 (26.3) | 11 (19.3) | 0.230 |
| 65 (56.5) | 33 (57.9) | 32 (56.1) | ||
| 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 13 (11.3) | 7 (12.3) | 6 (10.5) | ||
| 11 (9.6) | 2 (3.5) | 8 (14.0) | ||
| Decompensated graft prior to IK, n (%) | 64 (54.7) | 34 (57.6) | 30 (51.7) | 0.649 |
| Recurrent erosions, n (%) | 46 (36.8) | 26 (44.1) | 20 (34.5) | 0.383 |
| Dry eye syndrome, n (%) | 41 (32.8) | 20 (33.9) | 21 (36.2) | 0.946 |
| Perforation, n (%) | 16 (12.8) | 6 (10.2) | 9 (15.5) | 0.556 |
| Number of lesions, n (%) | ||||
| Multifocal Monofocal | 15 (12.8) | 7 (12.7) | 8 (14.5) | >0.999 |
| 102 (87.2) | 48 (87.3) | 47 (85.5) | ||
| Therapeutic transplant, n (%) | 19 (17.3) | 6 (11.5) | 11 (21.6) | 0.269 |
| Topical treatment, n (%) | ||||
| Monotherapy Politherapy | 22 (18.0) | 10 (16.9) | 11 (19.6) | 0.895 |
| 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Evisceration, n (%) | 10 (8.0) | 6 (10.2) | 4 (6.9) | 0.762 |
| Part of the cornea affected, n (%) | ||||
| Central Midperipheral Peripheral Whole cornea | 61 (54.0) | 29 (54.7) | 29 (54.7) | 0.736 2 |
| 24 (21.2) | 9 (17.0) | 13 (24.5) | ||
| 23 (20.4) | 12 (22.6) | 9 (17.0) | ||
| 5 (4.4) | 3 (5.7) | 2 (3.8) |
| Variable | Whole Cohort | Penetrating Keratoplasty (PKP) | Anterior Lamellar Keratoplasty (DALK) | Posterior Lamellar Keratoplasty (DSAEK) | p |
|---|---|---|---|---|---|
| BCVA—diagnosis, median (IQR) | 2.30 (1.50; 2.30) | 2.30 (1.52; 2.30) | 1.30 (0.50; 2.30) | 2.30 (2.10; 2.30) | 0.094 |
| BCVA—discharge, median (IQR) | 1.90 (1.30; 2.30) | 1.90 (1.30; 2.30) | 1.75 (0.50; 2.40) | 1.90 (1.90; 2.10) | 0.550 |
| BCVA—year one follow-up visit, median (IQR) | 1.90 (0.90; 2.30) | 1.90 (0.90; 2.30) | 2.10 (1.43; 3.03) | 1.40 (1.15; 1.65) | 0.439 |
| BCVA—visit preceding the microbial keratitis onset, median (IQR) | 1.60 (0.88; 2.30) | 1.60 (0.90; 2.30) | 1.30 (1.15; 2.00) | 1.30 (1.00; 1.30) | 0.714 |
| Variable | One Corneal Transplant | Multiple Corneal Transplants (2–5 Grafts) | p |
|---|---|---|---|
| Therapeutic transplant, n (%) | 11 (15.3) | 7 (19.4) | 0.784 |
| Amniotic membrane, n (%) | 17 (20.7) | 7 (17.5) | 0.858 |
| Evisceration, n (%) | 8 (9.8) | 2 (5.0) | 0.495 1 |
| Topical treatment, n (%) | |||
| Monotherapy Politherapy | 17 (21.0) | 5 (12.8) | 0.406 |
| 64 (79.0) | 34 (87.2) | ||
| General treatment | 47 (57.3) | 22 (55.0) | 0.962 |
| Variable | Univariate Model | Multivariate Model | ||||||
|---|---|---|---|---|---|---|---|---|
| ß | 95% CI for ß | Std. ß | p | ß | 95% CI for ß | Std. ß | p | |
| Age, years | 0.00 | −0.01 to 0.01 | 0.06 | 0.523 | - | - | - | - |
| Sex, female (vs. male) | −0.31 | −0.61 to −0.01 | −0.39 | 0.041 | - | - | - | - |
| Transplant type | ||||||||
| DALK (vs. PKP) | 0.53 | −0.06 to 1.11 | 0.65 | 0.077 | 0.65 | 0.09 to 1.21 | 0.83 | 0.024 |
| DSAEK (vs. PKP) | 0.16 | −0.97 to 1.30 | 0.20 | 0.775 | −0.30 | −1.71 to 1.11 | −0.38 | 0.677 |
| Number of transplants | −0.05 | −0.24 to 0.15 | −0.05 | 0.633 | - | - | - | - |
| Hospitalization, days | −0.02 | −0.05 to 0.00 | −0.16 | 0.109 | - | - | - | - |
| Recurrent infections | 0.32 | 0.01 to 0.63 | 0.40 | 0.040 | - | - | - | - |
| Time from the last transplant to microbial keratitis, months | 0.00 | 0.00 to 0.00 | −0.11 | 0.265 | - | - | - | - |
| Time from the first transplant to microbial keratitis, months | 0.00 | 0.00 to 0.00 | −0.32 | 0.072 | - | - | - | - |
| Indication | ||||||||
| Post-infection corneal scar (vs. active infection) | 0.01 | −0.49 to 0.52 | 0.02 | 0.960 | - | - | - | - |
| Keratoconus (vs. active infection) | −0.53 | −1.05 to −0.01 | −0.65 | 0.047 | - | - | - | - |
| Bullous keratopathy (vs. active infection) | −0.31 | −0.73 to 0.11 | −0.39 | 0.141 | - | - | - | - |
| Immunological (vs. active infection) | −0.76 | −1.70 to 0.18 | −0.94 | 0.110 | - | - | - | - |
| Chemical burn (vs. active infection) | −0.39 | −1.13 to 0.35 | −0.48 | 0.299 | - | - | - | - |
| Other (vs. active infection) | −0.35 | −1.17 to 0.47 | −0.44 | 0.394 | - | - | - | - |
| CL use | −0.05 | −1.21 to 1.11 | −0.07 | 0.927 | - | - | - | - |
| Trauma | −0.21 | −1.16 to 0.74 | −0.26 | 0.664 | - | - | - | - |
| Dry eye syndrome | −0.13 | −0.45 to 0.19 | −0.16 | 0.426 | - | - | - | - |
| Recurrent erosions | 0.04 | −0.27 to 0.35 | 0.05 | 0.785 | - | - | - | - |
| Perforation | 0.63 | 0.20 to 1.06 | 0.78 | 0.005 | 0.77 | 0.36 to 1.17 | 0.98 | <0.001 |
| Local immunosuppression at the time of infection | −0.24 | −0.54 to 0.06 | −0.29 | 0.123 | - | - | - | - |
| Systematic immunosuppression | 0.25 | −0.04 to 0.55 | 0.32 | 0.094 | 0.45 | 0.16 to 0.74 | 0.57 | 0.003 |
| Systematic immunosuppression—type | ||||||||
| Steroids (vs. MFM) | −0.06 | −0.69 to 0.56 | −0.07 | 0.843 | - | - | - | - |
| MFM + steroids (vs. MFM) | −0.04 | −0.58 to 0.50 | −0.05 | 0.873 | - | - | - | - |
| Others (vs. MFM) | 0.09 | −0.98 to 1.17 | 0.11 | 0.860 | - | - | - | - |
| Previous immunosuppression | 0.10 | −0.28 to 0.49 | 0.14 | 0.591 | - | - | - | - |
| Time from ceased immunosuppression, months | 0.00 | −0.01 to 0.00 | −0.09 | 0.619 | - | - | - | - |
| Active uveitis | 0.18 | −0.18 to 0.53 | 0.22 | 0.332 | - | - | - | - |
| Therapeutic transplant | −0.38 | −0.79 to 0.03 | −0.49 | 0.067 | - | - | - | - |
| BCVA—diagnosis | 0.13 | −0.11 to 0.36 | 0.11 | 0.282 | - | - | - | - |
| Topical treatment, monotherapy (vs. polytherapy) | −0.13 | −0.52 to 0.26 | −0.16 | 0.522 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybek-Skrzypecka, J.; Ryk-Adamska, M.; Skrzypecki, J.; Izdebska, J.; Udziela, M.; Major, J.; Szaflik, J.P. Outcomes of Post-Keratoplasty Microbial Keratitis: A 16-Year Analysis. J. Clin. Med. 2025, 14, 3165. https://doi.org/10.3390/jcm14093165
Przybek-Skrzypecka J, Ryk-Adamska M, Skrzypecki J, Izdebska J, Udziela M, Major J, Szaflik JP. Outcomes of Post-Keratoplasty Microbial Keratitis: A 16-Year Analysis. Journal of Clinical Medicine. 2025; 14(9):3165. https://doi.org/10.3390/jcm14093165
Chicago/Turabian StylePrzybek-Skrzypecka, Joanna, Małgorzata Ryk-Adamska, Janusz Skrzypecki, Justyna Izdebska, Monika Udziela, Joanna Major, and Jacek P. Szaflik. 2025. "Outcomes of Post-Keratoplasty Microbial Keratitis: A 16-Year Analysis" Journal of Clinical Medicine 14, no. 9: 3165. https://doi.org/10.3390/jcm14093165
APA StylePrzybek-Skrzypecka, J., Ryk-Adamska, M., Skrzypecki, J., Izdebska, J., Udziela, M., Major, J., & Szaflik, J. P. (2025). Outcomes of Post-Keratoplasty Microbial Keratitis: A 16-Year Analysis. Journal of Clinical Medicine, 14(9), 3165. https://doi.org/10.3390/jcm14093165





