Modifiable and Non-Modifiable Risk Factors and Vascular Damage Progression in Type 2 Diabetes: A Primary Care Analysis
Abstract
1. Introduction
2. Materials and Methods
- For each parameter, 0 was assigned in case of absence, 1 in case of mild–moderate, 2 in case of severe disease.
- Arterial hypertension was graded with score 1 assigned to first–second and 2 to third stage.
- Diabetic nephropathy was classified as mild–moderate in the presence of proteinuria ≤3.0 g/d with modest reduction in renal function (GFR > 30 mL/min) and severe in the presence of severe renal insufficiency (≤30 mL/min) and/or proteinuria >3.0 g/d.
- Diabetic peripheral neuropathy was considered mild–moderate in case of modest symptomatology with EMG confirmation, severe in case of invalidating symptomatology and EMG finding corresponding to clinical severity.
- Diabetic retinopathy was classified as mild–moderate in case of compatible instrumental finding in the absence of significant vision loss, severe when there was significant vision loss.
- Ischemic heart disease was considered mild–moderate when clinical and instrumental elements were compatible with ischemia without hearth necrosis, severe in the presence of previous hearth necrosis (NSTEMI/STEMI).
- Peripheral vascular disease was considered mild–moderate in presence of “intermittent claudication” and radiographic diagnostics compatible with perfusion deficit, severe in presence of previous revascularization interventions of various types.
- Abdominal aortic aneurysm was considered mild–moderate in absence of characteristics indicating vascular treatment, severe in remaining cases.
- Cerebral vascular disease was considered mild–moderate in presence of carotid stenosis indicating surgical intervention and/or previous history of TIA, severe in presence of previous ischemic/hemorrhagic stroke.
- In case of multiple results present in a time interval, the average of values was calculated.
- For glycated hemoglobin, serum creatinine, microalbuminuria, lipids, the number of determinations in the time interval also had to be reported (0 = absence of values).
- Regarding arterial hypertension, the presence or absence of measurement in the considered time interval was indicated, 0 indicating absence and 1 presence of one or more measurements.
3. Statistical Analysis
4. Results
4.1. Population
4.2. Comorbidities (Table 2)
| Disease | Total N = 1169 | Males N = 663 | Females N = 506 | p | ≤70 N = 425 | 71–80 N = 403 | ≥81 N = 341 | p |
|---|---|---|---|---|---|---|---|---|
| Cardiovascular * | 353 | 30.5% | 29.8% | 0.817 | 18.1% | 30.5% | 44.9% | <0.0001 |
| Respiratory | 237 | 22.9% | 16.8% | 0.010 | 16.7% | 24.8% | 19.4% | 0.278 |
| Esophagus–gastric | 326 | 22.9% | 34.4% | <0.0001 | 24.0% | 28.3% | 32.3% | 0.011 |
| Hepato-biliary | 300 | 25.9% | 25.3% | 0.802 | 25.4% | 27.0% | 24.3% | 0.775 |
| Hematologic | 211 | 18.9% | 17.0% | 0.413 | 9.4% | 18.9% | 27.9% | <0.0001 |
| Rheumatologic | 110 | 8.0% | 11.3% | 0.058 | 6.1% | 12.2% | 10.3% | 0.036 |
| Nephrology ** | 159 | 14.8% | 12.1% | 0.178 | 6.8% | 16.4% | 18.8% | <0.0001 |
| Neoplastic | 225 | 19.0% | 19.6% | 0.810 | 11.8% | 22.1% | 25.2% | <0.0001 |
| Thyroid | 332 | 17.2% | 43.1% | <0.0001 | 25.6% | 29.8% | 30.2% | 0.151 |
| Neurology *** | 200 | 14.5% | 20.6% | 0.006 | 6.4% | 19.9% | 27.3% | <0.0001 |
| Psychiatry Anxiety Depression Psychosis | 318 | 16.6% | 41.1% | <0.0001 | 26.6% | 26.6% | 28.7% | 0.522 |
| 150 | 34.0% | 66.0% | 0.922 | 38.0% | 36.0% | 26.0% | 0.018 | |
| 155 | 35.5% | 64.5% | 30.3% | 32.3% | 37.4% | |||
| 13 | 30.8% | 69.2% | 69.2% | 23.1% | 7.7% | |||
| ▲ CSHA > 3 | 388 | 27.3% | 40.9% | <0.0001 | 12.9% | 27.3% | 65.4% | <0.0001 |
| ▲ CSHA/Median = 3/Q1–Q3 = 2–4 | ||||||||
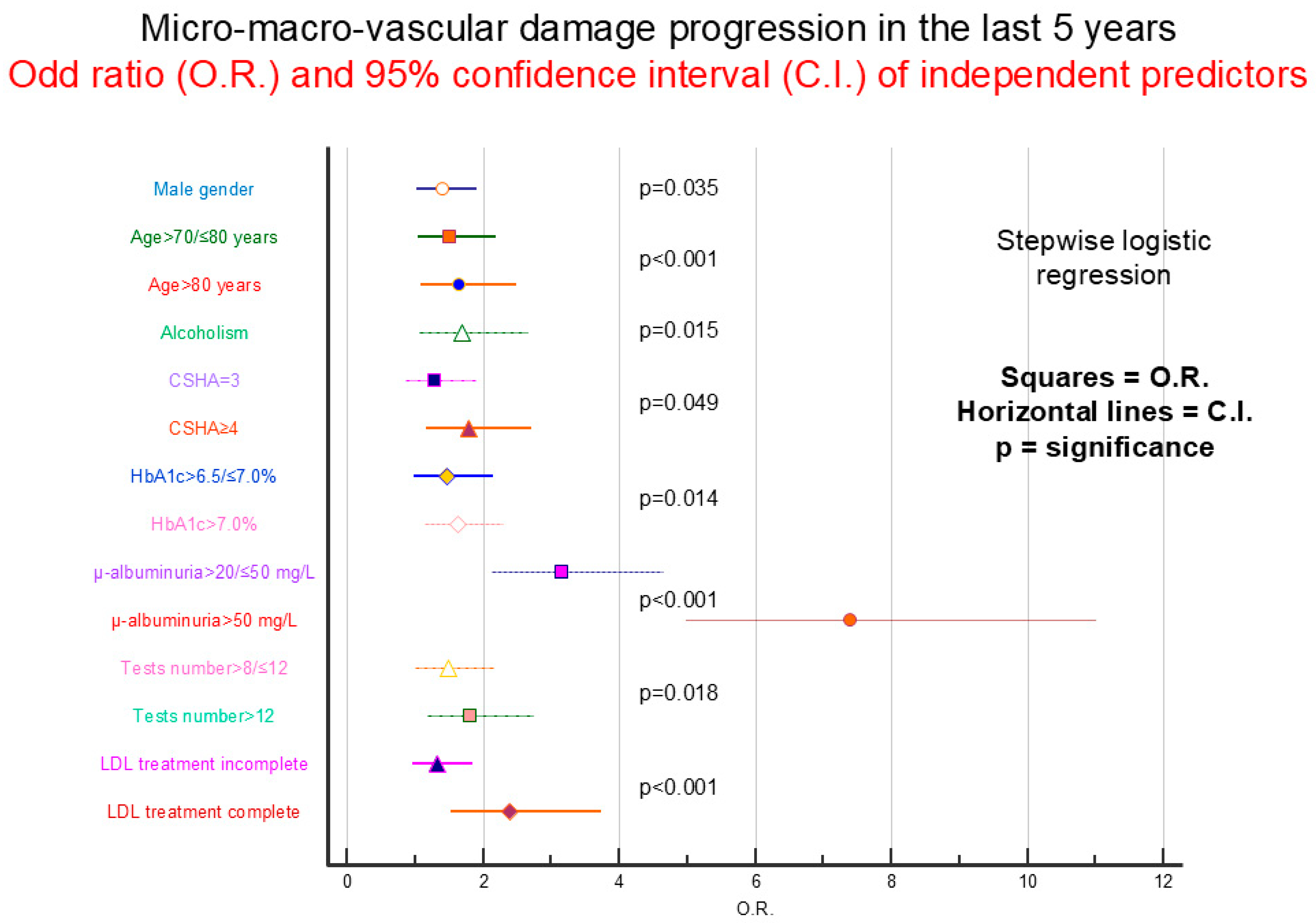
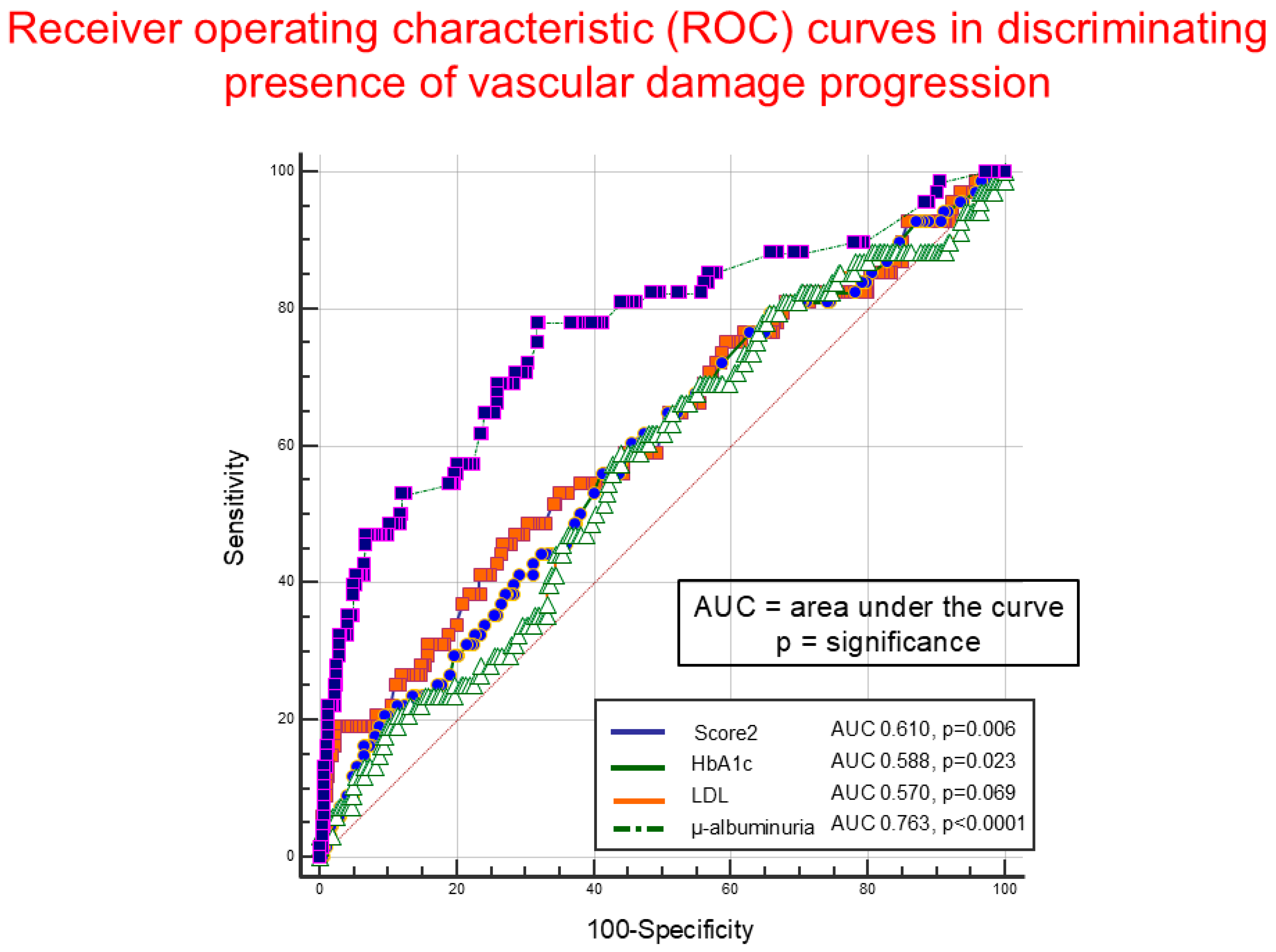
4.3. Organ Damage Progression
4.4. Gender/Age
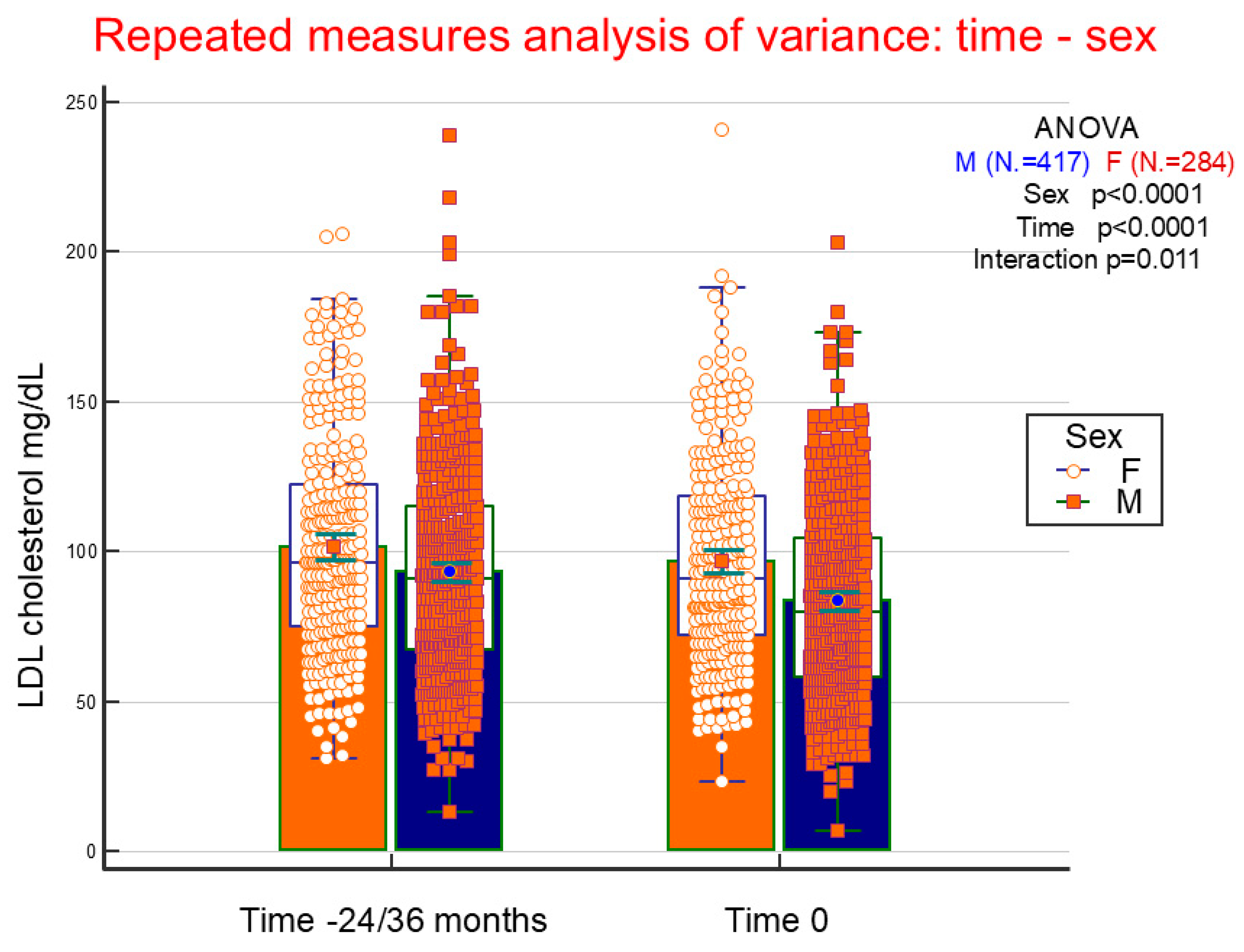
4.5. LDL Cholesterol Variations
4.6. Advanced Age, Male Gender, Smoking History, and Treatment
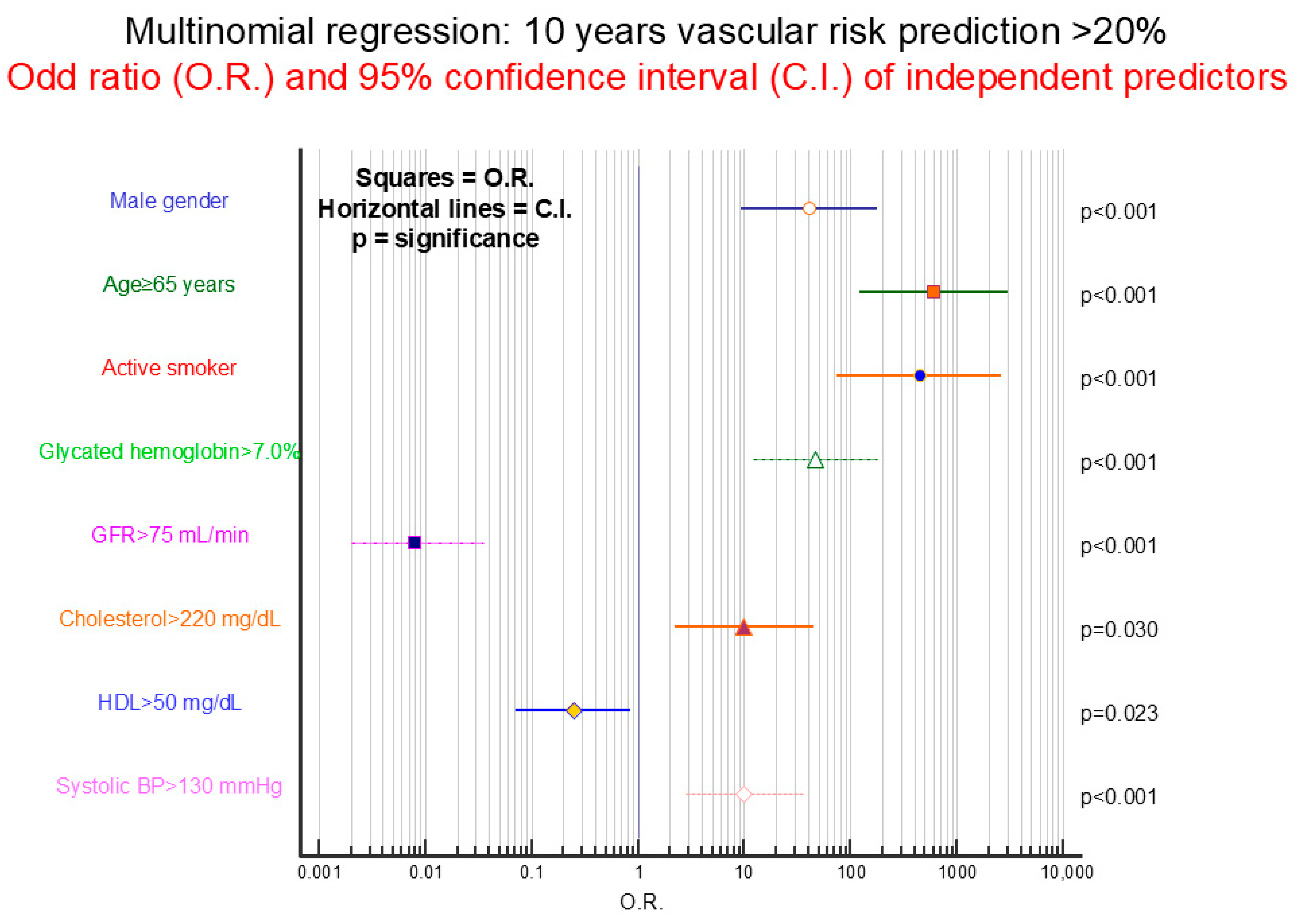
4.7. Current and 10-Year Cardiovascular Risk
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
- Type two diabetes mellitus: DM2
- General practitioner: GP
References
- Cho, S.W.; Kim, S.H.; Kim, Y.E.; Yoon, S.J.; Jo, M.W. Estimating Lifetime Duration of Diabetes by Age and Gender in the Korean Population Using a Markov Model. J. Korean Med. Sci. 2019, 34, e74. [Google Scholar] [CrossRef] [PubMed]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Rangel, E.B.; Rodrigues, C.O.; de Sa, J.R. Micro- and Macrovascular Complications in Diabetes Mellitus: Preclinical and Clinical Studies. J. Diabetes Res. 2019, 2019, 2161085. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; McMurray, J.; Boren, J.; Rawshani, A.; Omerovic, E.; Berg, N.; Halminen, J.; Skoglund, K.; Eliasson, B.; Gerstein, H.C.; et al. Twenty Years of Cardiovascular Complications and Risk Factors in Patients with Type 2 Diabetes: A Nationwide Swedish Cohort Study. Circulation 2023, 147, 1872–1886. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Franzen, S.; Sattar, N.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; McGuire, D.K.; Rosengren, A.; et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2018, 379, 633–644. [Google Scholar] [CrossRef]
- Ye, W.; Ding, X.; Putnam, N.; Farej, R.; Singh, R.; Wang, D.; Kuo, S.; Kong, S.X.; Elliott, J.C.; Lott, J.; et al. Development of clinical prediction models for renal and cardiovascular outcomes and mortality in patients with type 2 diabetes and chronic kidney disease using time-varying predictors. J. Diabetes Complicat. 2022, 36, 108180. [Google Scholar] [CrossRef]
- Ozturk, B.; Lawton, T.; Smith, S.; Habli, I. Predicting Progression of Type 2 Diabetes Using Primary Care Data with the Help of Machine Learning. Stud. Health Technol. Inform. 2023, 302, 38–42. [Google Scholar]
- Islam, R.; Sultana, A.; Tuhin, M.N.; Saikat, M.S.H.; Islam, M.R. Clinical Decision Support System for Diabetic Patients by Predicting Type 2 Diabetes Using Machine Learning Algorithms. J. Healthc. Eng. 2023, 2023, 6992441. [Google Scholar] [CrossRef] [PubMed]
- Hoek, A.G.; van Oort, S.; Elders, P.J.M.; Beulens, J.W.J. Causal Association of Cardiovascular Risk Factors and Lifestyle Behaviors With Peripheral Artery Disease: A Mendelian Randomization Approach. J. Am. Heart Assoc. 2022, 11, e025644. [Google Scholar] [CrossRef]
- Mansour, A.; Mousa, M.; Abdelmannan, D.; Tay, G.; Hassoun, A.; Alsafar, H. Microvascular and macrovascular complications of type 2 diabetes mellitus: Exome wide association analyses. Front, Endocrinol. 2023, 14, 1143067. [Google Scholar] [CrossRef]
- Collier, A.; Ghosh, S.; Hair, M.; Waugh, N. Impact of socioeconomic status and gender on glycaemic control, cardiovascular risk factors and diabetes complications in type 1 and 2 diabetes: A population based analysis from a Scottish region. Diabetes Metab. 2015, 41, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, Y.; Hu, Y.; Zong, G.; Li, S.; Rimm, E.B.; Hu, F.B.; Manson, J.E.; Rexrode, K.M.; Shin, H.J.; et al. Influence of Lifestyle on Incident Cardiovascular Disease and Mortality in Patients With Diabetes Mellitus. J. Am. Coll. Cardiol. 2018, 71, 2867–2876. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, A. Cardiovascular disease in diabetes type 2: Current concepts. J. Intern. Med. 2018, 284, 240–253. [Google Scholar] [CrossRef]
- Van der Leeuw, J.; Van Dieren, S.; Beulens, J.W.J.; Boeing, H.; Spijkerman, A.M.W.; Van der Graaf, Y.; Nöthlings, U.; Visseren, F.L.J.; Rutten, G.E.H.M.; Moons, K.G.M.; et al. The validation of cardiovascular risk scores for patients with type 2 diabetes mellitus. Heart 2015, 101, 222–229. [Google Scholar] [CrossRef]
- Hurst, R.T.; Lee, R.W. Increased incidence of coronary atherosclerosis in type 2 diabetes mellitus: Mechanisms and management. Ann. Intern. Med. 2003, 139, 824–834. [Google Scholar] [CrossRef] [PubMed]
- SCORE2-Diabetes Working Group; ESC Cardiovascular Risk Collaboration. SCORE2-Diabetes: 10-year cardiovascular risk estimation in type 2 diabetes in Europe. Eur. Heart J. 2023, 44, 2544–2556. [Google Scholar] [CrossRef]
- Blomster, J.I.; Zoungas, S.; Chalmers, J.; Li, Q.; Chow, C.K.; Woodward, M.; Mancia, G.; Poulter, N.; Williams, B.; Harrap, S.; et al. The relationship between alcohol consumption and vascular complications and mortality in individuals with type 2 diabetes. Diabetes Care 2014, 37, 1353–1359. [Google Scholar] [CrossRef]
- Bonekamp, N.E.; Visseren, F.L.J.; Cramer, M.J.; Dorresteijn, J.A.N.; van der Meer, M.G.; Ruigrok, Y.M.; van Sloten, T.T.; Teraa, M.; Geleijnse, J.M.; Koopal, C. Long-term lifestyle change and risk of mortality and Type 2 diabetes in patients with cardiovascular disease. Eur. J. Prev. Cardiol. 2024, 31, 205–213. [Google Scholar] [CrossRef]
- Mohan, V.; Venkatraman, J.V.; Pradeepa, R. Epidemiology of cardiovascular disease in type 2 diabetes: The Indian scenario. J. Diabetes Sci. Technol. 2010, 4, 158–170. [Google Scholar] [CrossRef]
- Dal Canto, E.; Ceriello, A.; Ryden, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 2019, 26, 25–32. [Google Scholar] [CrossRef]
- Heikkala, E.; Mikkola, I.; Jokelainen, J.; Timonen, M.; Hagnas, M. Multimorbidity and achievement of treatment goals among patients with type 2 diabetes: A primary care, real-world study. BMC Health Serv. Res. 2021, 21, 964. [Google Scholar] [CrossRef]
- Chiang, J.I.; Furler, J.; Mair, F.; Jani, B.D.; Nicholl, B.I.; Thuraisingam, S.; Manski-Nankervis, J.A. Associations between multimorbidity and glycaemia (HbA1c) in people with type 2 diabetes: Cross-sectional study in Australian general practice. BMJ Open 2020, 10, e039625. [Google Scholar] [CrossRef]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Laouenan, C.; Vallet-Pichard, A.; Vidal-Trecan, T.; Manchon, P.; Paradis, V.; Roulot, D.; Gault, N.; Boitard, C.; Terris, B.; et al. High Prevalence of NASH and Advanced Fibrosis in Type 2 Diabetes: A Prospective Study of 330 Outpatients Undergoing Liver Biopsies for Elevated ALT, Using a Low Threshold. Diabetes Care 2023, 46, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Damanik, J.; Yunir, E. Type 2 Diabetes Mellitus and Cognitive Impairment. Acta Med. Indones. 2021, 53, 213–220. [Google Scholar] [PubMed]
- Moheet, A.; Mangia, S.; Seaquist, E.R. Impact of diabetes on cognitive function and brain structure. Ann. N. Y. Acad. Sci. 2015, 1353, 60–71. [Google Scholar] [CrossRef]
- De Feo, M.; Del Pinto, R.; Pagliacci, S.; Grassi, D.; Ferri, C.; The Italian Society of Hypertension and Federfarma. Real-World Hypertension Prevalence, Awareness, Treatment, and Control in Adult Diabetic Individuals: An Italian Nationwide Epidemiological Survey. High Blood Press. Cardiovasc. Prev. 2021, 28, 301–307. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.L.; Liu, S.; Ye, Y.S.; Yang, Q.T.; Xia, L.N. Prevalence of hypertension among type 2 diabetes mellitus patients in China: A systematic review and meta-analysis. Int. Health 2024, 16, 144–151. [Google Scholar] [CrossRef]
- Russo, G.T.; Manicardi, V.; Rossi, M.C.; Orsi, E.; Solini, A. Sex- and gender-differences in chronic long-term complications of type 1 and type 2 diabetes mellitus in Italy. Nutr. Metab. Cardiovasc Dis. 2022, 32, 2297–2309. [Google Scholar] [CrossRef]
- Teliti, M.; Cogni, G.; Sacchi, L.; Dagliati, A.; Marini, S.; Tibollo, V.; De Cata, P.; Bellazzi, R.; Chiovato, L. Risk factors for the development of micro-vascular complications of type 2 diabetes in a single-centre cohort of patients. Diabetes Vasc. Dis. Res. 2018, 15, 424–432. [Google Scholar] [CrossRef]
- Patsouras, A.; Farmaki, P.; Garmpi, A.; Damaskos, C.; Garmpis, N.; Mantas, D.; Diamantis, E. Screening and Risk Assessment of Coronary Artery Disease in Patients With Type 2 Diabetes: An Updated Review. Vivo 2019, 33, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Kaze, A.D.; Fonarow, G.C.; Echouffo-Tcheugui, J.B. Cardiac Autonomic Dysfunction and Risk of Silent Myocardial Infarction Among Adults With Type 2 Diabetes. J. Am. Heart Assoc. 2023, 12, e029814. [Google Scholar] [CrossRef]
- Scognamiglio, R.; Negut, C.; Ramondo, A.; Tiengo, A.; Avogaro, A. Detection of coronary artery disease in asymptomatic patients with type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2006, 47, 65–71. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Tse, G.; Chan, J.S.K.; Wu, S.; Liu, T. Control for multiple risk factors and incident heart failure and mortality in patients with diabetes mellitus: Insights from the Kailuan cohort study. Curr. Probl. Cardiol. 2024, 49, 102737. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Zhang, H.Y.; Dai, H.J.; Gong, J.; Wang, Y.; He, Y.; Liu, Y.L.; Hao, W.R.; Wei, Y.H. A 3-year follow-up analysis of renal function in elderly patients with type 2 diabetes mellitus and an estimated glomerular filtration rate <90 mL/min/1.73m2: A retrospective cohort study. Medicine 2024, 103, e38865. [Google Scholar] [PubMed]
- Messina, G.; Alioto, A.; Parisi, M.C.; Mingrino, O.; Di Corrado, D.; Crescimanno, C.; Kulis, S.; Nese Sahin, F.; Padua, E.; Canzone, A.; et al. Experimental study on physical exercise in diabetes: Pathophysiology and therapeutic effects. Eur. J. Transl. Myol. 2023, 33, 11560. [Google Scholar] [CrossRef]
- McGill, J.B.; Haller, H.; Roy-Chaudhury, P.; Cherrington, A.; Wada, T.; Wanner, C.; Ji, L.; Rossing, P. Making an impact on kidney disease in people with type 2 diabetes: The importance of screening for albuminuria. BMJ Open Diabetes Res. Care 2022, 10, e002806. [Google Scholar] [CrossRef]
- Pietraszek, A.; Gregersen, S.; Hermansen, K. Alcohol and type 2 diabetes. A review. Nutr. Metab. Cardiovasc Dis. 2010, 20, 366–375. [Google Scholar] [CrossRef]
- Buyadaa, O.; Salim, A.; Morton, J.I.; Magliano, D.J.; Shaw, J.E. Rate of decline in kidney function and known age-of-onset or duration of type 2 diabetes. Sci. Rep. 2021, 11, 14705. [Google Scholar] [CrossRef]
- Solini, A.; Penno, G.; Bonora, E.; Fondelli, C.; Orsi, E.; Trevisan, R.; Vedovato, M.; Cavalot, F.; Cignarelli, M.; Morano, S.; et al. Age, renal dysfunction, cardiovascular disease, and antihyperglycemic treatment in type 2 diabetes mellitus: Findings from the Renal Insufficiency and Cardiovascular Events Italian Multicenter Study. J. Am. Geriatr. Soc. 2013, 61, 1253–1261. [Google Scholar] [CrossRef]
- Maric-Bilkan, C. Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clin. Sci. 2017, 131, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Shepard, B.D. Sex differences in diabetes and kidney disease: Mechanisms and consequences. Am. J. Physiol. Renal Physiol. 2019, 317, F456–F462. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, T.; Iwase, M.; Fujii, H.; Kitazono, T. Sex differences in cardiovascular risk, lifestyle, and psychological factors in patients with type 2 diabetes: The Fukuoka Diabetes Registry. Biol. Sex Differ. 2023, 14, 32. [Google Scholar] [CrossRef]
- Rajendran, A.; Minhas, A.S.; Kazzi, B.; Varma, B.; Choi, E.; Thakkar, A.; Michos, E.D. Sex-specific differences in cardiovascular risk factors and implications for cardiovascular disease prevention in women. Atherosclerosis 2023, 384, 117269. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Gebhard, C. Gender medicine: Effects of sex and gender on cardiovascular disease manifestation and outcomes. Nat. Rev. Cardiol. 2023, 20, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.E.; Byrne, M.; Galvin, R.; Boland, F.; Fahey, T.; Smith, S.M. Improving risk factor management for patients with poorly controlled type 2 diabetes: A systematic review of healthcare interventions in primary care and community settings. BMJ Open 2017, 7, e015135. [Google Scholar] [CrossRef]
- Hodgson, S.; Morgan-Harrisskitt, J.; Hounkpatin, H.; Stuart, B.; Dambha-Miller, H. Primary care service utilisation and outcomes in type 2 diabetes: A longitudinal cohort analysis. BMJ Open 2022, 12, e054654. [Google Scholar] [CrossRef]
- Guillen-Aguinaga, S.; Forga, L.; Brugos-Larumbe, A.; Guillen-Grima, F.; Guillen-Aguinaga, L.; Aguinaga-Ontoso, I. Variability in the Control of Type 2 Diabetes in Primary Care and Its Association with Hospital Admissions for Vascular Events. The APNA Study. J. Clin. Med. 2021, 10, 5854. [Google Scholar] [CrossRef]
- Kramer, H.U.; Raum, E.; Ruter, G.; Schottker, B.; Rothenbacher, D.; Rosemann, T.; Szecsenyi, J.; Brenner, H. Gender disparities in diabetes and coronary heart disease medication among patients with type 2 diabetes: Results from the DIANA study. Cardiovasc. Diabetol. 2012, 11, 88. [Google Scholar] [CrossRef]
- Al-Salameh, A.; Bucher, S.; Bauduceau, B.; Benattar-Zibi, L.; Berrut, G.; Bertin, P.; Corruble, E.; Danchin, N.; Derumeaux, G.; Doucet, J.; et al. Gender-Related Differences in the Control of Cardiovascular Risk Factors in Primary Care for Elderly Patients with Type 2 Diabetes: A Cohort Study. Can. J. Diabetes 2018, 42, 365–371.e2. [Google Scholar] [CrossRef]
- Zhao, M.; Woodward, M.; Vaartjes, I.; Millett, E.R.C.; Klipstein-Grobusch, K.; Hyun, K.; Carcel, C.; Peters, S.A.E. Sex Differences in Cardiovascular Medication Prescription in Primary Care: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e014742. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Y.; Zheng, S.; Yang, X.; Wang, T.; Zeng, J. Moderate alcohol consumption and atherosclerosis: Meta-analysis of effects on lipids and inflammation. Wien. Klin. Wochenschr. 2017, 129, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.H.; Yajnik, C.; Phatak, S.; Hanson, R.L.; Knowler, W.C. Racial/ethnic differences in the burden of type 2 diabetes over the life course: A focus on the USA and India. Diabetologia 2019, 62, 1751–1760. [Google Scholar] [CrossRef]
- Ma, R.C.; Chan, J.C. Type 2 diabetes in East Asians: Similarities and differences with populations in Europe and the United States. Ann. N. Y. Acad. Sci. 2013, 1281, 64–91. [Google Scholar] [CrossRef] [PubMed]
- SCORE2 Working Group; ESC Cardiovascular Risk Collaboration. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef]
- Crowley, M.J.; Zullig, L.L.; Shah, B.R.; Shaw, R.J.; Lindquist, J.H.; Peterson, E.D.; Bosworth, H.B. Medication non-adherence after myocardial infarction: An exploration of modifying factors. J. Gen. Intern. Med. 2015, 30, 83–90. [Google Scholar] [CrossRef]
- Warren, J.R.; Falster, M.O.; Tran, B.; Jorm, L. Association of Continuity of Primary Care and Statin Adherence. PLoS ONE 2015, 10, e0140008. [Google Scholar] [CrossRef]
| Male Gender N (%) | 663 (56.7) | |
| Age Years ME (SD) | 73.5 (11.0) | |
| Age in years at diagnosis ME (SD) | 61.6 (11.6) | |
| Caucasian ethnicity N (%) | 1145 (97.9) | |
| Body mass index (Kg/m2) ME (SD) | 28.4 (5.2) | |
| Menopause | Not applicable N (%) | 663 (56.7) |
| Absent N (%) | 14 (1.2) | |
| Present N (%) | 492 (42.1) | |
| Smoking | Never N (%) | 602 (51.5) |
| Past N (%) | 443 (37.9) | |
| Current N (%) | 124 (10.6) | |
| Alcohol | Never N (%) | 619 (52.9) |
| Mild * N (%) | 424 (36.3) | |
| Severe * N (%) | 126 (10.8) | |
| School | Elementary/middle N (%) | 691 (59.1) |
| Primary N (%) | 382 (32.7) | |
| Graduate N (%) | 96 (8.2) | |
| Work/income | Absent N (%) | 48 (4.1) |
| Low N (%) | 168 (14.4) | |
| Sufficient N (%) | 953 (81.5) | |
| Family support | Alone N (%) | 269 (23.0) |
| Married N (%) | 703 (60.1) | |
| With others N (%) | 197 (16.9) | |
| Physical activity | Absent N (%) | 556 (47.6) |
| Low N (%) | 486 (41.6) | |
| Regular N (%) | 127 (10.9) | |
| Five Years Before | At the Entry | |||||||
|---|---|---|---|---|---|---|---|---|
| No N (%) | Mild/Moderate N (%) | Severe N (%) | No N (%) | Mild/Moderate N (%) | Severe N (%) | p | ||
| Arterial hypertension | 332 (28.4) | 697 (59.6) | 140 (12.0) | 252 (21.6) | 702 (60.1) | 215 (18.4) | <0.0001 | |
| Microangiopathy | Diabetic nephropathy | 1035 (88.5) | 126 (10.8) | 8 (0.7) | 909 (77.8) | 232 (19.8) | 28 (2.4) | <0.0001 |
| Diabetic neuropathy | 1126 (96.3) | 38 (3.3) | 5 (0.4) | 1087 (93.0) | 72 (6.2) | 10 (0.9) | <0.0001 | |
| Diabetic retinopathy | 1115 (95.4) | 48 (4.1) | 6 (0.5) | 1091 (93.3) | 69 (5.9) | 9 (0.8) | <0.0001 | |
| Macroangiopathy | Ischemic cardiopathy | 1046 (89.5) | 28 (2.4) | 95 (8.1) | 997 (85.3) | 48 (4.1) | 124 (10.6) | <0.0001 |
| Peripheral vasculopathy | 1103 (94.4) | 53 (4.5) | 13 (1.1) | 1084 (92.7) | 66 (5.6) | 19 (1.6) | <0.0001 | |
| Abdominal aortic aneurism | 1157 (99.0) | 8 (0.7) | 4 (0.3) | 1146 (98.0) | 19 (1.6) | 4 (0.3) | 0.0009 | |
| Cerebral vasculopathy | 1081 (92.5) | 55 (4.7) | 33 (2.8) | 1013 (86.7) | 99 (8.5) | 57 (4.9) | <0.0001 | |
| Microangiopathy * | 977 (83.6) | 151 (12.9) | 41 (3.5) | 825 (70.6) | 255 (21.8) | 89 (7.6) | <0.0001 | |
| Macroangiopathy * | 924 (79.0) | 102 (8.7) | 143 (12.2) | 819 (70.1) | 144 (12.3) | 206 (17.6) | <0.0001 | |
| Micro/macroangiopathy * | 803 (68.7) | 167 (14.3) | 199 (17.0) | 618 (52.9) | 247 (21.1) | 304 (26.0) | <0.0001 | |
| Presence and Percentage of Micro/Macroangiopathy Progression | p | ||||
|---|---|---|---|---|---|
| Gender | Female N = 109 (21.5) | Male N = 205 (30.9) | 0.0003 | ||
| Age years | ≤70 N = 75 18% | 71–80 N = 120 30% | ≥81 N = 119 35% | <0.0001 | |
| Years of diabetes | ≤10 N = 109 21% | 11–15 N = 80 28% | ≥16 N = 125 36% | <0.0001 | |
| BMI kg/m2 | ≤25 N = 92 31% | 26–30 N = 119 24% | ≥31 N = 103 27% | 0.337 | |
| Smoking | Absent N = 142 24% | Present N = 172 30% | 0.009 | ||
| Heavy alcohol | Absent N = 267 26% | Present N = 47 37% | 0.005 | ||
| School | Middle N = 190 28% | Diploma N = 97 25% | Degree N = 27 28% | 0.744 | |
| Income | Poor N = 16 33% | Modest N = 45 27% | Good N = 253 27% | 0.428 | |
| Family | Alone N = 75 28% | Spouse N = 184 26% | Others N = 55 28% | 0.945 | |
| Physical activity | No N = 173 31% | Light N = 114 24% | Good N = 27 21% | 0.002 | |
| CSHA | ≤2 N = 64 18% | 3 N = 111 27% | ≥4 N = 139 36% | <0.0001 | |
| Hypertension * | Absent N = 66 20% | Present N = 248 30% | 0.0007 | ||
| HbA1c % ** | ≤6.5 N = 99 21% | 6.6–7.0 N = 78 28% | >7.0 N = 137 33% | <0.0001 | |
| GFR mL/min ** | ≤45 N = 57 48% | 46–60 N = 64 33% | >60 N = 193 23% | <0.0001 | |
| µALBU mg/L ** | ≤20 N = 146 17% | 21–50 N = 66 44% | >50 N = 102 65% | <0.0001 | |
| HDL mg/dL ** | ≤40 N = 52 29% | 41–60 N = 172 27% | >60 N = 90 25% | 0.300 | |
| LDL mg/dL ** | ≤70 N = 115 38% | 71–130 N = 170 24% | >130 N = 29 18% | <0.0001 | |
| Tests number *** | ≤8 N = 57 18% | 9–12 N = 152 27% | >12 N = 105 35% | <0.0001 | |
| LDL therapy ^ | No N = 98 20% | Partial N = 157 30% | Regular N = 59 39% | <0.0001 | |
| Males | N = 663 | Females | N = 506 | ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | |||||
| Age in years | 72.5 | 10.6 | 74.8 | 11.4 | 0.0003 | ||||
| Years at diagnosis | 60.4 | 11.0 | 63.2 | 12.2 | <0.0001 | Correlation with age | ANCOVA | ||
| Years with the disease | 12.1 | 7.8 | 11.6 | 7.6 | 0.319 | Coefficient | F | p | p |
| BMI (kg/m2) | 28.4 | 4.8 | 28.5 | 5.6 | 0.867 | −0.088 | 42.8 | <0.0001 | 0.386 |
| CSHA | 3.16 | 1.44 | 3.56 | 1.52 | <0.0001 | 0.064 | 336 | <0.0001 | 0.001 |
| Mean serum glucose (mg/dL) | 134 | 33 | 130 | 33 | 0.034 | −0.267 | 9.42 | 0.002 | 0.070 |
| Mean glycated hemoglobin (%) | 6.92 | 1.04 | 6.88 | 0.95 | 0.577 | −0.009 | 11.1 | 0.0009 | 0.834 |
| Mean serum creatinine (mg/dL) | 1.11 | 0.66 | 0.88 | 0.30 | <0.0001 | 0.004 | 7.99 | 0.005 | <0.001 |
| Mean glomerular filtration rate (mL/min) | 75.2 | 21.1 | 71.2 | 22.2 | 0.002 | −0.977 | 383 | <0.0001 | 0.120 |
| Mean cholesterol (mg/dL) | 165 | 35 | 183 | 37 | <0.0001 | −0.444 | 20.6 | <0.0001 | <0.001 |
| Mean HDL cholesterol (mg/dL) | 51 | 13 | 58 | 15 | <0.0001 | 0.117 | 9.22 | 0.002 | <0.001 |
| Mean triglycerides (mg/dL) | 126 | 59 | 128 | 54 | 0.546 | −0.785 | 27.7 | <0.0001 | 0.240 |
| Mean LDL cholesterol (mg/dL) | 89 | 31 | 99 | 33 | <0.0001 | −0.402 | 22.9 | <0.0001 | <0.001 |
| Mean Cholesterol Values 71–130 mg/dL | Mean Cholesterol Values ≤ 70 mg/dL | ||||||
|---|---|---|---|---|---|---|---|
| Coefficient | O.R. | 95% C.I. | Coefficient | O.R. | 95% C.I. | p | |
| Male gender | 0.683 | 2.0 | 1.3–3.0 | 1.174 | 3.2 | 1.9–5.4 | <0.001 |
| Age > 75 years | 0.690 | 2.0 | 1.3–3.0 | 0.766 | 2.2 | 1.4–3.4 | 0.006 |
| Smoking history | 0.348 | 1.4 | 0.97–2.10 | 0.662 | 1.9 | 1.2–3.0 | 0.016 |
| Alcohol consumption > 40 gr/day | −0.322 | 0.72 | 0.49–1.1 | −0.769 | 0.46 | 0.29–0.74 | 0.008 |
| Family components ≥ 2 | 0.447 | 1.6 | 1.1–2.3 | 0.704 | 2.0 | 1.2–3.3 | 0.019 |
| Diabetes present from >10 years | −0.036 | 0.96 | 0.67–1.4 | 0.684 | 2.0 | 1.3–3.1 | <0.001 |
| Arterial hypertension from ≥5 years | −0.078 | 0.92 | 0.62–1.4 | 0.568 | 1.8 | 1.1–2.9 | 0.001 |
| Mean creatinine > 0.90 mg/dL | −0.428 | 0.65 | 0.44–0.96 | −0.082 | 0.92 | 0.58–1.5 | 0.017 |
| Cholesterol treatment present | 0.895 | 2.4 | 1.7–3.6 | 2.384 | 11 | 6.8–17 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabris, C.; Rizzo, E.; Bertolissi, S.; Casatta, L.; Pavan, M.; Toniutto, P. Modifiable and Non-Modifiable Risk Factors and Vascular Damage Progression in Type 2 Diabetes: A Primary Care Analysis. J. Clin. Med. 2025, 14, 3155. https://doi.org/10.3390/jcm14093155
Fabris C, Rizzo E, Bertolissi S, Casatta L, Pavan M, Toniutto P. Modifiable and Non-Modifiable Risk Factors and Vascular Damage Progression in Type 2 Diabetes: A Primary Care Analysis. Journal of Clinical Medicine. 2025; 14(9):3155. https://doi.org/10.3390/jcm14093155
Chicago/Turabian StyleFabris, Carlo, Elena Rizzo, Stefano Bertolissi, Lucia Casatta, Massimo Pavan, and Pierluigi Toniutto. 2025. "Modifiable and Non-Modifiable Risk Factors and Vascular Damage Progression in Type 2 Diabetes: A Primary Care Analysis" Journal of Clinical Medicine 14, no. 9: 3155. https://doi.org/10.3390/jcm14093155
APA StyleFabris, C., Rizzo, E., Bertolissi, S., Casatta, L., Pavan, M., & Toniutto, P. (2025). Modifiable and Non-Modifiable Risk Factors and Vascular Damage Progression in Type 2 Diabetes: A Primary Care Analysis. Journal of Clinical Medicine, 14(9), 3155. https://doi.org/10.3390/jcm14093155





