Pneumatic Displacement and Anti-VEGF Therapy for Submacular Hemorrhage in Neovascular Age-Related Macular Degeneration: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Treatment Methods
2.4. Statical Analysis
3. Results
3.1. Clinical Characteristics of Patients
3.2. Treatment Outcomes
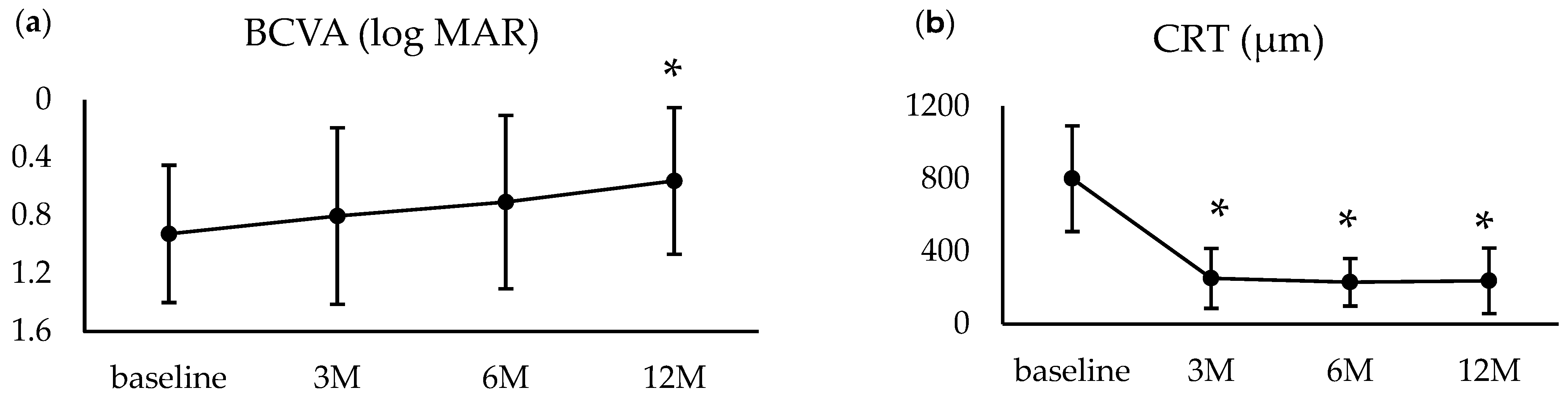
3.3. One-Year Outcomes
3.4. Complications
3.5. Factors Associated with Visual Outcomes
3.6. Factors Associated with VH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bressler, N.M.; Bressler, S.B.; Congdon, N.G.; Ferris, F.L.; Friedman, D.S.; Klein, R.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M. Potential Public Health Impact of Age-Related Eye Disease Study Results: AREDS Report No. 11. Arch. Ophthalmol. 2003, 121, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.S.; O’Colmain, B.J.; Muñoz, B.; Tomany, S.C.; McCarty, C.; de Jong, P.T.; Nemesure, B.; Mitchell, P.; Kempen, J.; the Eye Diseases Prevalence Research Group. Prevalence of Age-Related Macular Degeneration in the United States. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar] [CrossRef]
- Toth, C.A.; Morse, L.S.; Hjelmeland, L.M.; Landers, M.B. Fibrin Directs Early Retinal Damage After Experimental Subretinal Hemorrhage. Arch. Ophthalmol. 1991, 109, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Gabrielle, P.; Maitrias, S.; Nguyen, V.; Arnold, J.J.; Squirrell, D.; Arnould, L.; Sanchez-Monroy, J.; Viola, F.; O’Toole, L.; Barthelmes, D.; et al. Incidence, Risk Factors and Outcomes of Submacular Haemorrhage with Loss of Vision in Neovascular Age-related Macular Degeneration in Daily Clinical Practice: Data from the FRB! Registry. Acta Ophthalmol. 2022, 100, e1569–e1578. [Google Scholar] [CrossRef]
- Kim, H.S.; Cho, H.J.; Yoo, S.G.; Kim, J.H.; Han, J.I.; Lee, T.G.; Kim, J.W. Intravitreal Anti-Vascular Endothelial Growth Factor Monotherapy for Large Submacular Hemorrhage Secondary to Neovascular Age-Related Macular Degeneration. Eye 2015, 29, 1141–1151. [Google Scholar] [CrossRef]
- Shin, J.Y.; Lee, J.; Byeon, S.H. Anti–Vascular Endothelial Growth Factor with or Without Pneumatic Displacement for Submacular Hemorrhage. Am. J. Ophthalmol. 2015, 159, 904–914.e1. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Hara, C.; Shiraki, A.; Shiraki, N.; Sayanagi, K.; Sakimoto, S.; Sato, S.; Sakaguchi, H.; Nishida, K. Simultaneous Intravitreal Aflibercept and Gas Injections for Submacular Hemorrhage Secondary to Polypoidal Choroidal Vasculopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Stanescu-Segall, D.; Balta, F.; Jackson, T.L. Submacular Hemorrhage in Neovascular Age-Related Macular Degeneration: A Synthesis of the Literature. Surv. Ophthalmol. 2016, 61, 18–32. [Google Scholar] [CrossRef]
- Grohmann, C.; Dimopoulos, S.; Bartz-Schmidt, K.U.; Schindler, P.; Katz, T.; Spitzer, M.S.; Skevas, C. Surgical Management of Submacular Hemorrhage Due to N-AMD: A Comparison of Three Surgical Methods. Int. J. Retin. Vitr. 2020, 6, 27. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to Use the Bonferroni Correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Yamashiro, K.; Oishi, A.; Hata, M.; Takahashi, A.; Tsujikawa, A. Visual Acuity Outcomes of Anti-VEGF Treatment for Neovascular Age-Related Macular Degeneration in Clinical Trials. Jpn. J. Ophthalmol. 2021, 65, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Kato, A.; Araki, T.; Kimura, T.; Kinoshita, T.; Okamoto, F.; Murakami, T.; Mitamura, Y.; Sakamoto, T.; Miki, A.; et al. Visual Prognosis of Submacular Hemorrhage Secondary to Age-Related Macular Degeneration: A Retrospective Multicenter Survey. PLoS ONE 2022, 17, e0271447. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, Y.J.; Park, S.J.; Park, K.H.; Yeo, J.H.; Kim, J.; Yoon, Y.H.; Lee, J.Y.; Woo, S.J. Comparison of Visual Outcomes of Polypoidal Choroidal Vasculopathy and Typical Neovascular Age-related Macular Degeneration—Up to 10 Years of Follow-up. Acta Ophthalmol. 2022, 100, e1579–e1588. [Google Scholar] [CrossRef]
- Ohji, M.; Okada, A.A.; Sasaki, K.; Moon, S.C.; Machewitz, T.; Takahashi, K.; ALTAIR Investigators. Relationship between Retinal Fluid and Visual Acuity in Patients with Exudative Age-Related Macular Degeneration Treated with Intravitreal Aflibercept Using a Treat-and-Extend Regimen: Subgroup and Post-Hoc Analyses from the ALTAIR Study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 3637–3647. [Google Scholar] [CrossRef] [PubMed]
- González-López, J.J.; McGowan, G.; Chapman, E.; Yorston, D. Vitrectomy with Subretinal Tissue Plasminogen Activator and Ranibizumab for Submacular Haemorrhages Secondary to Age-Related Macular Degeneration: Retrospective Case Series of 45 Consecutive Cases. Eye 2016, 30, 929–935. [Google Scholar] [CrossRef]
- Lee, K.; Park, Y.G.; Park, Y.-H. Visual Prognosis after Pneumatic Displacement of Submacular Hemorrhage According to Age-Related Macular. Degeneration Subtypes. Retina 2020, 40, 2304–2311. [Google Scholar] [CrossRef]
- Haupert, C.L.; McCuen, B.W.; Jaffe, G.J.; Steuer, E.R.; Cox, T.A.; Toth, C.A.; Fekrat, S.; Postel, E.A. Pars Plana Vitrectomy, Subretinal Injection of Tissue Plasminogen Activator, and Fluid–Gas Exchange for Displacement of Thick Submacular Hemorrhage in Age-Related Macular Degeneration. Am. J. Ophthalmol. 2001, 131, 208–215. [Google Scholar] [CrossRef]
- Schulze, S.D.; Hesse, L. Tissue Plasminogen Activator plus Gas Injection in Patients with Subretinal Hemorrhage Caused by Age-Related Macular Degeneration: Predictive Variables for Visual Outcome. Graefe S Arch. Clin. Exp. Ophthalmol. 2002, 240, 717–720. [Google Scholar] [CrossRef]
- Wu, T.-T.; Kung, Y.-H.; Hong, M.-C. Vitreous Hemorrhage Complicating Intravitreal Tissue Plasminogen Activator and Pneumatic Displacement of Submacular Hemorrhage. Retina 2011, 31, 2071–2077. [Google Scholar] [CrossRef]
- Matsuo, M.; Takai, Y.; Ohira, A.; Hara, K.; Imamachi, K.; Tanito, M. Comparison of the Short-Term Efficacy of Intravitreal Aflibercept and Ranibizumab for Macular Edema Caused by Branch Retinal Vein Occlusion. Shimane J. Med. Sci. 2024, 41, 41–51. [Google Scholar] [CrossRef]
- Silva, R.M.; Ruiz-Moreno, J.M.; Gomez-Ulla, F.; Montero, J.A.; Gregório, T.; Cachulo, M.L.; Pires, I.A.; Cunha-Vaz, J.G.; Murta, J.N. Photodynamic Therapy for Chronic Central Serous Chorioretinopathy. Retina 2013, 33, 309–315. [Google Scholar] [CrossRef]
- De Silva, S.R.; Bindra, M.S. Early Treatment of Acute Submacular Haemorrhage Secondary to Wet AMD Using Intravitreal Tissue Plasminogen Activator, C3F8, and an Anti-VEGF Agent. Eye 2016, 30, 952–957. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hooper, C.; Chiu, D.; Chamberlain, M.; Karia, N.; Heriot, W.J. Management of Submacular Hemorrhage with Intravitreal Injection of Tissue Plasminogen Activator and Expansile Gas. Retina 2007, 27, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Handwerger, B.A.; Blodi, B.A.; Chandra, S.R.; Olsen, T.W.; Stevens, T.S. Treatment of Submacular Hemorrhage with Low-Dose Intravitreal Tissue Plasminogen Activator Injection and Pneumatic Displacement. Arch. Ophthalmol. 2001, 119, 28–32. [Google Scholar]
- Fassbender, J.M.; Sherman, M.P.; Barr, C.C.; Schaal, S. Tissue Plasminogen Activator for Subfoveal Hemorrhage Due to Age-Related Macular Degeneration. Retina 2016, 36, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Çakir, M.; Çekiç, O.; Yilmaz, Ö.F. Pneumatic Displacement of Acute Submacular Hemorrhage with and without the Use of Tissue Plasminogen Activator. Eur. J. Ophthalmol. 2009, 20, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, M.; Sawada, O.; Miyake, T.; Kakinoki, M.; Sawada, T.; Kawamura, H.; Sakaguchi, H.; Gomi, F.; Ohji, M. Comparison Of Pneumatic Displacement for Submacular Hemorrhages with Gas Alone and Gas Plus Tissue Plasminogen Activator. Retina 2013, 33, 1908–1914. [Google Scholar] [CrossRef]
- Kadonosono, K.; Arakawa, A.; Yamane, S.; Inoue, M.; Yamakawa, T.; Uchio, E.; Yanagi, Y. Displacement of Submacular Hemorrhages in Age-Related Macular Degeneration with Subretinal Tissue Plasminogen Activator and Air. Ophthalmology 2015, 122, 123–128. [Google Scholar] [CrossRef]
- Kamei, M.; Tano, Y.; Maeno, T.; Ikuno, Y.; Mitsuda, H.; Yuasa, T. Surgical Removal of Submacular Hemorrhage Using Tissue Plasminogen Activator and Perfluorocarbon Liquid. Am. J. Ophthalmol. 1996, 121, 267–275. [Google Scholar] [CrossRef]
- Niwa, Y.; Kakinoki, M.; Sawada, T.; Wang, X.; Ohji, M. Ranibizumab and Aflibercept: Intraocular Pharmacokinetics and Their Effects on Aqueous VEGF Level in Vitrectomized and Nonvitrectomized Macaque Eyes. Investig. Opthalmol. Vis. Sci. 2015, 56, 6501–6505. [Google Scholar] [CrossRef]
- Gabrielle, P.-H.; Delyfer, M.-N.; Glacet-Bernard, A.; Conart, J.B.; Uzzan, J.; Kodjikian, L.; Arndt, C.; Tadayoni, R.; Soudry-Faure, A.; Garcher, C.P.C. STAR: A Randomized Controlled Trial for Submacular Hemorrhage Secondary to Age-Related Macular Degeneration. Ophthalmology 2023, 130, 947–957. [Google Scholar] [CrossRef]
- Jeong, S.; Park, D.-G.; Sagong, M. Management of a Submacular Hemorrhage Secondary to Age-Related Macular Degeneration: A Comparison of Three Treatment Modalities. J. Clin. Med. 2020, 9, 3088. [Google Scholar] [CrossRef] [PubMed]
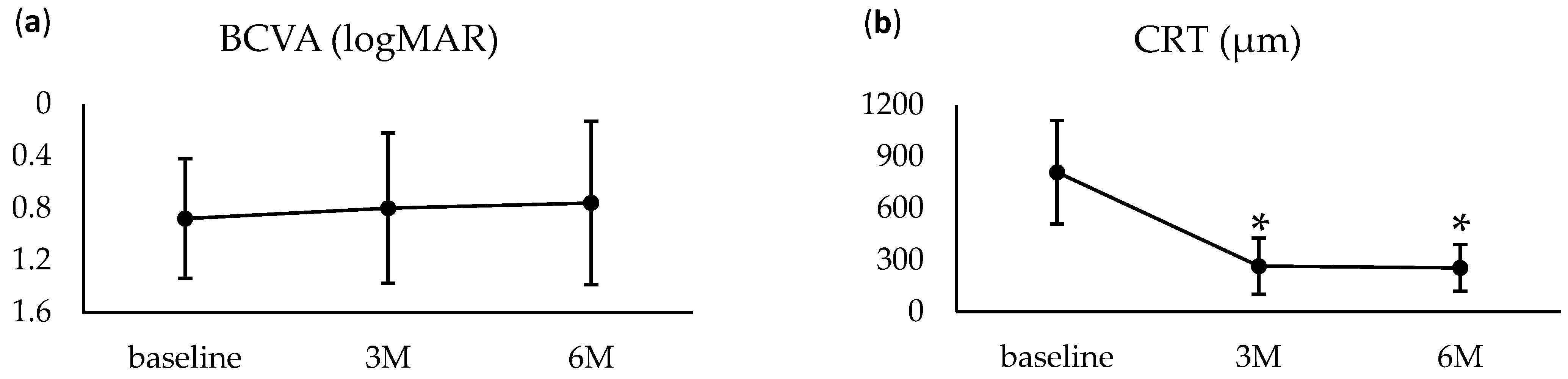
| 6 Months’ Follow-Up | 12 Months’ Follow-Up | |
|---|---|---|
| Number of eyes/ patients | 22/22 | 15/15 |
| Age (year; mean ± SD, range) | 75.5 ± 9.25 | 74.0 ± 9.88 |
| Sex, eyes (%) | ||
| Male | 19 (86.4) | 13 (15.4) |
| Female | 3 (13.6) | 2 (13.3) |
| Subtype, eyes (%) | ||
| Type 1 MNV | 5 (22.7) | 2 (13.3) |
| PCV | 17 (77.3) | 13 (86.7) |
| Anticoagulant treatment, yes (%) | 2 (9.10) | 1 (6.67) |
| Duration of SMH (days; mean ± SD, range) | 7.63 ± 5.12 | 8.40 ± 5.43 |
| Previous anti-VEGF treatment, yes (%) | 8 (36.4) | 5 (33.3) |
| BCVA (logMAR; mean ± SD) | 0.88 ± 0.46 | 0.92 ± 0.47 |
| CRT (μm; mean ± SD, range) | 810.3 ± 300.8 | 801.6 ± 290.9 |
| Size of SMH (DD; mean ± SD, range) | 5.84 ± 3.48 | 5.58 ± 3.59 |
| Height of retinal detachment(μm; mean ± SD, range) | 482.2 ± 245.1 | 473.1 ± 286.6 |
| Patients with Visual Improvement (n = 15) | Patients Without Visual Improvement (n = 7) | p (Univariate) | p (Multivariate) | Odds Ratio (95% Cl) | |
|---|---|---|---|---|---|
| Age (year; mean) | 77.7 ± 7.70 | 70.7 ± 11.1 | 0.10 | ||
| Sex | 0.23 | ||||
| Male | 14 (63.6) | 5 (22.7) | |||
| Female | 1 (6.67) | 2 (9.1) | |||
| Subtype, eyes (%) | 0.48 | ||||
| Type 1 MNV | 4 (18.1) | 1 (4.50) | |||
| PCV | 11 (50.0) | 6 (27.3) | |||
| Duration of SMH (days) | 5.11 ± 3.86 | 9.38 ± 5.29 | <0.01 | 0.02 | 0.51–0.95 |
| BCVA (logMAR) | 0.89 ± 0.37 | 0.85 ± 0.64 | 0.86 | 0.65 | 0.14–23.2 |
| Size of SMH (DD) | 5.60 ± 2.53 | 6.33 ± 5.19 | 0.90 | 0.40 | 0.79–1.82 |
| Height of retinal detachment (μm) | 420.4 ± 246.8 | 545.7 ± 287.4 | 0.42 | 0.29 | 0.99–1.00 |
| Patients with VH (n = 7) | Patients Without VH (n = 15) | p (Univariate) | p (Multivariate) | Odds Ratio (95% Cl) | |
|---|---|---|---|---|---|
| Age (year; mean) | 74.7 ± 9.59 | 75.8 ± 9.41 | 0.81 | ||
| Sex | 0.71 | ||||
| Male | 6 (27.3) | 13 (59.1) | |||
| Female | 1 (4.55) | 2 (9.1) | |||
| Subtype, eyes (%) | 0.11 | ||||
| Type 1 MNV | 0 (0) | 5 (22.7) | |||
| PCV | 7 (31.8) | 10 (45.5) | |||
| Duration of SMH (days) | 8.00 ± 6.40 | 7.47 ± 4.66 | 0.83 | 0.22 | 0.42–1.22 |
| BCVA (logMAR) | 0.84 ± 0.56 | 0.90 ± 0.42 | 0.80 | 0.26 | 0.01–3.93 |
| Size of SMH (DD) | 8.61 ± 4.77 | 4.55 ± 1.68 | 0.02 | 0.06 | 0.95–6.02 |
| Height of retinal detachment (μm) | 409.0 ± 343.3 | 484.2 ± 221.9 | 0.95 | 0.42 | 1.00–1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ota, H.; Takeuchi, J.; Nonogaki, R.; Tamura, K.; Kominami, T. Pneumatic Displacement and Anti-VEGF Therapy for Submacular Hemorrhage in Neovascular Age-Related Macular Degeneration: A Retrospective Study. J. Clin. Med. 2025, 14, 3154. https://doi.org/10.3390/jcm14093154
Ota H, Takeuchi J, Nonogaki R, Tamura K, Kominami T. Pneumatic Displacement and Anti-VEGF Therapy for Submacular Hemorrhage in Neovascular Age-Related Macular Degeneration: A Retrospective Study. Journal of Clinical Medicine. 2025; 14(9):3154. https://doi.org/10.3390/jcm14093154
Chicago/Turabian StyleOta, Hikaru, Jun Takeuchi, Ryo Nonogaki, Kazuma Tamura, and Taro Kominami. 2025. "Pneumatic Displacement and Anti-VEGF Therapy for Submacular Hemorrhage in Neovascular Age-Related Macular Degeneration: A Retrospective Study" Journal of Clinical Medicine 14, no. 9: 3154. https://doi.org/10.3390/jcm14093154
APA StyleOta, H., Takeuchi, J., Nonogaki, R., Tamura, K., & Kominami, T. (2025). Pneumatic Displacement and Anti-VEGF Therapy for Submacular Hemorrhage in Neovascular Age-Related Macular Degeneration: A Retrospective Study. Journal of Clinical Medicine, 14(9), 3154. https://doi.org/10.3390/jcm14093154






