Barbed and Non-Barbed Suture Materials for Ventral Hernia Repair: An Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. Study Outcomes
2.4. Surgery
2.5. Follow-Up
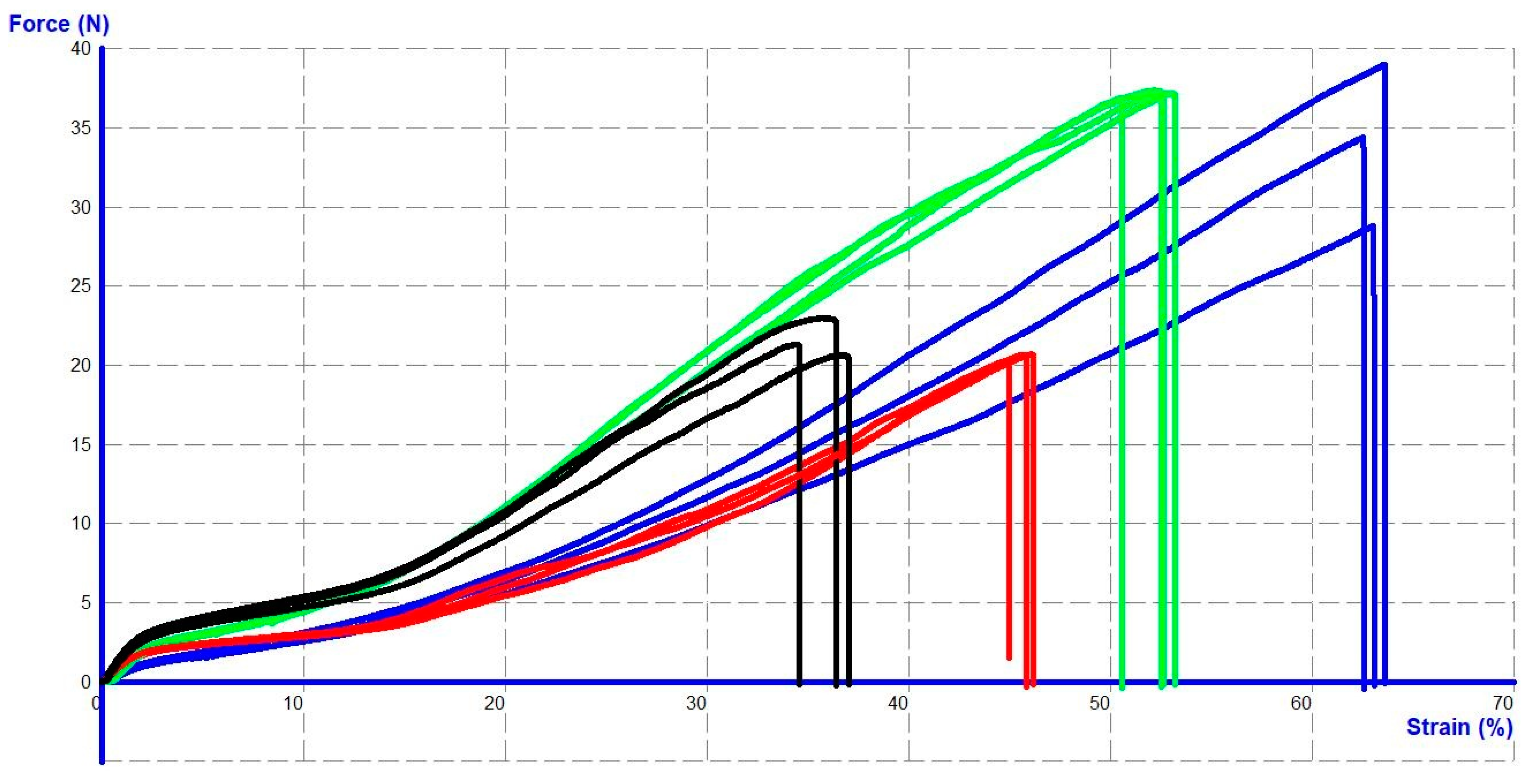
2.6. Mechanical Tensile Tests
2.7. Histopathology
2.8. Statistical Analysis
3. Results
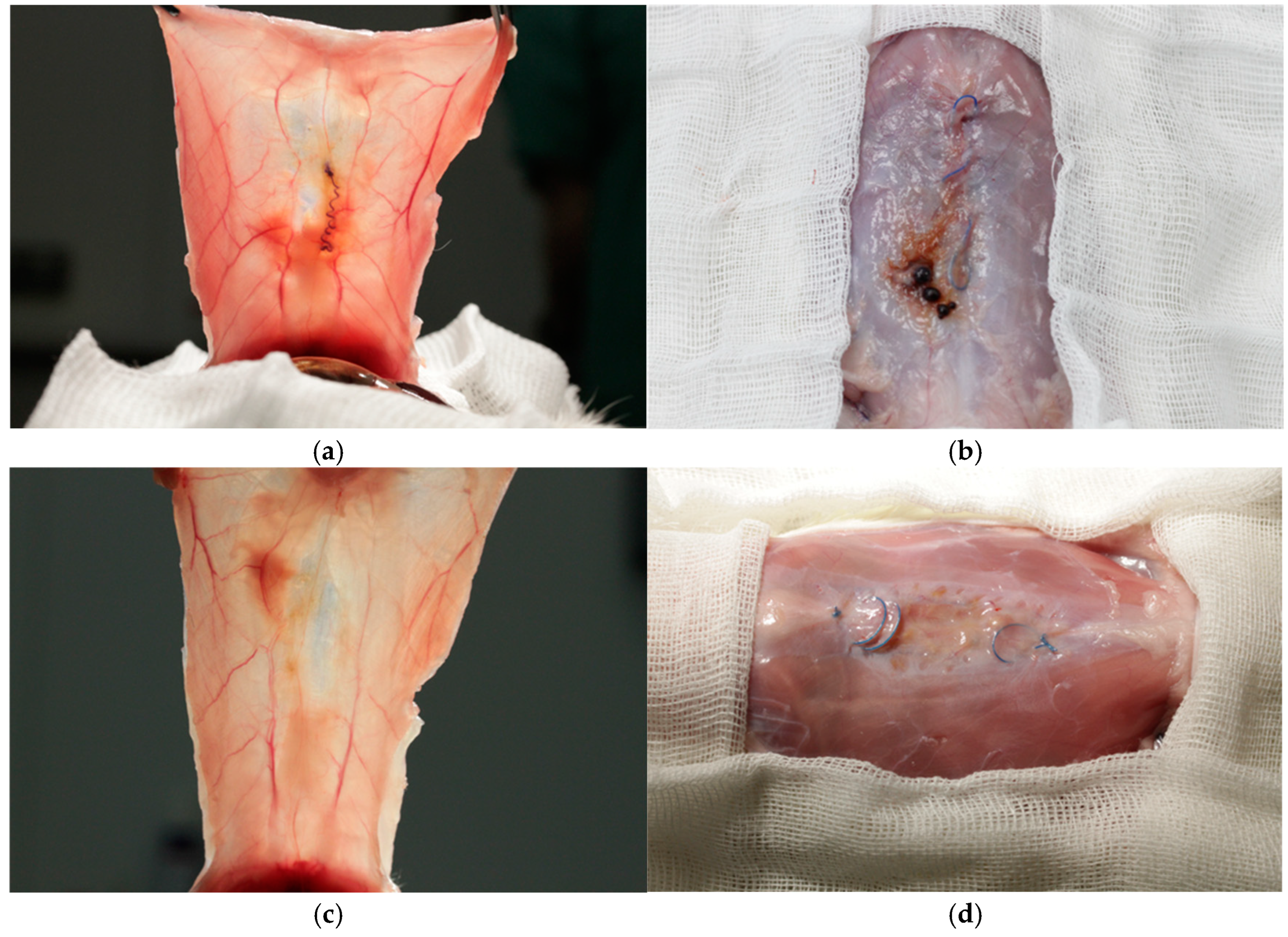
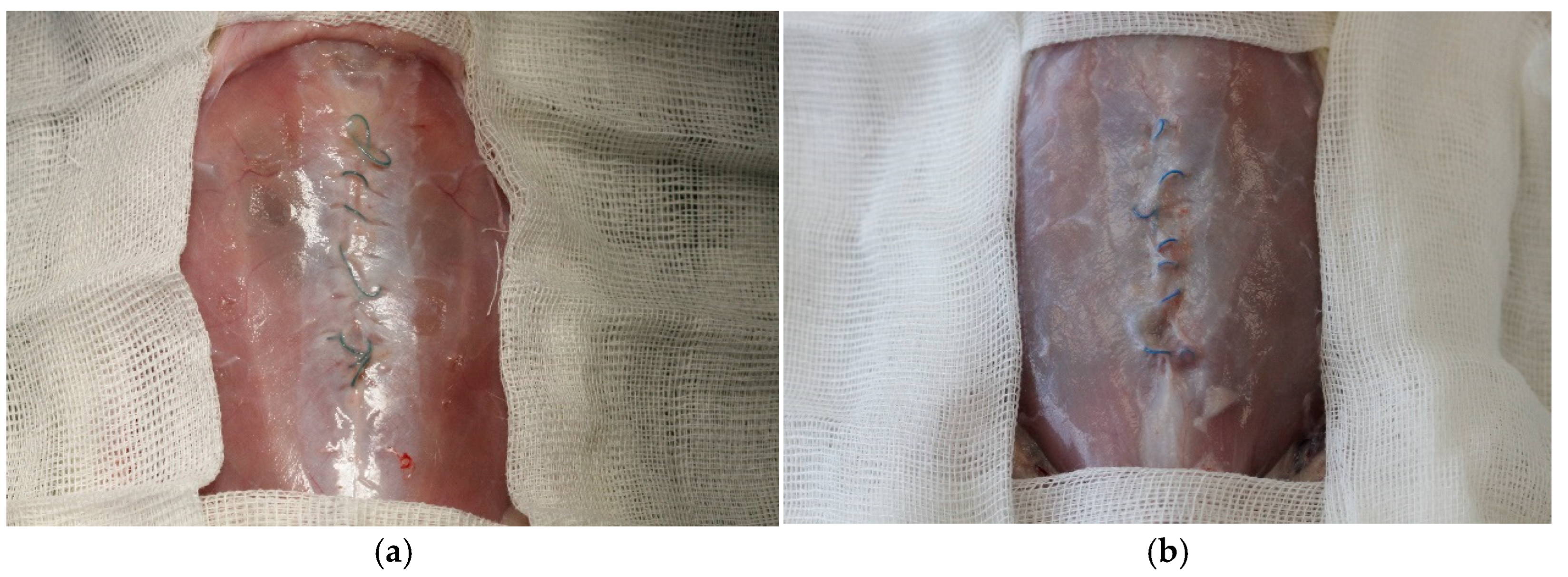
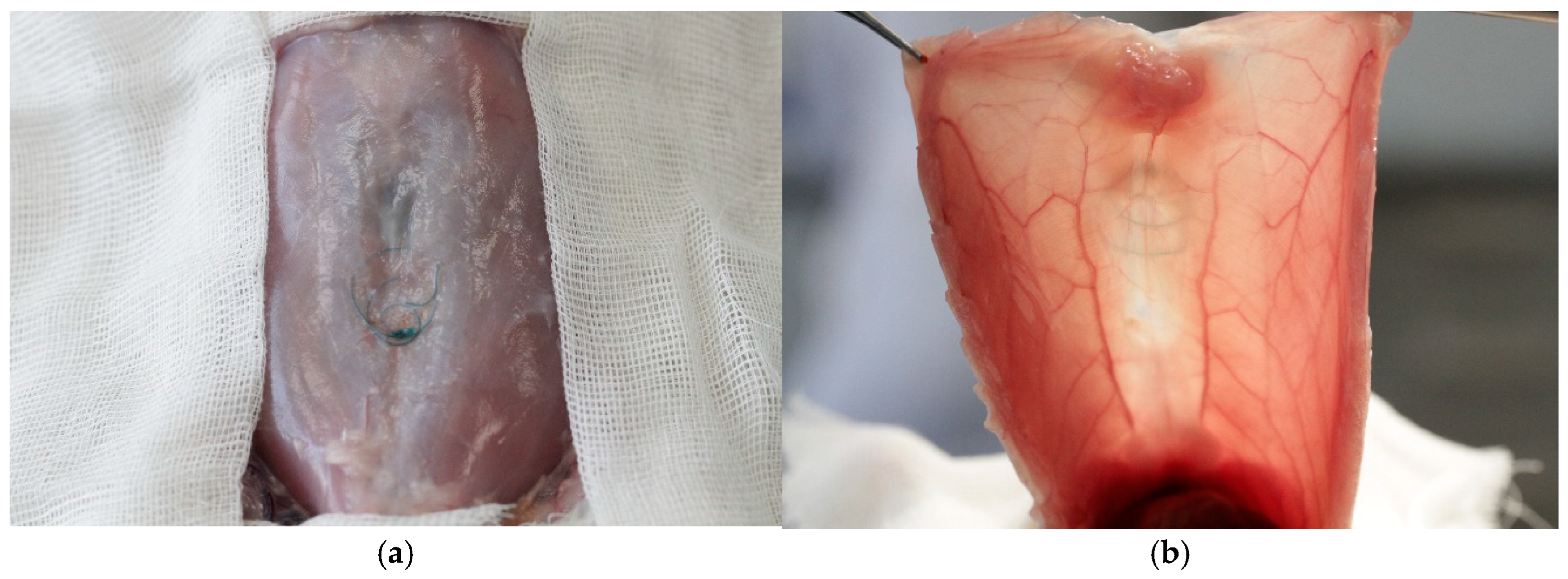
3.1. Complications
3.2. Structural and Mechanical Properties of Intact Sutures
3.3. The Mechanical Properties of the Sutures Depend on the Location and Timing of Implantation
3.4. Histology
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogmark, P.; Petersson, U.; Bringman, S.; Eklund, A.; Ezra, E.; Sevonius, D.; Smedberg, S.; Österberg, J.; Montgomery, A. Short-term outcomes for open and laparoscopic midline incisional hernia repair: A randomized multicenter controlled trial: The ProLOVE (prospective randomized trial on open versus laparoscopic operation of ventral eventrations) trial. Ann. Surg. 2013, 258, 37–45. [Google Scholar]
- Natarajan, S.; Meenaa, S.; Thimmaiah, K. A randomised prospective study to evaluate preperitoneal mesh repair versus onlay mesh repair and laparo-scopic IPOM in incisional hernia surgery. Indian J. Surg. 2017, 79, 96–100. [Google Scholar] [PubMed]
- Eker, H.H.; Hansson, B.M.; Buunen, M.; Janssen, I.M.; Pierik, R.E.; Hop, W.C.; Bonjer, H.J.; Jeekel, J.; Lange, J.F. Laparoscopic vs. open incisional hernia repair: A randomized clinical trial. JAMA Surg. 2013, 148, 259–263. [Google Scholar] [PubMed]
- Olmi, S.; Scaini, A.; Cesana, G.C.; Erba, L.; Croce, E. Laparoscopic versus open incisional hernia repair: An open randomized controlled study. Surg. Endosc. 2007, 21, 555–559. [Google Scholar]
- Navarra, G.; Musolino, C.; De Marco, M.L.; Bartolotta, M.; Barbera, A.; Centorrino, T. Retromuscular sutured incisional hernia repair: A randomized controlled trial to com-pare open and laparoscopic approach. Surg. Laparosc. Endosc. Percutaneous Tech. 2007, 17, 86–90. [Google Scholar]
- Villa, M.T.; White, L.E.; Alam, M.; Yoo, S.S.; Walton, R.L. Barbed sutures: A review of the literature. Plast. Reconstr. Surg. 2008, 121, 102e–108e. [Google Scholar]
- Iavazzo, C.; Mamais, I.; Gkegkes, I.D. The role of knotless barbed suture in gynecologic surgery: Systematic review and meta-analysis. Surg. Innov. 2015, 22, 528–539. [Google Scholar] [CrossRef]
- Bautista, T.; Shabbir, A.; Rao, J.; So, J.; Kono, K.; Durai, P. Enterotomy closure using knot-less and barbed suture in laparoscopic upper gastrointestinal surgeries. Surg. Innov. 2016, 30, 1699–1703. [Google Scholar]
- Rosen, A.; Hartman, T. Repair of the midline fascial defect in abdominoplasty with long-acting barbed and smooth absorbable sutures. Aesthetic Surg. J. 2011, 31, 668–673. [Google Scholar]
- Su, X.; Lin, Y.; Wu, Y.; Feng, K.; Xiang, N.; Hu, Z.; Zhou, J.; Guo, Q.; Chen, Z.; Liao, G.; et al. Effectiveness and safety of knotless barbed sutures in cosmetic surgery: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthetic Surg. 2023, 87, 416–429. [Google Scholar]
- Kathopoulis, N.; Prodromidou, A.; Douligeris, A.; Diakosavvas, M.; Zacharakis, D.; Kypriotis, K.; Chatzipapas, I.; Grigoriadis, T.; Protopapas, A. Barbed Sutures Compared With Conventional Sutures During Laparoscopic Myomectomy: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2024, 144, e81–e100. [Google Scholar]
- Hafermann, J.; Silas, U.; Saunders, R. Efficacy and safety of V-Loc™ barbed sutures versus conventional suture techniques in gynecological surgery: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2024, 309, 1249–1265. [Google Scholar] [PubMed]
- Feroci, F.; Giani, I.; Baraghini, M.; Romoli, L.; Zalla, T.; Quattromani, R.; Cantafio, S.; Scatizzi, M. Barbed versus traditional suture for enterotomy closure after laparoscopic right colectomy with intracorporeal mechanical anastomosis: A case–control study. Updates Surg. 2018, 70, 433–439. [Google Scholar] [PubMed]
- Anand, S.; Jukić, M.; Krishnan, N.; Pogorelić, Z. Barbed Versus Non-Barbed Suture for Pyeloplasty via the Minimally Invasive Approach: A Systematic Review and Meta-Analysis. J. Laparoendosc. Adv. Surg. Tech. 2022, 32, 1056–1063. [Google Scholar]
- Shehata, M.A.; Negm, M.A.; Shalaby, M.M.; Mansour, M.A.; Elhaddad, A.A. Unidirectional barbed sutures vs. interrupted intracorporeal knots in thoracoscopic repair of congenital diaphragmatic hernia in pediatrics. Front. Pediatr. 2024, 12, 1348753. [Google Scholar]
- Ataya, K.; Patel, N.; Yang, W.; Aljaafreh, A.; Melebari, S.S.; Global Obesity Collaborative. Safety and Efficacy of Barbed Sutures Compared to Non-barbed Sutures in Bariatric Surgery: An Updated Systematic Review and Meta-analysis. Obes. Surg. 2024, 34, 3324–3334. [Google Scholar]
- Nambi Gowri, K.; King, M.W. A Review of Barbed Sutures—Evolution, Applications and Clinical Significance. Bioengineering 2023, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Cortez, R.; Lazcano, E.; Miller, T.; Hein, R.E.; Constantine, R.S.; Anigian, K.; Davis, K.E.; Kenkel, J.M. Barbed Sutures and Wound Complications in Plastic Surgery: An Analysis of Outcomes. Aesthetic Surg. J. 2015, 35, 178–188. [Google Scholar]
- Stabile, G.; Romano, F.; De Santo, D.; Sorrentino, F.; Nappi, L.; Cracco, F.; Ricci, G. Case Report: Bowel Occlusion Following the Use of Barbed Sutures in Abdominal Surgery. A Single-Center Experience and Literature Review. Front. Surg. 2021, 8, 626505. [Google Scholar] [CrossRef]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab. Anim. 2017, 52, 135–141. [Google Scholar] [CrossRef]
- Zaruby, J.; Gingras, K.; Taylor, J.; Maul, D. An in vivo comparison of barbed suture devices and conventional monofilament sutures for cosmetic skin closure:biomechanical wound strength and histology. Aesthetic Surg. J. 2011, 31, 232–240. [Google Scholar]
- Sanders, D.L.; Pawlak, M.M.; Simons, M.P.; Aufenacker, T.; Balla, A.; Berger, C.; Berrevoet, F.; de Beaux, A.C.; East, B.; Henriksen, N.A.; et al. Midline incisional hernia guidelines: The European Hernia Society. Br. J. Surg. 2023, 110, 1732–1768. [Google Scholar]
- Arias-Espinosa, L.; Wang, A.; Wermelinger, J.P.; Olson, M.A.; Phillips, S.; Xie, W.; de Pena Pena, X.; Pereira, X.; Damani, T.; Malcher, F. The current role of barbed sutures in fascial closure of ventral hernia repair: A multicenter study using the abdominal core health quality collaborative database. Surg. Endosc. 2024, 38, 6657–6670. [Google Scholar]
- Hernández-Granados, P.; Henriksen, N.A.; Berrevoet, F.; Cuccurullo, D.; López-Cano, M.; Nienhuijs, S.; Ross, D.; Montgomery, A. European Hernia Society guidelines on management of rectus diastasis. Br. J. Surg. 2021, 108, 1189–1191. [Google Scholar]
- Xiang, N.; Lin, Y.; Su, X.; Hu, Z.; Zhou, J.; Wu, Y.; Du, L.; Huang, J. Assessing the application of barbed sutures in comparison to conventional sutures for surgical applications: A global systematic review and meta-analysis of preclinical animal studies. Int. J. Surg. 2024, 110, 3060–3071. [Google Scholar]
- van Uchelen, J.; Kon, M.; Werker, P.M.N. The long-term durability of plication of the anterior rectus sheath assessed by ultrasonography. Plast. Reconstr. Surg. 2001, 107, 1578–1584. [Google Scholar] [PubMed]
- Law, A.Y.; Butler, J.R.; Patnaik, S.S.; Cooley, J.A.; Elder, S.H. Biomechanical Testing and Histologic Examination of Intradermal Skin Closure in Dogs Using Barbed Suture Device and Non-Barbed Monofilament Suture. Vet. Surg. 2017, 46, 59–66. [Google Scholar] [PubMed]
- Hoer, J.J.; Junge, K.; Schachtrupp, A.; Klinge, U.; Schumpelick, V. Influence of laparotomy closure technique on collagen synthesis in the incisional region. Hernia 2002, 6, 93–98. [Google Scholar]
- Höer, J.; Töns, C.; Schachtrupp, A.; Anurov, M.; Titkova, S.; Oettinger, A.; Wetter, O.; Schumpelick, V. Quantitative evaluation of abdominal wall perfusion after different types of laparotomy closure using laser-fluorescence videography. Hernia 2002, 6, 11–16. [Google Scholar]
- Kushner, B.S.; Arefanian, S.; McAllister, J.; Tan, W.H.; Grant, M.; MacGregor, R.; Majumder, A.; Blatnik, J.A. Examination of abdominal wall perfusion using varying suture techniques for midline abdominal laparotomy closure. Surg. Endosc. 2022, 36, 3843–3851. [Google Scholar]
- Hong, W.; Chen, I.-C.; Su, C.-Y.; Perng, C.-K.; Ma, H.; Fang, H.-W. Evaluating Pull-Out Strength of Barbed Suture In Vitro by Using Porcine Tissue and Polydimethylsiloxane (PDMS). Polymers 2022, 14, 2170. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, Y.; Zhao, X.; Lang, D.; Zhou, Z. Biomechanical and tissue reaction: The effects of varying sutures size on canine abdominal wall stitching. Front. Vet. Sci. 2023, 10, 1254998. [Google Scholar] [CrossRef]
- Thankam, F.G.; Palanikumar, G.; Fitzgibbons, R.J.; Agrawal, D.K. Molecular mechanisms and potential therapeutic targets in incisional hernia. J. Surg. Res. 2019, 236, 134–143. [Google Scholar] [PubMed]
- Pogorelić, Z.; Stričević, L.; Elezović Baloević, S.; Todorić, J.; Budimir, D. Safety and Effectiveness of Triclosan-Coated Polydioxanone (PDS Plus) versus Uncoated Polydioxanone (PDS II) Sutures for Prevention of Surgical Site Infection after Hypospadias Repair in Children: A 10-Year Single Center Experience with 550 Hypospadias. Biomedicines 2024, 12, 583. [Google Scholar] [CrossRef]
- Ruiz-Tovar, J.; Llavero, C.; Jimenez-Fuertes, M.; Duran, M.; Perez-Lopez, M.; Garcia-Marin, A. Incisional Surgical Site Infection after Abdominal Fascial Closure with Triclosan-Coated Barbed Suture vs Triclosan-Coated Polydioxanone Loop Suture vs Polydioxanone Loop Suture in Emergent Abdominal Surgery: A Randomized Clinical Trial. J. Am. Coll. Surg. 2020, 230, 766–774. [Google Scholar]
- Dilday, J.; McGillen, P.; Park, S.; Gallagher, S.; Lee, H.; Schellenberg, M.; Matsushima, K.; Inaba, K.; Martin, M.J. Is barbed better? Evaluation of triclosan-coated barbed suture on wound complications following emergency laparotomy. J. Trauma Acute Care Surg. 2024, 97, 149–157. [Google Scholar]
- Depuydt, M.; Van Egmond, S.; Petersen, S.M.; Muysoms, F.; Henriksen, N.; Deerenberg, E. Systematic review and meta-analysis comparing surgical site infection in abdominal surgery between triclosan-coated and uncoated sutures. Hernia 2024, 28, 1017–1027. [Google Scholar]
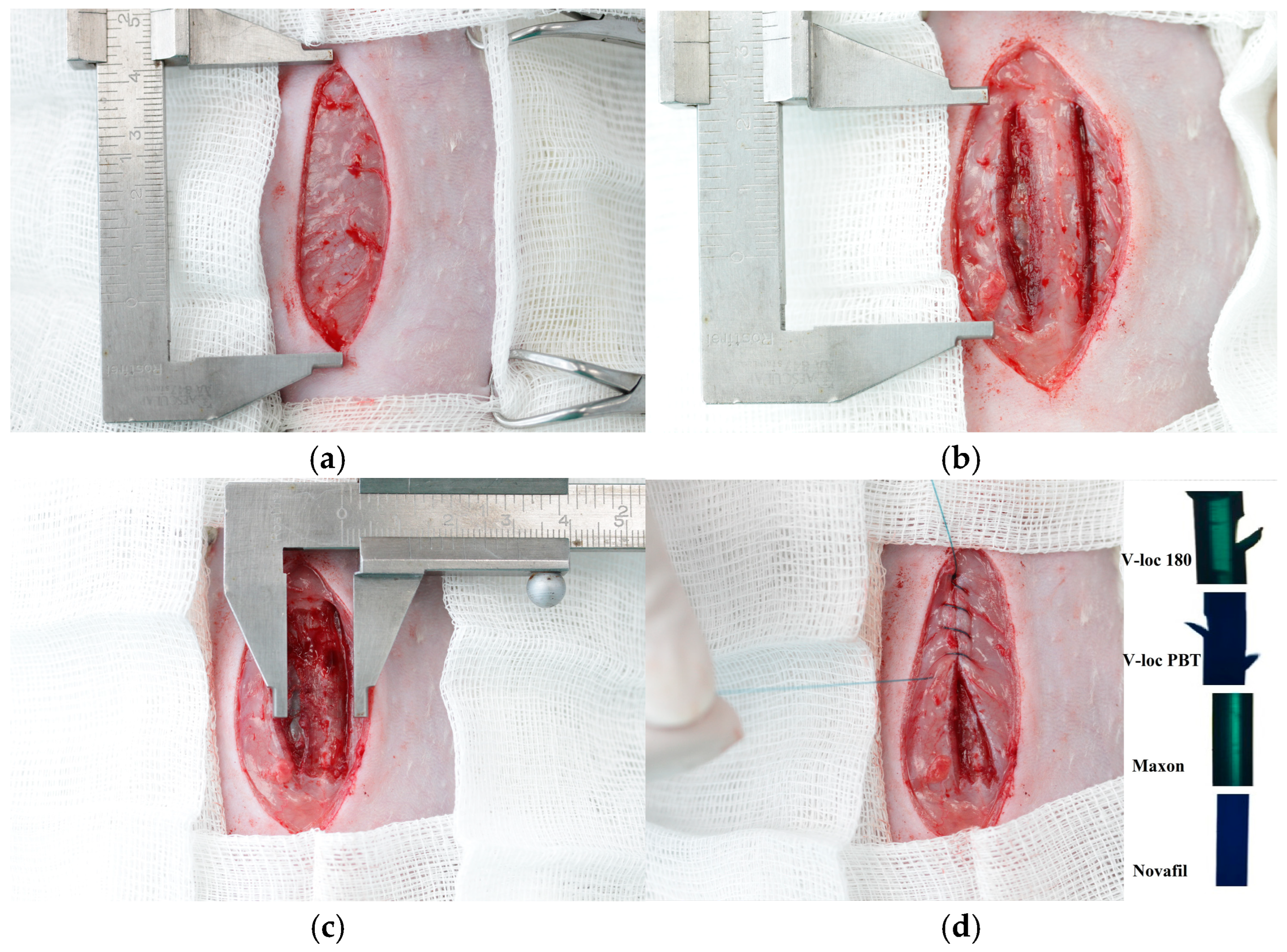
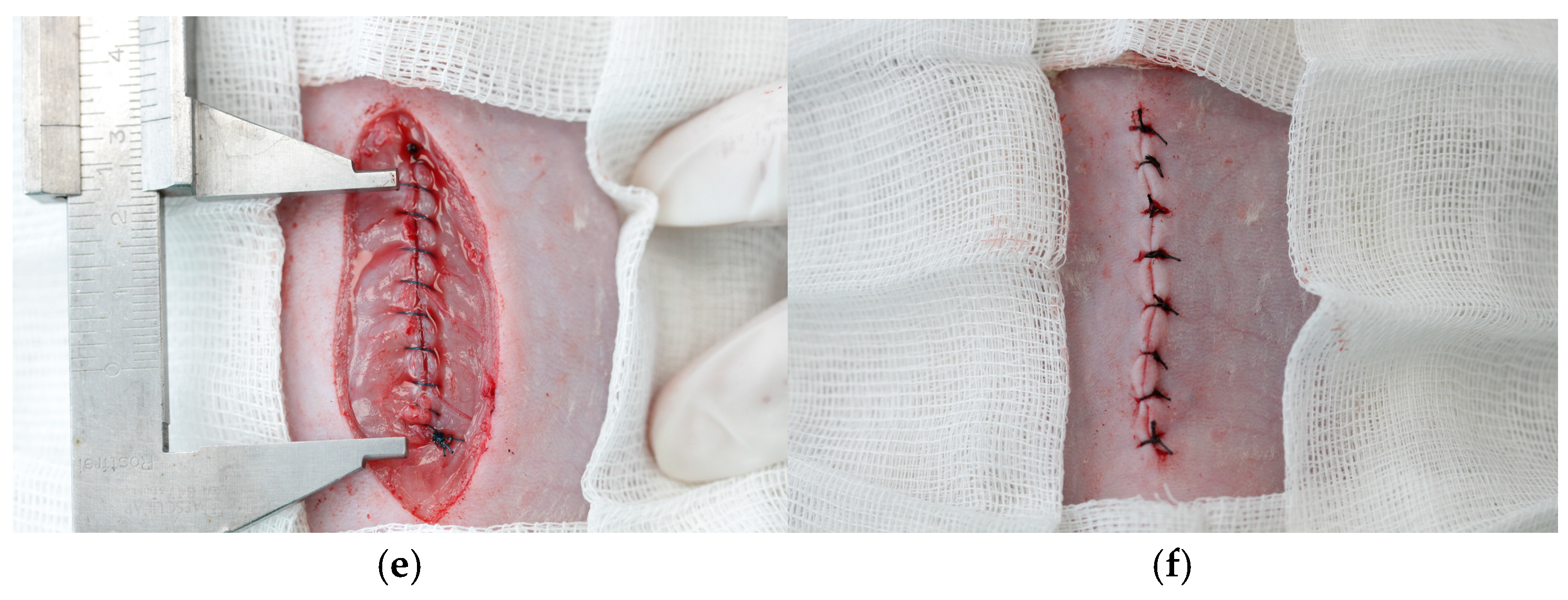
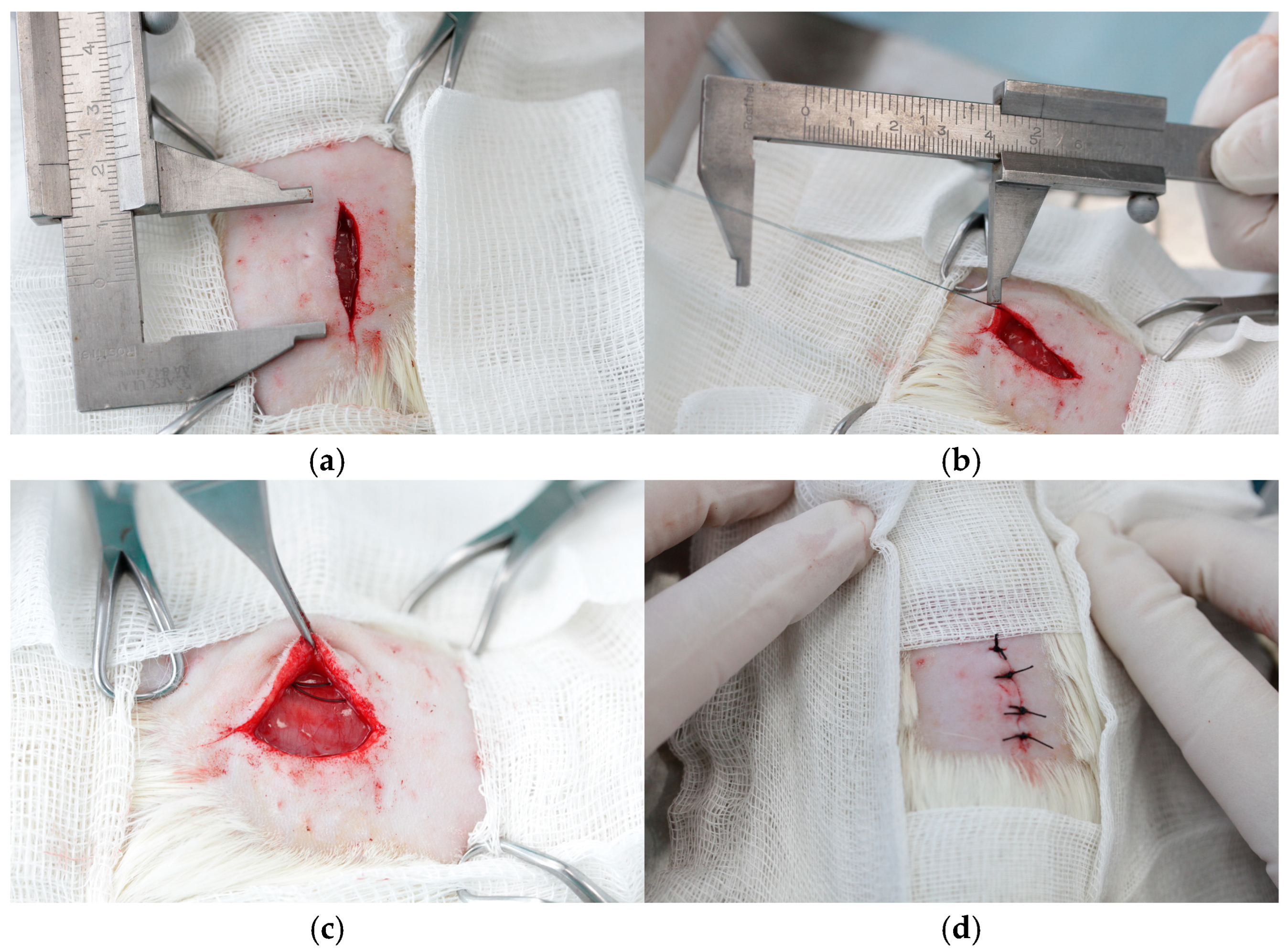
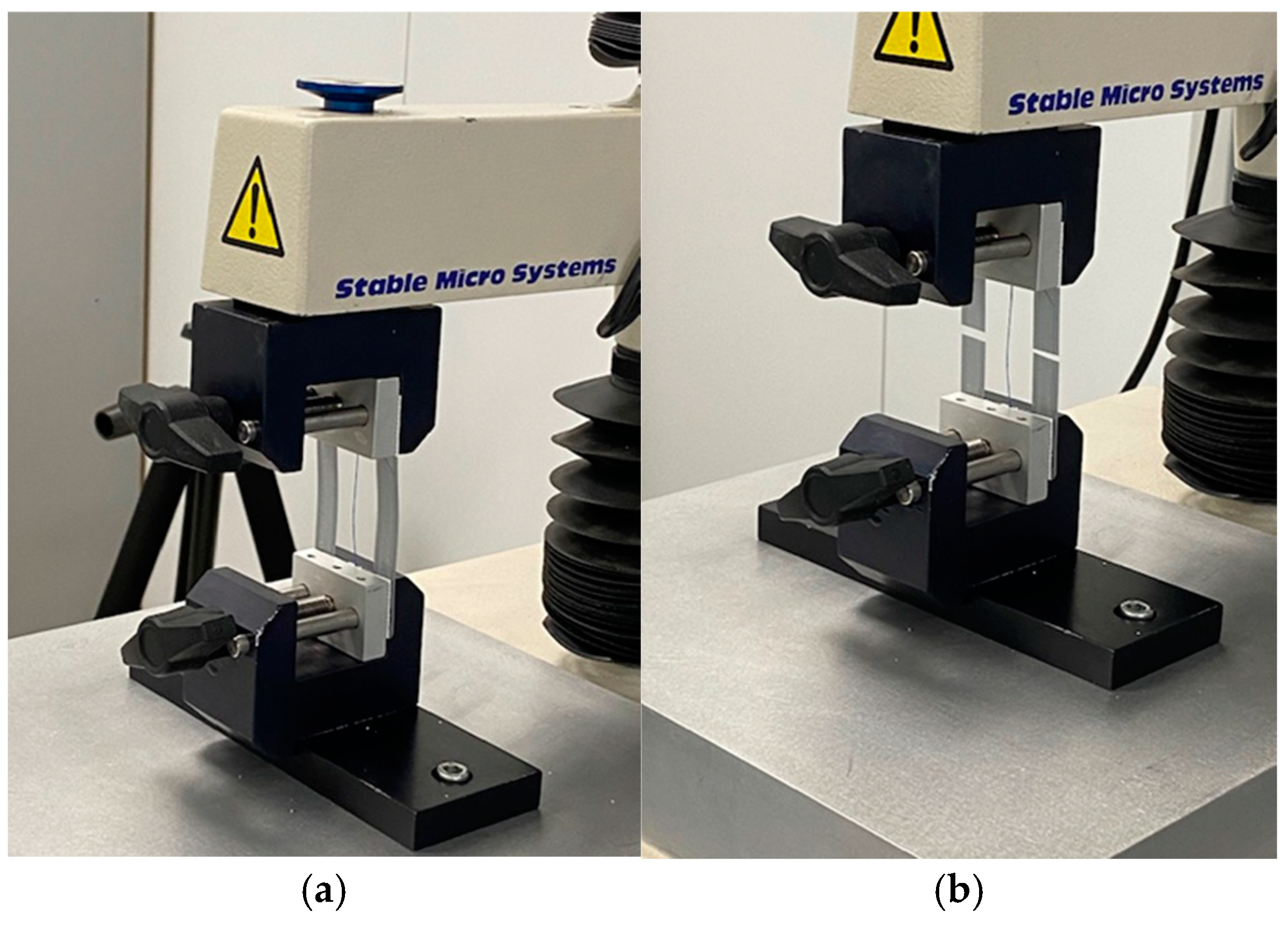

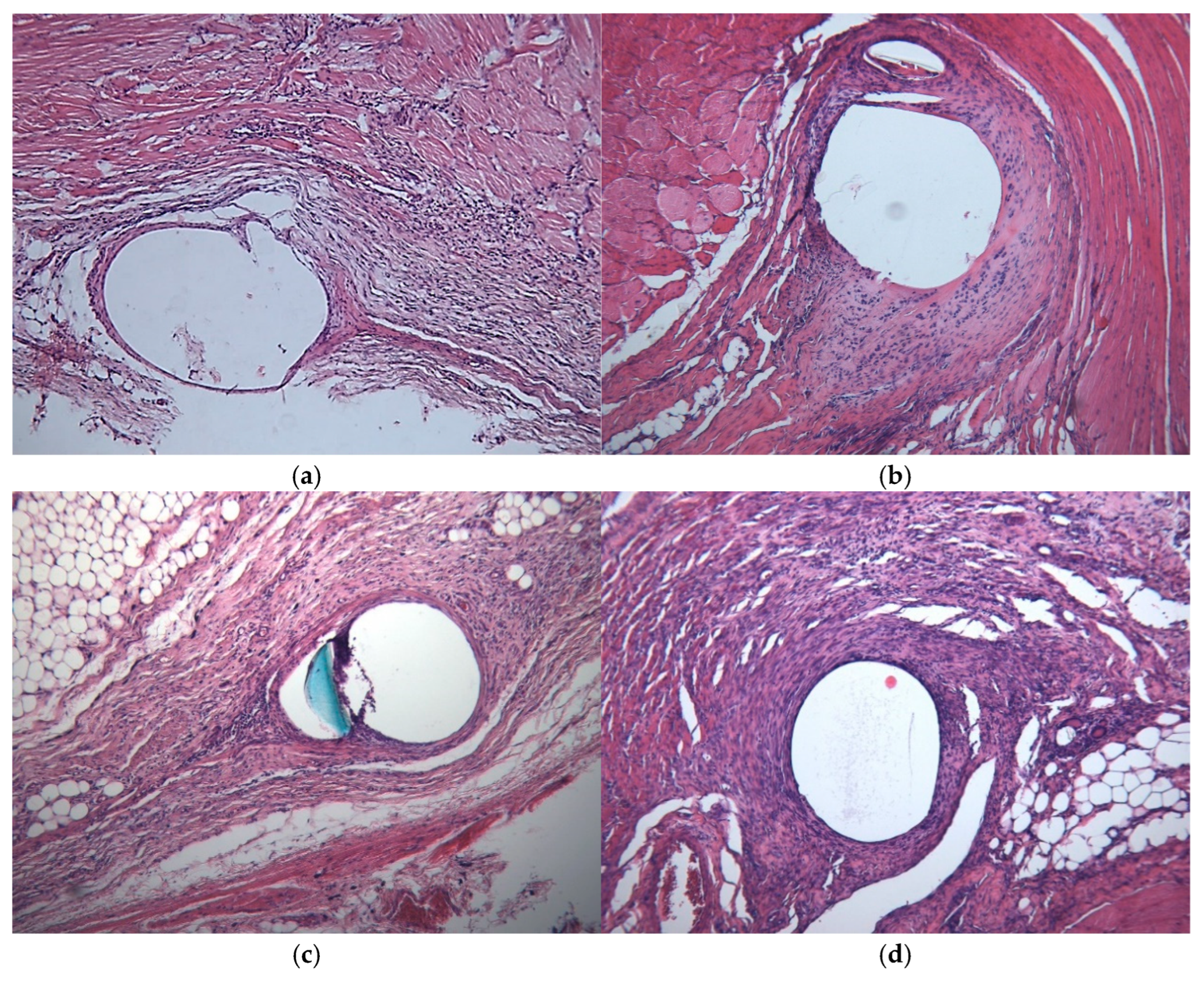
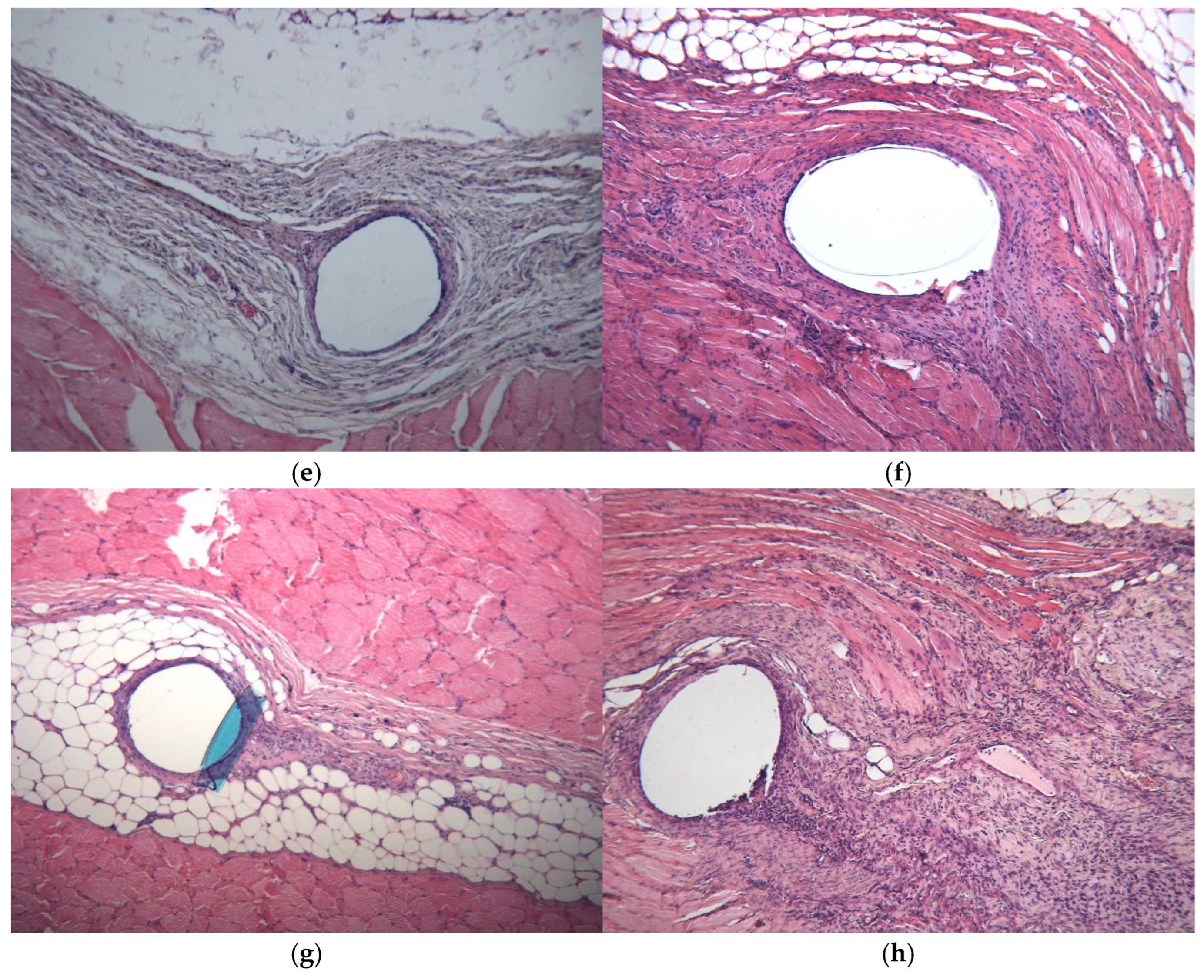
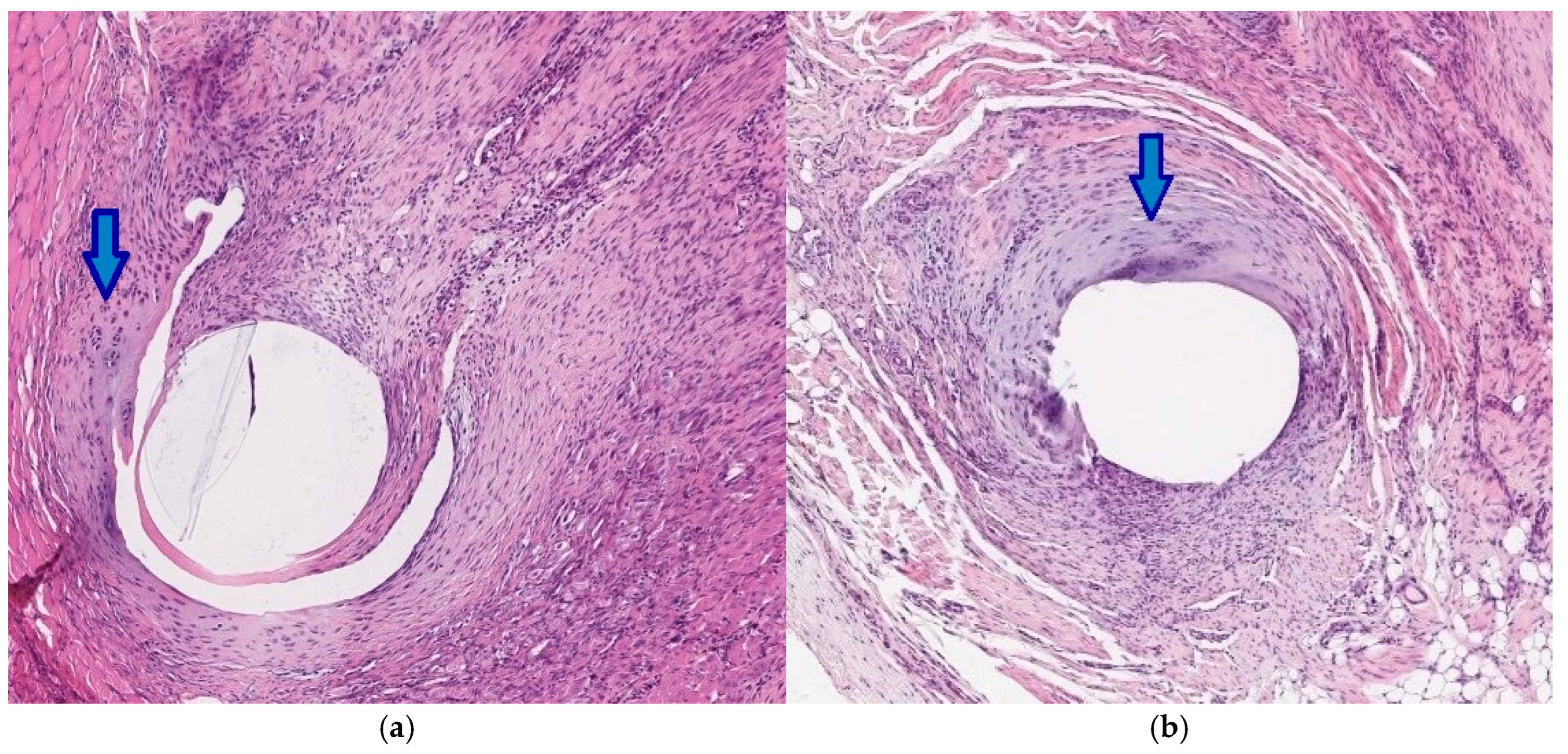
| Histologic Category | Scoring System |
|---|---|
| Degree of necrosis | 0–4 |
| Congestion/edema | 0–4 |
| Cellular infiltrate | 0–4 * |
| Neutrophils | |
| Lymphocytes | |
| Plasma cells | |
| Macrophages | |
| Eosinophils | |
| Giant cells | 0–4 |
| Fibrosis | 0–4 |
| Neovascularity | 0–4 |
| Calcification | 0–4 |
| Fatty infiltration | 0–4 |
| Foreign body reaction | 0/1 |
| V-LocTM 180 | V-LocTM PBT | MaxonTM | NovafilTM | |
|---|---|---|---|---|
| Thickness, mm 3 weeks | 2.52 ± 0.24 | 2.38 ± 0.22 | 2.37 ± 0.21 | 2.24 ± 0.43 |
| Thickness, mm 6 weeks | 3.18 ± 0.43 | 3.42 ± 0.15 | 2.43 ± 0.76 | 2.57 ± 0.23 |
| p | 0.028 | 0.008 | 1 | 0.291 |
| Type of Suture Material | Maximum Load to Failure, N Mean ± SD (Min–Max) | p |
|---|---|---|
| V-LocTM 180 | 37.02 ± 0.42 (36.21–37.44) | <0.001 vs. V-locTM PBT <0.01 vs. MaxonTM <0.001 vs. NovafilTM |
| V-LocTM PBT | 21.31 ± 1.14 (19.59–23.05) | <0.001 vs. V-locTM 180 <0.001 vs. MaxonTM |
| MaxonTM | 33.61 ± 3.04 (28.88–39.10) | <0.01 vs. V-locTM 180 <0.001 vs. V-locTM PBT <0.001 vs. NovafilTM |
| NovafilTM | 20.71 ± 0.87 (19.47–22.13) | <0.001 vs. V-locTM 180 <0.001 vs. MaxonTM |
| Time | Group/Place | Abdominal Wall | Back | p |
|---|---|---|---|---|
| 3 weeks | V-locTM 180 | 33.86 + 1.05 | 34.41 + 1.07 | 0.997 |
| V-locTM PBT | 23.20 + 1.82 | 21.69 + 1.37 | 0.678 | |
| MaxonTM | 36.45 + 4.13 | 31.54 + 3.75 | 0.245 | |
| NovafilTM | 17.59 + 4.22 | 14.69 + 3.54 | 0.371 | |
| 6 weeks | V-locTM 180 | 13.52 + 5.45 | 19.18 + 1.80 | 0.005 |
| V-locTM PBT | 20.94 + 3.06 | 21.51 + 1.36 | 0.987 | |
| MaxonTM | 13.39 + 1.60 | 14.44 + 2.22 | 0.993 | |
| NovafilTM | 19.21 + 2.71 | 19.57 + 1.19 | 0.999 |
| Time | Group/Place | Abdominal Wall | Back | p |
|---|---|---|---|---|
| 3 weeks | V-locTM 180 | 0.79 [0.67–0.96] | 0.34 [0.25–0.50] | 0.028 |
| V-locTM PBT | 0.83 [0.63–0.88] | 0.25 [0.21–0.46] | 0.041 | |
| MaxonTM | 0.83 [0.75–0.92] | 0.42 [0.29–0.54] | 0.021 | |
| NovafilTM | 1.0 [0.84–1.64] | 0.29 [0.21–0.50] | 0.021 | |
| p | 0.483 | 0.814 | ||
| 6 weeks | V-locTM 180 | 0.42 [0.34–0.46] * | 0.42 [0.25–0.58] | 0.859 |
| V-locTM PBT | 0.33 [0.29–0.42] * | 0.54 [0.46–0.63] | 0.061 | |
| MaxonTM | 0.29 [0.17–0.54] * | 0.42 [0.42–0.50] | 0.289 | |
| NovafilTM | 0.21 [0.13–0.29] * | 0.25 [0.17–0.50] | 0.479 | |
| p | 0.252 | 0.281 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivakhov, G.B.; Titkova, S.M.; Anurov, M.V.; Kalinina, A.A.; Shadin, K.I.; Suglob, V.V.; Andriyashkin, A.V.; Sazhin, A.V. Barbed and Non-Barbed Suture Materials for Ventral Hernia Repair: An Experimental Study. J. Clin. Med. 2025, 14, 3139. https://doi.org/10.3390/jcm14093139
Ivakhov GB, Titkova SM, Anurov MV, Kalinina AA, Shadin KI, Suglob VV, Andriyashkin AV, Sazhin AV. Barbed and Non-Barbed Suture Materials for Ventral Hernia Repair: An Experimental Study. Journal of Clinical Medicine. 2025; 14(9):3139. https://doi.org/10.3390/jcm14093139
Chicago/Turabian StyleIvakhov, Georgy B., Svetlana M. Titkova, Mikhail V. Anurov, Aleksandra A. Kalinina, Konstantin I. Shadin, Vladimir V. Suglob, Andrey V. Andriyashkin, and Alexander V. Sazhin. 2025. "Barbed and Non-Barbed Suture Materials for Ventral Hernia Repair: An Experimental Study" Journal of Clinical Medicine 14, no. 9: 3139. https://doi.org/10.3390/jcm14093139
APA StyleIvakhov, G. B., Titkova, S. M., Anurov, M. V., Kalinina, A. A., Shadin, K. I., Suglob, V. V., Andriyashkin, A. V., & Sazhin, A. V. (2025). Barbed and Non-Barbed Suture Materials for Ventral Hernia Repair: An Experimental Study. Journal of Clinical Medicine, 14(9), 3139. https://doi.org/10.3390/jcm14093139







