Long-Term Cardiovascular Risk and Maternal History of Pre-Eclampsia
Abstract
:1. Introduction
2. Cardiovascular Risk in Women with a History of Pre-Eclampsia
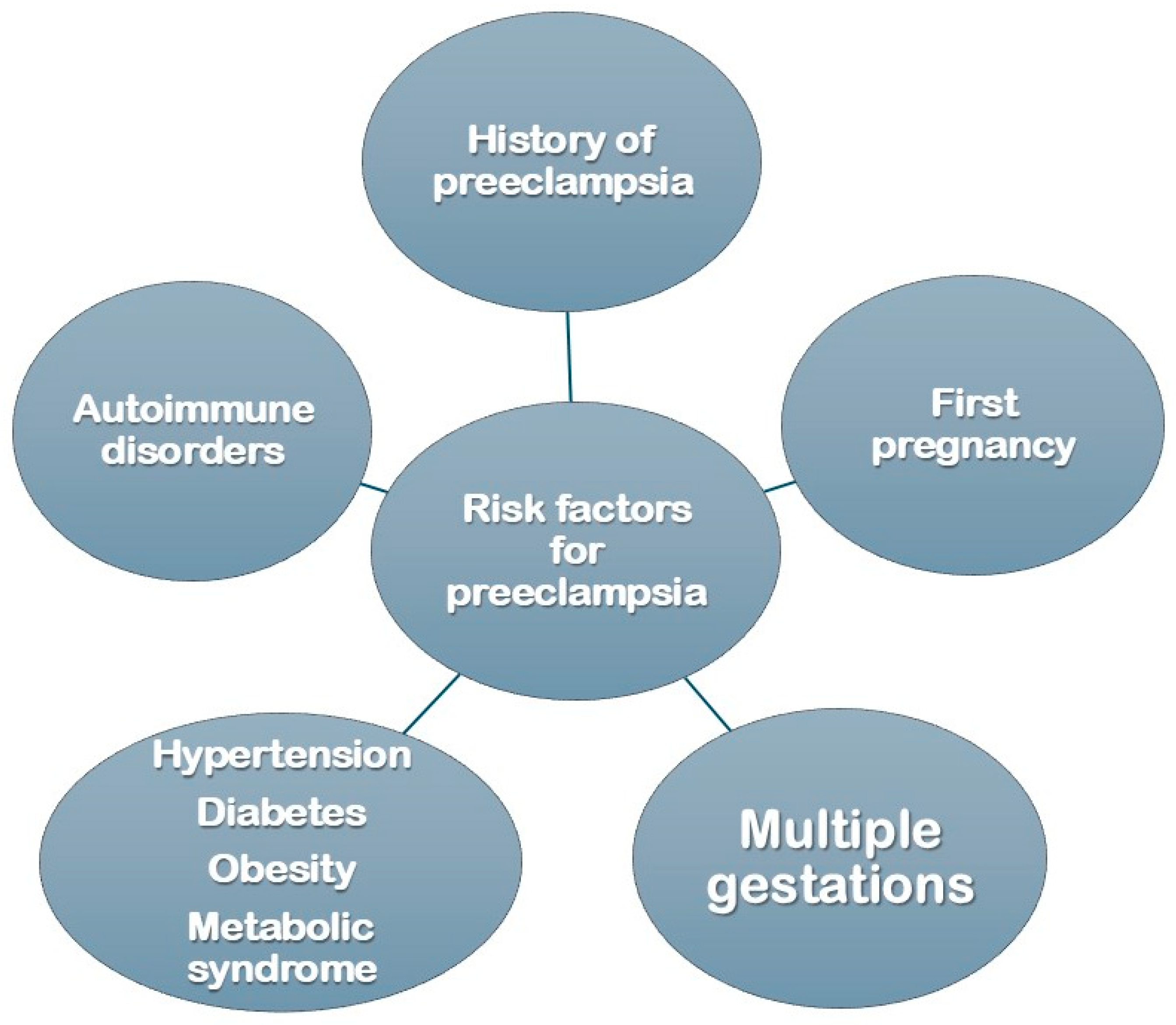
3. Maternal History and Genetic Implications
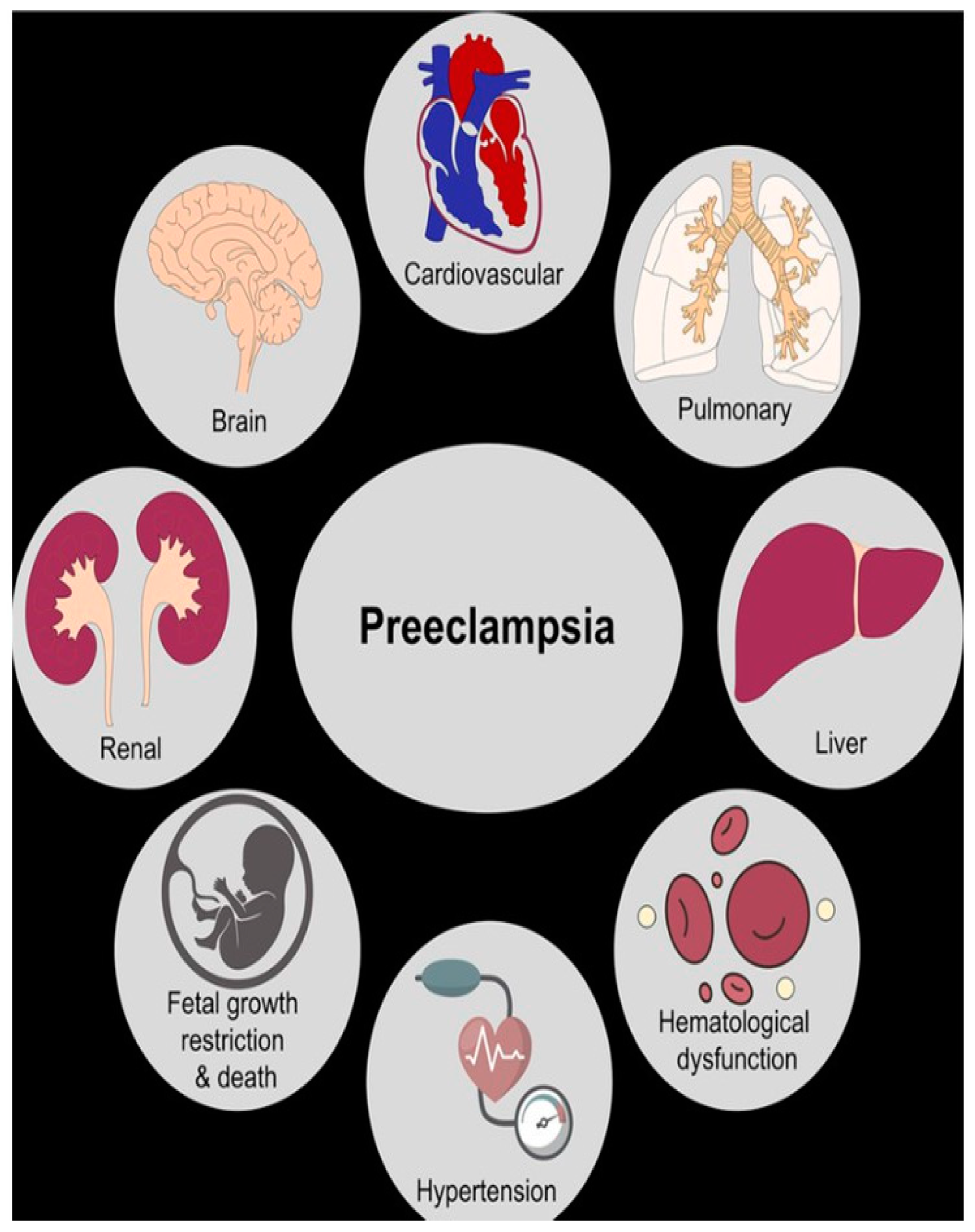
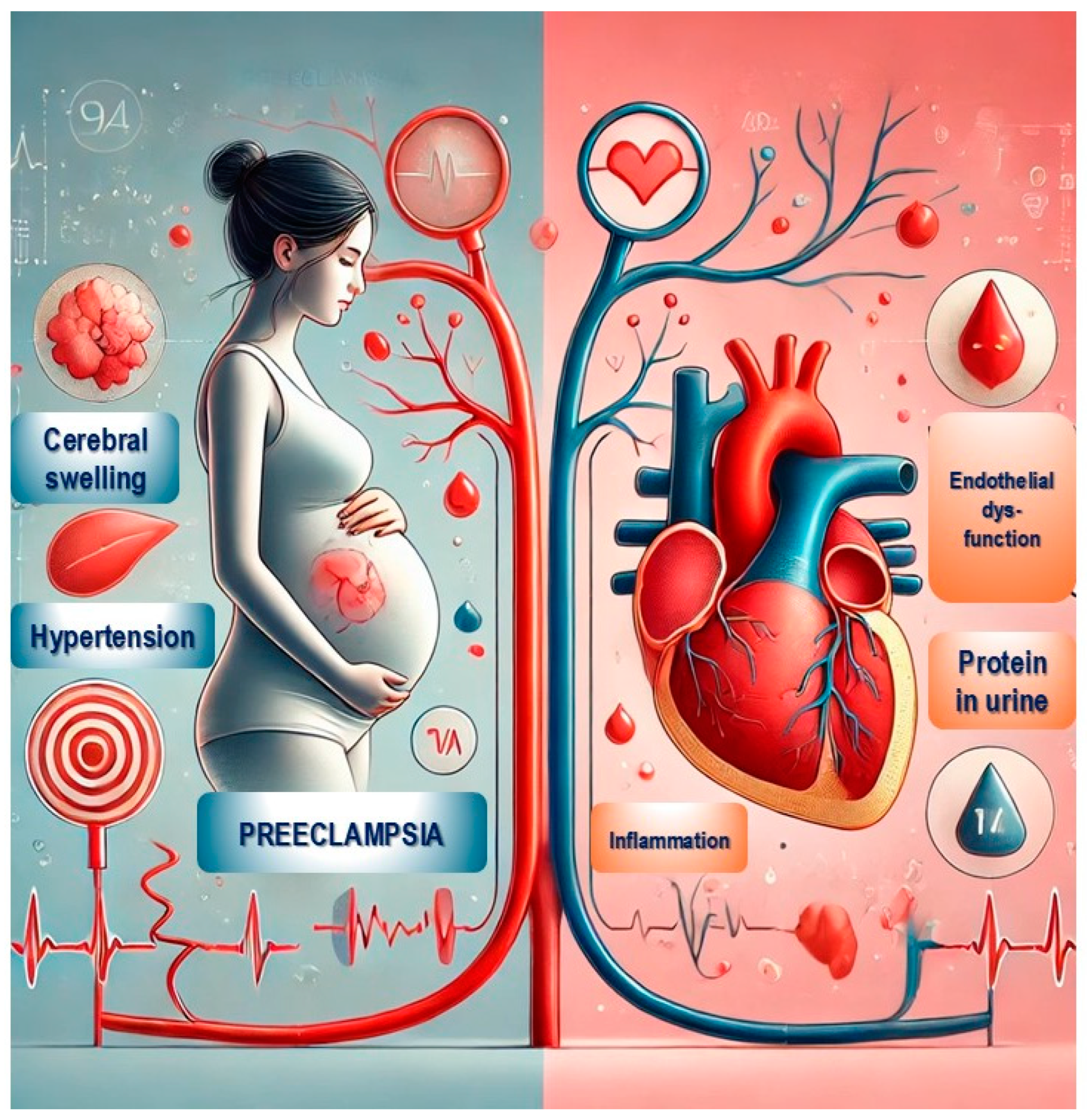
4. Hypertensive Disorders of Pregnancy (HDP)
5. Pre-Eclampsia and the Development of Arterial Hypertension
6. Pre-Eclampsia and Its Impact on Cardiac Structure
7. Pathophysiology of Left Ventricular Hypertrophy in Pre-Eclampsia
8. Pre-Eclampsia and Diastolic Dysfunction
9. Atherosclerosis and Pre-Eclampsia
10. Coronary Disease and Pre-Eclampsia
11. Relationship Between Pre-Eclampsia and HFpEF
12. Pre-Eclampsia and Peripartum Cardiomyopathy
13. Pre-Eclampsia and Postmenopausal Cardiovascular Risk
14. Clinical Implications
15. Future Implications
16. Future Research Directions
17. Conclusions
Funding
Conflicts of Interest
References
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007, 335, 974. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Vermeulen, M.J.; Schull, M.J.; Redelmeier, D.A. Cardiovascular health after maternal placental syndromes (CHAMPS): Population-based retrospective cohort study. Lancet 2005, 366, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Stuart, J.J.; Tanz, L.J.; Missmer, S.A.; Rimm, E.B.; Spiegelman, D.; James-Todd, T.M.; Rich-Edwards, J.W. Hypertensive Disorders of Pregnancy and Maternal Cardiovascular Disease Risk Factor Development: An Observational Cohort Study. Ann. Intern. Med. 2018, 169, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Seely, E.W.; Tsigas, E.; Rich-Edwards, J.W. Preeclampsia and future cardiovascular disease in women: How good are the data and how can we manage our patients? Semin. Perinatol. 2015, 39, 276–283. [Google Scholar] [CrossRef]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef]
- Wu, P.; Green, M.; Myers, J.E. Hypertensive disorders of pregnancy. BMJ 2023, 381, e071653. [Google Scholar] [CrossRef]
- Brown, M.C.; Best, K.E.; Pearce, M.S.; Waugh, J.; Robson, S.C.; Bell, R. Cardiovascular disease risk in women with pre-eclampsia: Systematic review and meta-analysis. Eur. J. Epidemiol. 2013, 28, 1–19. [Google Scholar] [CrossRef]
- Roberts, J.; Hubel, C. The two stage model of preeclampsia: Variations on the theme. Placenta 2009, 30 (Suppl. A), S32–S37. [Google Scholar] [CrossRef]
- Carty, D.M.; Delles, C.; Dominiczak, A.F. Preeclampsia and future maternal health. J. Hypertens. 2010, 28, 1349–1355. [Google Scholar] [CrossRef]
- Battarbee, A.N.; Sinkey, R.G.; Harper, L.M.; Oparil, S.; Tita, A.T.N. Chronic hypertension in pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 532–541. [Google Scholar] [CrossRef]
- Palmiero, P.; Maiello, M.; Zito, A.; Ciccone, M.M. Hypertensive Cardiomyopathy in asymptomatic patients: A neglected diagnosis. Curr. Hypertens. Rev. 2014, 10, 239–245. [Google Scholar] [CrossRef]
- Palmiero, P.; Maiello, M.; Daly, D.D.; Ciccone, M.M.; Nanda, N.C. Nanda Aortic stiffness assessed by global pulse wave velocity in postmenopausal women: An. ultrasonographic study. Echocardiography 2012, 29, 1233–1238. [Google Scholar] [CrossRef]
- Melchiorre, K.; Sharma, R.; Khalil, A.; Thilaganathan, B. Maternal Cardiovascular Function in Normal Pregnancy: Evidence of Maladaptation to Chronic Volume Overload. Hypertension 2016, 67, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, M.; Oktay, A.A.; Stewart, M.H.; Milani, R.V.; Ventura, H.O.; Lavie, C.J. Left ventricular hypertrophy and hypertension. Prog. Cardiovasc. Dis. 2020, 63, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Raymond, D.; Peterson, E. A critical review of early-onset and late-onset preeclampsia. Obstet. Gynecol. Surv. 2011, 66, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Palmiero, P.; Maiello, M. Chapter 13—Abnormalities in the cardiac relaxation. In Pathophysiology, Risk Factors, and Management of Chronic Heart Failure; Singh, R.B., Fedacko, J., Hristova, K., Elkilany, G.N., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 159–167. [Google Scholar] [CrossRef]
- Nagueh, S.F. Left Ventricular Diastolic Function: Understanding Pathophysiology, Diagnosis, and Prognosis With Echocardiography. JACC Cardiovasc. Imaging 2020, 13 Pt 2, 228–244. [Google Scholar] [CrossRef]
- Silbiger, J.J. Pathophysiology and Echocardiographic Diagnosis of Left Ventricular Diastolic Dysfunction. J. Am. Soc. Echocardiogr. 2019, 32, 216–232.e2. [Google Scholar] [CrossRef]
- Steinert, J.R.; Wyatt, A.W.; Poston, L.; Jacob, R.; Mann, G.E. Preeclampsia is associated with altered Ca2+ regulation and NO production in human fetal venous endothelial cells. FASEB J. 2002, 16, 721–723. [Google Scholar] [CrossRef]
- Melchiorre, K.; Sutherland, G.R.; Liberati, M.; Thilaganathan, B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension 2011, 58, 709–715. [Google Scholar] [CrossRef]
- Hauspurg, A.; Countouris, M.E.; Catov, J.M. Hypertensive Disorders of Pregnancy and Future Maternal Health: How Can the Evidence Guide Postpartum Management? Curr. Hypertens. Rep. 2019, 21, 96. [Google Scholar] [CrossRef]
- Khosla, K.; Heimberger, S.; Nieman, K.M.; Tung, A.; Shahul, S.; Staff, A.C.; Rana, S. Long-Term Cardiovascular Disease Risk in Women After Hypertensive Disorders of Pregnancy: Recent Advances in Hypertension. Hypertension 2021, 78, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Uzan, J.; Carbonnel, M.; Piconne, O.; Asmar, R.; Ayoubi, J.-M. Pre-eclampsia: Pathophysiology, diagnosis, and management. Vasc. Health Risk Manag. 2011, 7, 467–474. [Google Scholar] [CrossRef]
- Mongraw-Chaffin, M.L.; Cirillo, P.M.; Cohn, B.A. Preeclampsia and cardiovascular disease death: Prospective evidence from the child health and development studies cohort. Hypertension 2010, 56, 166–171. [Google Scholar] [CrossRef] [PubMed]
- ACOG. The American College of Obstetricians and Gynecologists, 2020.Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet. Gynecol. 2020, 135, 1492–1495. [Google Scholar]
- Hauge, M.G.; Damm, P.; Kofoed, K.F.; Ersbøll, A.S.; Johansen, M.; Sigvardsen, P.E.; Møller, M.B.; Fuchs, A.; Kühl, J.T.; Nordestgaard, B.G.; et al. Early Coronary Atherosclerosis in Women with Previous Preeclampsia. J. Am. Coll. Cardiol. 2022, 79, 2310–2321. [Google Scholar] [CrossRef]
- Honigberg, M.C.; Jowell, A.R. Accelerated Coronary Atherosclerosis After Preeclampsia: Seeing Is Believing. J. Am. Coll. Cardiol. 2022, 79, 2322–2324. [Google Scholar] [CrossRef]
- Honigberg, M.C.; Economy, K.E.; Pabón, M.A.; Wang, X.; Castro, C.; Brown, J.M.; Divakaran, S.; Weber, B.N.; Barrett, L.; Perillo, A.; et al. Coronary Microvascular Function Following Severe Preeclampsia. Hypertension 2024, 81, 1272–1284. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Nuckols, V.R.; Pierce, G.L. Pierce Maternal microvascular dysfunction during preeclamptic pregnancy. Clin. Sci. 2021, 135, 1083–1101. [Google Scholar] [CrossRef]
- Rana, S.; Burke, S.D.; Karumanchi, S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obstet. Gynecol. 2022, 226, S1019–S1034. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes: Developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A. The pathophysiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2014, 11, 507–515. [Google Scholar] [CrossRef]
- Palmiero, P.; Caretto, P.; Zito, A.; Ciccone, M.M.; Pelliccia, F.; Maiello, M. Left ventricular diastolic function in atrial fibrillation: Methodological implications and clinical considerations. Echocardiography 2024, 41, e15818. [Google Scholar] [CrossRef]
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Ljung Faxén, U.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Sweitzer, N. Ventricular-Arterial Coupling in Chronic Heart Failure. Card. Fail. Rev. 2017, 3, 12–18. [Google Scholar] [CrossRef]
- Shaw, L.J.; Patel, K.; Lala-Trindade, A.; Feltovich, H.; Vieira, L.; Kontorovich, A.; Ananth, C.V.; Taqueti, V.R.; Mitrani, L.; Stern, T.; et al. Pathophysiology of Preeclampsia-Induced Vascular Dysfunction and Implications for Subclinical Myocardial Damage and Heart Failure. JACC Adv. 2024, 3, 100980. [Google Scholar] [CrossRef]
- Pfaller, B.; Siu, S.C.; D’Souza, R.; Wichert-Schmitt, B.; Nair, G.K.K.; Haberer, K.; Maxwell, C.; Silversides, C.K. Impact of Obesity on Outcomes of Pregnancy in Women With Heart Disease. J. Am. Coll. Cardiol. 2021, 77, 1317–1326. [Google Scholar] [CrossRef]
- Ghossein-Doha, C.; van Neer, J.; Wissink, B.; Breetveld, N.M.; de Windt, L.J.; van Dijk, A.P.J.; van der Vlugt, M.J.; Janssen, M.C.H.; Heidema, W.M.; Scholten, R.R.; et al. Pre-eclampsia: An important risk factor for asymptomatic heart failure. Ultrasound Obstet. Gynecol. 2017, 49, 143–149. [Google Scholar] [CrossRef]
- Honigberg, M.C.; Zekavat, S.M.; Aragam, K.; Klarin, D.; Bhatt, D.L.; Scott, N.S.; Peloso, G.M.; Natarajan, P. Long-Term Cardiovascular Risk in Women With Hypertension During Pregnancy. J. Am. Coll. Cardiol. 2019, 74, 2743–2754. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Søndergaard, M.M.; Hlatky, M.A.; Vittinghof, E.; Nah, G.; Stefanick, M.L.; Manson, J.E.; Farland, L.V.; Wells, G.L.; Mongraw-Chaffin, M.; et al. Adverse Pregnancy Outcomes and Incident Heart Failure in the Women’s Health Initiative. JAMA Netw. Open 2021, 4, e2138071. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Stout, M.J.; Rosenbloom, J.I.; Olsen, M.A.; Maddox, K.E.J.; Deych, E.; Davila-Roman, V.G.; Lindley, K.J. Preeclampsia Predicts Risk of Hospitalization for Heart Failure With Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2021, 78, 2281–2290. [Google Scholar] [CrossRef]
- Gevaert, A.B.; Boen, J.R.A.; Segers, V.F.; Van Craenenbroeck, E.M. Heart failure with preserved ejection fraction: A review of cardiac and noncardiac pathophysiology. Front. Physiol. 2019, 10, 638. [Google Scholar] [CrossRef]
- Clemmensen, T.S.; Christensen, M.; Kronborg, C.J.S.; Knudsen, U.B.; Løgstrup, B.B. Long-term follow-up of women with early onset pre-eclampsia shows subclinical impairment of the left ventricular function by two-dimensional speckle tracking echocardiography. Pregnancy Hypertens. 2018, 14, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Shahul, S.; Medvedofsky, D.; Wenger, J.B.; Nizamuddin, J.; Brown, S.M.; Bajracharya, S.; Salahuddin, S.; Thadhani, R.; Mueller, A.; Tung, A.; et al. Circulating antiangiogenic factors and myocardial dysfunction in hypertensive disorders of pregnancy. Hypertension 2016, 67, 1273–1280. [Google Scholar] [CrossRef]
- Hamad, R.R.; Larsson, A.; Pernow, J.; Bremme, K.; Eriksson, M.J. Assessment of left ventricular structure and function in preeclampsia by echocardiography and cardiovascular biomarkers. J. Hypertens. 2009, 27, 2257–2264. [Google Scholar] [CrossRef]
- Melchiorre, K.; Sutherland, G.R.; Watt-Coote, I.; Liberati, M.; Thilaganathan, B. Severe Myocardial Impairment and Chamber Dysfunction in Preterm Preeclampsia. Hypertens. Pregnancy 2012, 31, 454–471. [Google Scholar] [CrossRef]
- Vaught, A.J.; Kovell, L.C.; Szymanski, L.M.; Mayer, S.A.; Seifert, S.M.; Vaidya, D.; Murphy, J.D.; Argani, C.; O’kelly, A.; York, S.; et al. Acute Cardiac Effects of Severe Pre-Eclampsia. J. Am. Coll. Cardiol. 2018, 72, 1–11. [Google Scholar] [CrossRef]
- Popescu, M.R.; Bouariu, A.; Ciobanu, A.M.; Gică, N.; Panaitescu, A.M. Pregnancy Complications Lead to Subclinical Maternal Heart Dysfunction—The Importance and Benefits of Follow-Up Using Speckle Tracking Echocardiography. Medicina 2022, 58, 296. [Google Scholar] [CrossRef]
- Kuć, A.; Kubik, D.; Kościelecka, K.; Szymanek, W.; Męcik-Kronenberg, T. The Relationship Between Peripartum Cardiomyopathy and Preeclampsia—Pathogenesis, Diagnosis and Management. J. Multidiscip. Healthc. 2022, 15, 857–867. [Google Scholar] [CrossRef]
- Bello, N.; Rendon, I.S.H.; Arany, Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2013, 62, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Mebazaa, A.; Hilfiker-Kleiner, D.; Petrie, M.C.; Maggioni, A.P.; Laroche, C.; Regitz-Zagrosek, V.; Schaufelberger, M.; Tavazzi, L.; van der Meer, P.; et al. Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM): EURObservational Research Programme in conjunction with the Heart Failure Association of the European Society of Cardiology Study Group on PPCM. Eur. J. Heart Fail. 2017, 19, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.B.; Arany, Z.; McNamara, D.M.; Goland, S.; Elkayam, U. Peripartum Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Reuwer, A.Q.; Reuwer, P.J.H.M.; van der Post, J.A.; Cramer, M.J.; Kastelein, J.J.P.; Twickler, M.T.B. Prolactin fragmentation by trophoblastic matrix metalloproteinases as a possible contributor to peripartum cardiomyopathy and pre-eclampsia. Med. Hypotheses 2010, 74, 348–352. [Google Scholar] [CrossRef]
- Patten, I.S.; Rana, S.; Shahul, S.; Rowe, G.C.; Jang, C.; Liu, L.; Hacker, M.R.; Rhee, J.S.; Mitchell, J.; Mahmood, F.; et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012, 485, 333–338. [Google Scholar] [CrossRef]
- Young, B.C.; Levine, R.J.; Karumanchi, S.A. Pathogenesis of Preeclampsia. Annu. Rev. Pathol. 2010, 5, 173–192. [Google Scholar] [CrossRef]
- Fett, J.D. Peripartum cardiomyopathy: A puzzle closer to solution. World J. Cardiol. 2014, 6, 87–99. [Google Scholar] [CrossRef]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Zeisler, H.; Calda, P.; Sabria, J.; Markfeld-Erol, F.; Galindo, A.; Schoofs, K.; et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension 2014, 63, 346–352. [Google Scholar] [CrossRef]
- Patel, P.A.; Hernandez, A.F. Targeting anti-beta-1-adrenergic receptor antibodies for dilated cardiomyopathy. Eur. J. Heart Fail. 2013, 15, 724–729. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmed, A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ. Res. 2004, 95, 884–891. [Google Scholar] [CrossRef]
- Maas, A.H.E.M.; Rosano, G.; Cifkova, R.; Chieffo, A.; van Dijken, D.; Hamoda, H.; Kunadian, V.; Laan, E.; Lambrinoudaki, I.; Maclaran, K.; et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: A consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur. Heart J. 2021, 42, 967–984, Erratum in Eur. Heart J. 2022, 43, 2372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uddenberg, E.R.; Safwan, N.; Saadedine, M.; Hurtado, M.D.; Faubion, S.S.; Shufelt, C.L. Menopause transition and cardiovascular disease risk. Maturitas 2024, 185, 107974. [Google Scholar] [CrossRef] [PubMed]
- Macphail, M.G.; Juul, S.; Wollny, K.; Negre, J.Y.; Metcalfe, A.; Chaput, K.H.; Butalia, S.; Nerenberg, K.A. Nutrition Interventions for Lowering Cardiovascular Risk After Hypertensive Disorders of Pregnancy: A Systematic Review. CJC Open 2024, 6, 195–204. [Google Scholar] [CrossRef]
- Glisic, M.; Muka, T.; Franco, O.H. Cardiovascular screening and prevention strategies in women with history of preeclampsia: One size does not fit all. Eur. J. Prev. Cardiol. 2020, 27, 1386–1388. [Google Scholar] [CrossRef]
- Riemer, M.; Schulze, S.; Wagner, L.; Richter, M.; Ayerle, G.; Simm, A.; Seeger, S.; Schwesig, R.; Tchirikov, M.; Seliger, G. Cardiovascular Risk Reduction in Women Following Hypertensive Disorders of Pregnancy—A Prospective, Randomised, Controlled Interventional Study. Geburtshilfe Frauenheilkd 2021, 81, 966–978. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nerenberg, K.A.; Cooke, C.-L.; Smith, G.N.; Davidge, S.T. Optimising Women’s Cardiovascular Health After Hypertensive Disorders of Pregnancy: A Translational Approach to Cardiovascular Disease Prevention. Can. J. Cardiol. 2021, 37, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef]
- Roberge, S.; Bujold, E.; Nicolaides, K.H. Aspirin for the prevention of preterm and term preeclampsia: Systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2018, 218, 287–293.e281. [Google Scholar] [CrossRef]
- Smith, D.D.; Costantine, M.M. The role of statins in the prevention of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S1171–S1181. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Singh, J.; Khan, Y.; Seshan, S.V.; Girardi, G. A new mouse model to explore therapies for preeclampsia. PLoS ONE 2010, 5, e13663. [Google Scholar] [CrossRef] [PubMed]
- Costantine, M.M.; Tamayo, E.; Lu, F.; Bytautiene, E.; Longo, M.; Hankins, G.D.V.; Saade, G.R. Using pravastatin to improve the vascular reactivity in a mouse model of soluble fms-like tyrosine kinase-1-induced preeclampsia. Obstet. Gynecol. 2010, 116, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Ehret, G. Genes for Preeclampsia: An Opportunity for Blood Pressure Genomics. Hypertension 2018, 72, 285–286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ashraf, U.M.; Hall, D.L.; Rawls, A.Z.; Alexander, B.T. Epigenetic processes during preeclampsia and effects on fetal development and chronic health. Clin. Sci. 2021, 135, 2307–2327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmiero, P.; Caretto, P.; Ciccone, M.M.; Maiello, M.; on behalf of the I.C.I.S.C.U. (Italian Chapter of International Society Cardiovascular Ultrasound). Long-Term Cardiovascular Risk and Maternal History of Pre-Eclampsia. J. Clin. Med. 2025, 14, 3121. https://doi.org/10.3390/jcm14093121
Palmiero P, Caretto P, Ciccone MM, Maiello M, on behalf of the I.C.I.S.C.U. (Italian Chapter of International Society Cardiovascular Ultrasound). Long-Term Cardiovascular Risk and Maternal History of Pre-Eclampsia. Journal of Clinical Medicine. 2025; 14(9):3121. https://doi.org/10.3390/jcm14093121
Chicago/Turabian StylePalmiero, Pasquale, Pierpaolo Caretto, Marco Matteo Ciccone, Maria Maiello, and on behalf of the I.C.I.S.C.U. (Italian Chapter of International Society Cardiovascular Ultrasound). 2025. "Long-Term Cardiovascular Risk and Maternal History of Pre-Eclampsia" Journal of Clinical Medicine 14, no. 9: 3121. https://doi.org/10.3390/jcm14093121
APA StylePalmiero, P., Caretto, P., Ciccone, M. M., Maiello, M., & on behalf of the I.C.I.S.C.U. (Italian Chapter of International Society Cardiovascular Ultrasound). (2025). Long-Term Cardiovascular Risk and Maternal History of Pre-Eclampsia. Journal of Clinical Medicine, 14(9), 3121. https://doi.org/10.3390/jcm14093121





