Long-Term Outcomes of Revisional Powered Endoscopic Dacryocystorhinostomy (EnDCR) with Intraoperative Application of Mitomycin C in Patients After Failed Laser-Assisted (LDCR) or External Dacryocystorhinostomy (ExDCR)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Group
- Adult patients (≥18 years) with general health permitting at least 12 months of follow-up;
- Documented epiphora graded ≥3 on the Munk scale after previous DCR;
- Endoscopic confirmation of ostium closure or significant narrowing.
2.3. Outcome Measures
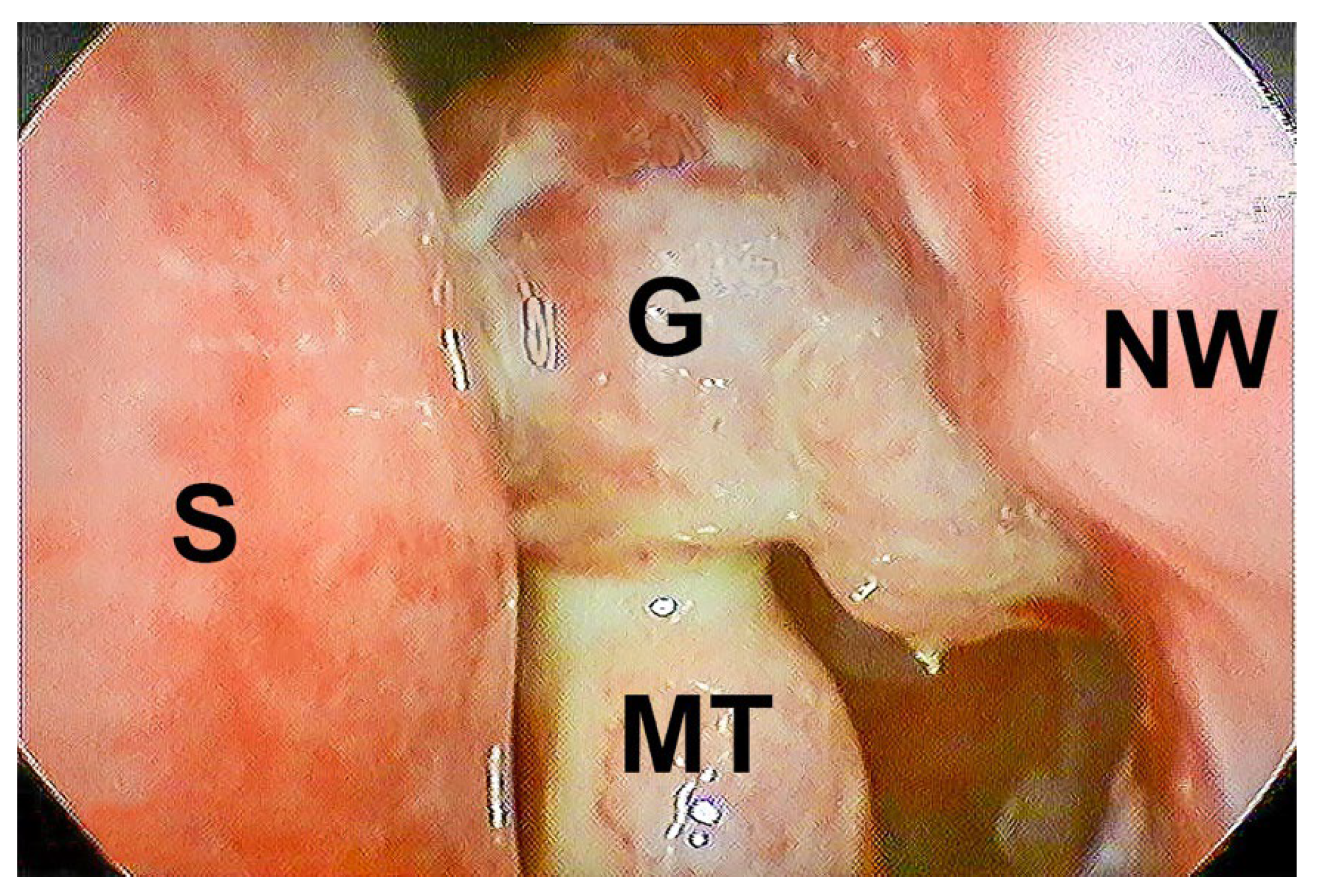
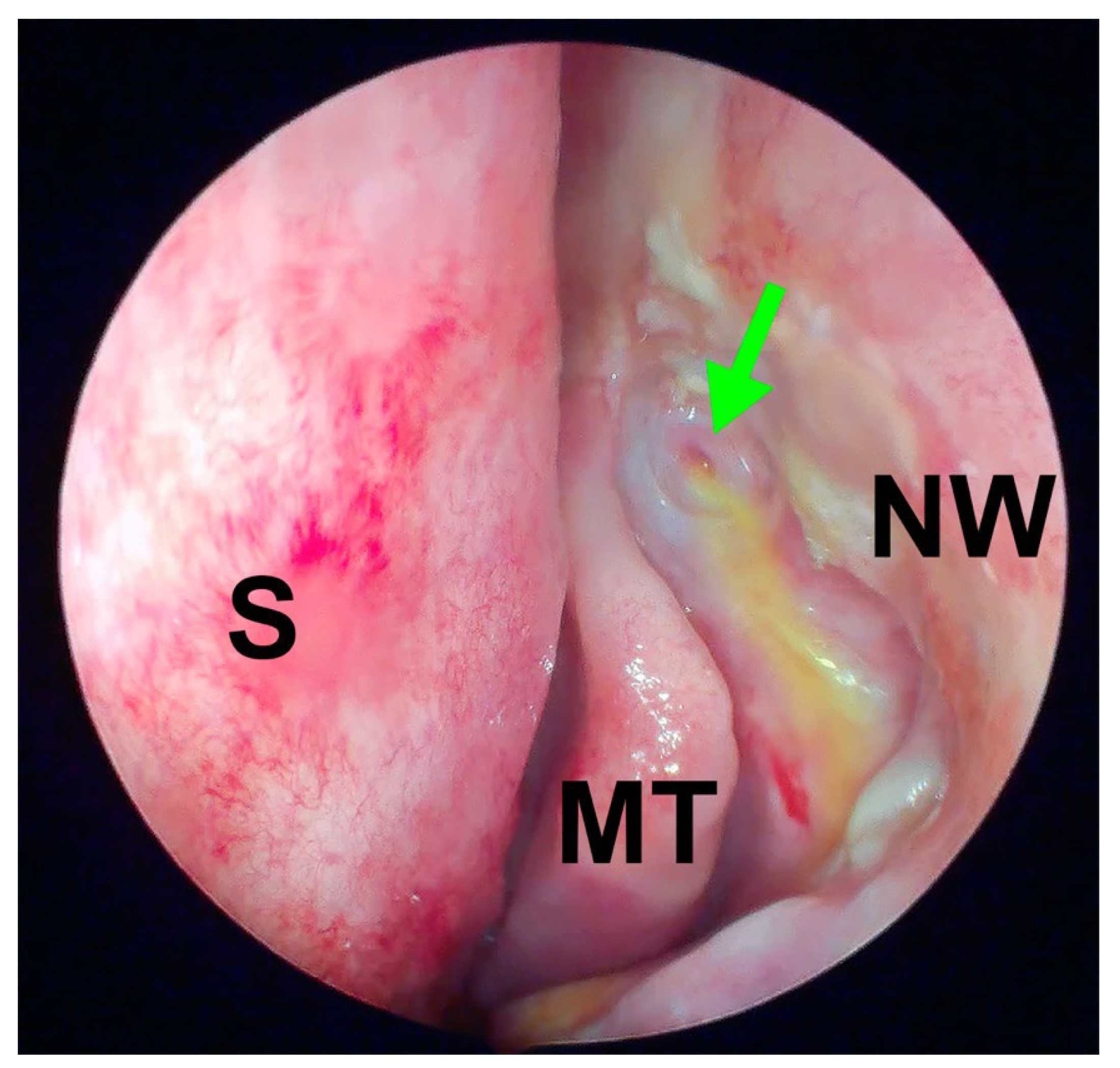
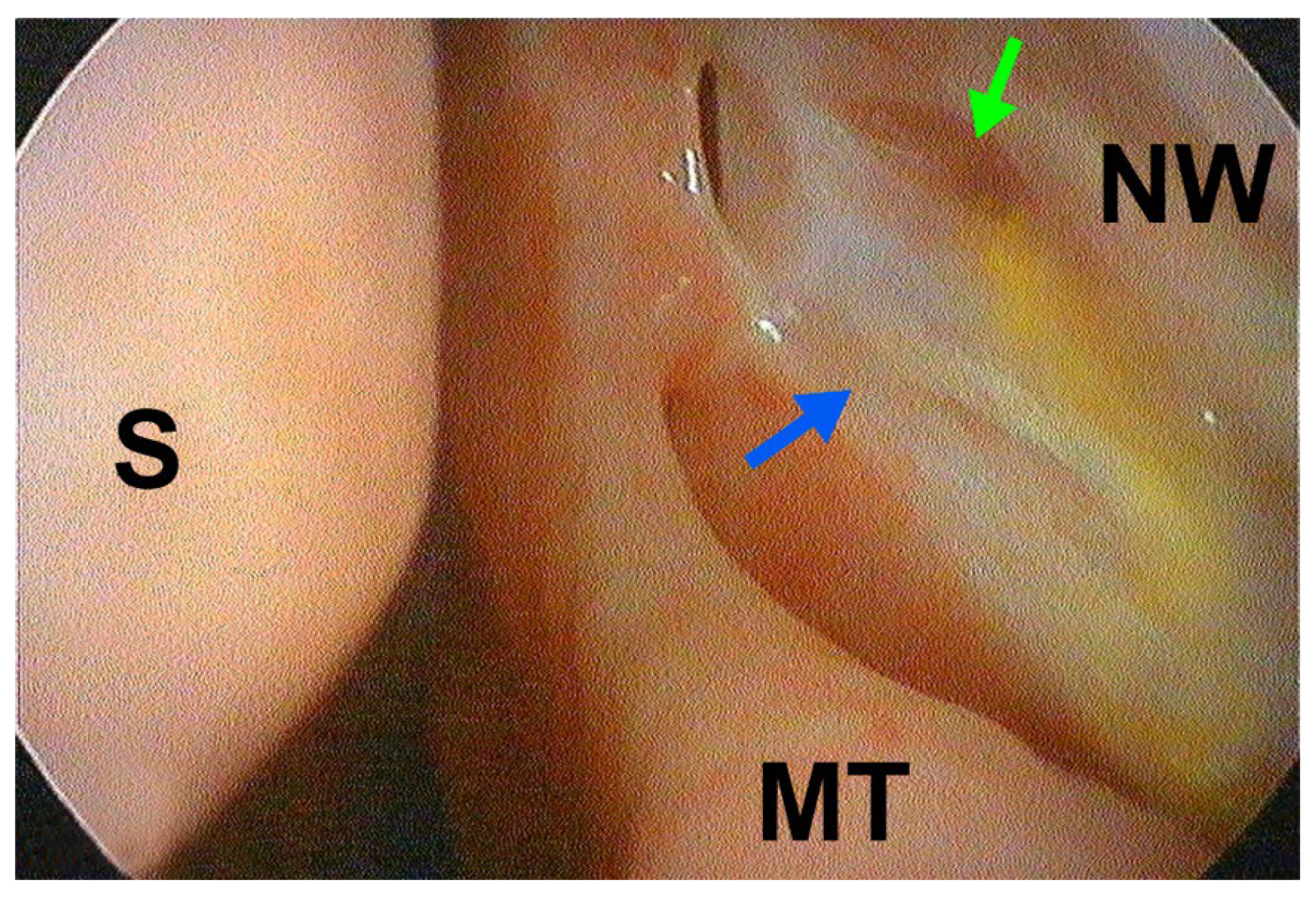
2.4. Surgical Technique
2.5. Postoperative Care
2.6. Statistical Analysis
3. Results
- A total of 12 patients underwent external dacryocystorhinostomy (ExDCR);
- A total of 12 patients underwent laser-assisted dacryocystorhinostomy (LDCR).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCVA | best-corrected visual acuity |
| DCR | dacryocystorhinostomy |
| EnDCR | endoscopic dacryocystorhinostomy |
| ExDCR | external dacryocystorhinostomy |
| FDDT | fluorescein dye disappearance test |
| LDCR | laser-assisted dacryocystorhinostomy |
| MMC | Mitomycin C |
| NLDO | nasolacrimal duct obstruction |
References
- Bulut, A.; Aslan, M.G.; Oner, V. Transcanalicular Multidiode Laser Versus External Dacryocystorhinostomy in the Treatment of Acquired Nasolacrimal Duct Obstruction. Beyoglu Eye J. 2021, 6, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Helmi, K.W.; Abdulhamid, A.S.; Alomari, M.S.; Alsudais, A.S.; Alqurashi, B.S.; Alsharif, A.; Alzahrani, A.H.; Bahalaq, A.M.; Qutub, M.F.; Almarzouki, H.S. Transcanalicular laser-assisted and external dacryocystorhinostomy anatomical and functional success in primary acquired nasolacrimal duct obstruction: Systematic review and meta-analysis. BMC Ophthalmol. 2025, 25, 5. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.J.; Psaltis, A.J.; Murphy, J.; Wormald, P.J. Powered endoscopic dacryocystorhinostomy: A decade of experience. Ophthalmic Plast. Reconstr. Surg. 2015, 31, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Sobel, R.K.; Aakalu, V.K.; Wladis, E.J.; Bilyk, J.R.; Yen, M.T.; Mawn, L.A. A Comparison of Endonasal Dacryocystorhinostomy and External Dacryocystorhinostomy: A Report by the American Academy of Ophthalmology. Ophthalmology 2019, 126, 1580–1585. [Google Scholar] [CrossRef]
- Ben Simon, G.J.; Joseph, J.; Lee, S.; Schwarcz, R.M.; McCann, J.D.; Goldberg, R.A. External versus endoscopic dacryocystorhinostomy for acquired nasolacrimal duct obstruction in a tertiary referral center. Ophthalmology 2005, 112, 1463–1468. [Google Scholar] [CrossRef]
- Tsirbas, A.; Davis, G.; Wormald, P.J. Mechanical endonasal dacryocystorhinostomy versus external dacryocystorhinostomy. Ophthalmic Plast. Reconstr. Surg. 2004, 20, 50–56. [Google Scholar] [CrossRef]
- Jawaheer, L.; MacEwen, C.J.; Anijeet, D. Endonasal versus external dacryocystorhinostomy for nasolacrimal duct obstruction. Cochrane Database Syst. Rev. 2017, 2, CD007097. [Google Scholar] [CrossRef]
- Wormald, P.J. Powered endoscopic dacryocystorhinostomy. Otolaryngol. Clin. N. Am. 2006, 39, 539–549. [Google Scholar] [CrossRef]
- Dogan, M.; Alizada, A.; Yavas, G.F.; Kahveci, O.K.; Bakan, O. Laser-assisted dacryocystorhinostomy in nasolacrimal duct obstruction: 5-year follow-up. Int. J. Ophthalmol. 2018, 11, 1616–1620. [Google Scholar] [CrossRef]
- Henson, R.D.; Henson, R.G., Jr.; Cruz, H.L., Jr.; Camara, J.G. Use of the diode laser with intraoperative mitomycin C in endocanalicular laser dacryocystorhinostomy. Ophthalmic Plast. Reconstr. Surg. 2007, 23, 134–137. [Google Scholar] [CrossRef]
- Nowak, R.; Rekas, M.; Gospodarowicz, I.N.; Ali, M.J. Long-term outcomes of primary transcanalicular laser dacryocystorhinostomy. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Maeso Riera, J.; Sellares Fabres, M.T. Trans-canalicular diode laser dacryocystorhinostomy: Technical variations and results. Acta Otorrinolaringol. Esp. 2007, 58, 10–15. [Google Scholar] [CrossRef] [PubMed]
- McClintic, S.M.; Yoon, M.K.; Bidar, M.; Dutton, J.J.; Vagefi, M.R.; Kersten, R.C. Tissue necrosis following diode laser-assisted transcanalicular dacryocystorhinostomy. Ophthalmic Plast. Reconstr. Surg. 2015, 31, e18–e22. [Google Scholar] [CrossRef] [PubMed]
- Evereklioglu, C.; Sener, H.; Polat, O.A.; Sonmez, H.K.; Gunay Sener, A.B.; Horozoglu, F. Success rate of external, endonasal, and transcanalicular laser DCR with or without silicone stent intubation for NLD obstruction: A network meta-analysis of randomized controlled trials. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 3369–3384. [Google Scholar] [CrossRef]
- Mutlu, D.; Bayram, N.; Arici, M.K.; Ozec, A.V.; Erdogan, H.; Toker, M.I. Comparison of Outcomes of External Dacryocystorhinostomy and Transcanalicular Laser-Assisted Dacryocystorhinostomy in Patients with Primary Acquired Nasolacrimal Duct Obstruction. Klin. Monbl Augenheilkd. 2022, 239, 799–803. [Google Scholar] [CrossRef]
- Tokat, T.; Tokat, S.; Kusbeci, T. Long-term outcomes of transcanalicular laser dacryocystorhinostomy versus endonasal dacryocystorhinostomy and a review of the literature. Niger. J. Clin. Pract. 2023, 26, 1069–1074. [Google Scholar] [CrossRef]
- Tsirbas, A.; Wormald, P.J. Mechanical endonasal dacryocystorhinostomy with mucosal flaps. Br. J. Ophthalmol. 2003, 87, 43–47. [Google Scholar] [CrossRef]
- Wormald, P.J. Powered endoscopic dacryocystorhinostomy. Laryngoscope 2002, 112, 69–72. [Google Scholar] [CrossRef]
- Kamal, S.; Ali, M.J.; Naik, M.N. Circumostial injection of mitomycin C (COS-MMC) in external and endoscopic dacryocystorhinostomy: Efficacy, safety profile, and outcomes. Ophthalmic Plast. Reconstr. Surg. 2014, 30, 187–190. [Google Scholar] [CrossRef]
- Niedrożność Dróg Łzowych—Podstawy Diagnostyki i Leczenia; Rekas, M., Nowak, R., Eds.; Medical Education: Warsaw, Poland, 2022. [Google Scholar]
- Hull, S.; Lalchan, S.A.; Olver, J.M. Success rates in powered endonasal revision surgery for failed dacryocystorhinostomy in a tertiary referral center. Ophthalmic Plast. Reconstr. Surg. 2013, 29, 267–271. [Google Scholar] [CrossRef]
- Freitag, S.K.; Aakalu, V.K.; Foster, J.A.; McCulley, T.J.; Tao, J.P.; Vagefi, M.R.; Yen, M.T.; Kim, S.J.; Wladis, E.J. Use of Mitomycin C in Dacryocystorhinostomy. Ophthalmology 2023, 130, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Munk, P.L.; Lin, D.T.; Morris, D.C. Epiphora: Treatment by means of dacryocystoplasty with balloon dilation of the nasolacrimal drainage apparatus. Radiology 1990, 177, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Ozturker, C.; Purevdorj, B.; Karabulut, G.O.; Seif, G.; Fazil, K.; Khan, Y.A.; Kaynak, P. A Comparison of Transcanalicular, Endonasal, and External Dacryocystorhinostomy in Functional Epiphora: A Minimum Two-Year Follow-Up Study. J. Ophthalmol. 2022, 2022, 3996854. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.B.; Nayak, B.; Mohapatra, S.; Thakur, S.; Vishwanath, S. Success and complications of endoscopic laser dacryocystorhinostomy vs. external dacryocystorhinostomy: A systematic review and meta-analysis. Indian. J. Ophthalmol. 2023, 71, 3290–3298. [Google Scholar] [CrossRef]
- Nowak, R.; Ali, M.J. Experience of the First Three Years of Independent Practice Following Surgical Training: Time Taken and Long-Term Outcomes of Powered Endoscopic Dacryocystorhinostomy. Semin. Ophthalmol. 2023, 38, 665–669. [Google Scholar] [CrossRef]
- Agarwal, A.; Doctor, S.; Mohan, E.R.; Ali, M.J.; Bothra, N. Outcomes of Powered Revision Endoscopic DCR and Utility of Three-Dimensional Computed Tomography Dacryocystography (3D CT-DCG). Curr. Eye Res. 2025, 1–6. [Google Scholar] [CrossRef]
- Ali, M.J.; Bothra, N. Long-term outcomes of revision endoscopic dacryocystorhinostomy aided by 4-mm coronary balloon catheter dacryoplasty. Indian. J. Ophthalmol. 2021, 69, 751–754. [Google Scholar] [CrossRef]
- Allon, R.; Cohen, O.; Bavnik, Y.; Milstein, A.; Halperin, D.; Warman, M. Long-term Outcomes for Revision Endoscopic Dacryocystorhinostomy-The Effect of the Primary Approach. Laryngoscope 2021, 131, E682–E688. [Google Scholar] [CrossRef]
- Korkut, A.Y.; Teker, A.M.; Yazici, M.Z.; Kahya, V.; Gedikli, O.; Kayhan, F.T. Surgical outcomes of primary and revision endoscopic dacryocystorhinostomy. J. Craniofac Surg. 2010, 21, 1706–1708. [Google Scholar] [CrossRef]
- Dolman, P.J. Comparison of external dacryocystorhinostomy with nonlaser endonasal dacryocystorhinostomy. Ophthalmology 2003, 110, 78–84. [Google Scholar] [CrossRef]
- Van Swol, J.M.; Myers, W.K.; Nguyen, S.A.; Eiseman, A.S. Revision dacryocystorhinostomy: Systematic review and meta-analysis. Orbit 2023, 42, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N. Endoscopic Dacryocystorhinostomy; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Vagefi, M.R.; Winn, B.J.; Lin, C.C.; Sires, B.S.; LauKaitis, S.J.; Anderson, R.L.; McCann, J.D. Facial nerve injury during external dacryocystorhinostomy. Ophthalmology 2009, 116, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Garg, S.; Nagpal, S.; Kumar, S.; Kamal, S. Naso-cutaneous fistula following transcanalicular laser dacrocystorhinostomy. Saudi J. Ophthalmol. 2014, 28, 69–71. [Google Scholar] [CrossRef]
| LDCR Group | ExDCR Group | |
|---|---|---|
| Age | 62 ± 20 | 62 ± 20 |
| Sex/Gender | F = 6, M = 6 | F = 5, M = 7 |
| Side | R = 6, L = 6 | R = 5, L = 7 |
| Munk score baseline | 3.67 ± 0.49 | 3.58 ± 0.51 |
| LDCR | ExDCR | p-Value | |
|---|---|---|---|
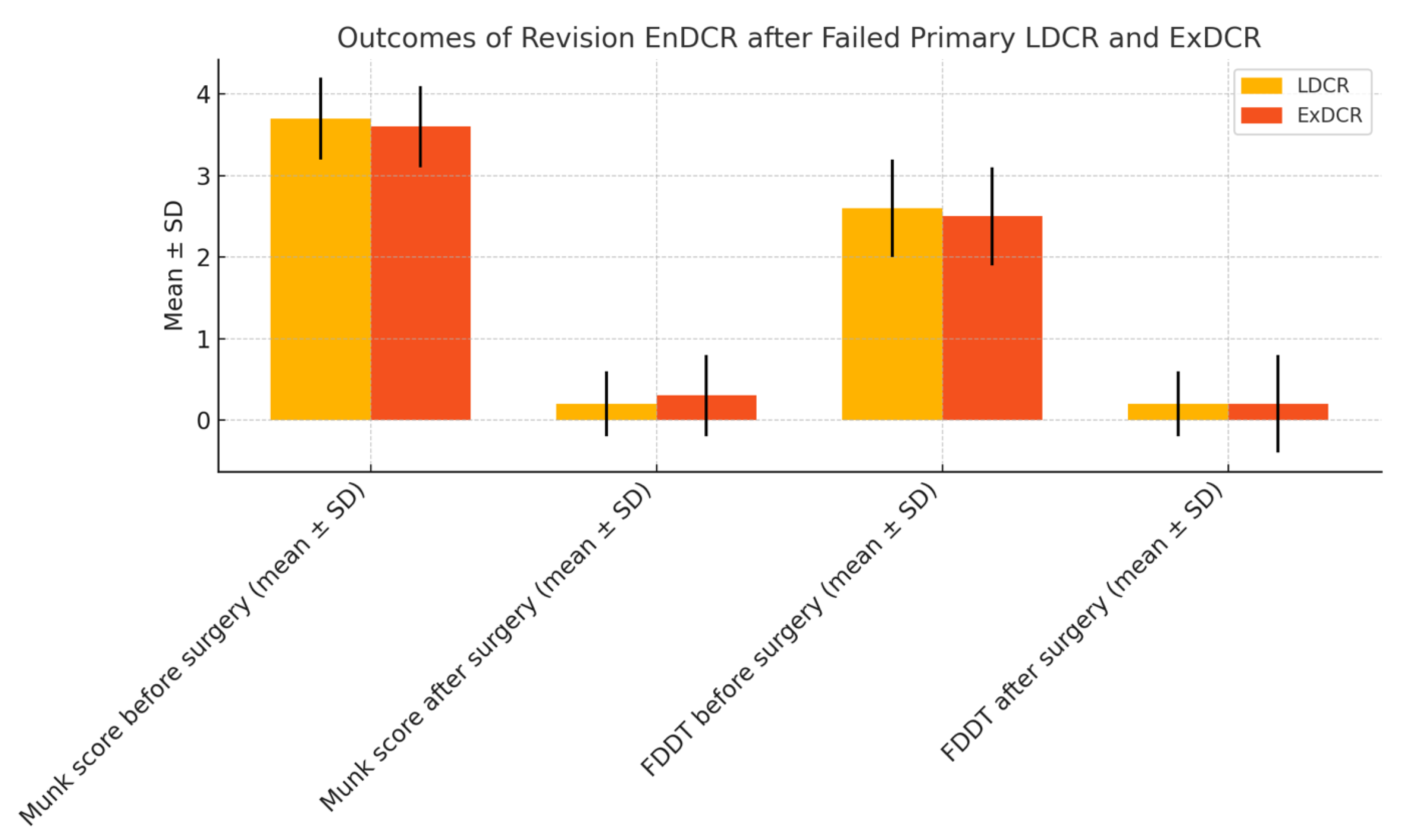
| Munk score before surgery (mean ± SD) | 3.67 ± 0.49 | 3.58 ± 0.51 | p = 0.689 |
| Munk score after surgery (mean ± SD) | 0.17 ± 0.39 | 0.25 ± 0.62 | p = 0.243 |
| FDDT before surgery (mean ± SD) | 2.67 ± 0.49 | 2.58 ± 0.51 | p = 0.689 |
| FDDT after surgery (mean ± SD) | 0.17 ± 0.39 | 0.17 ± 0.58 | p = 0.104 |
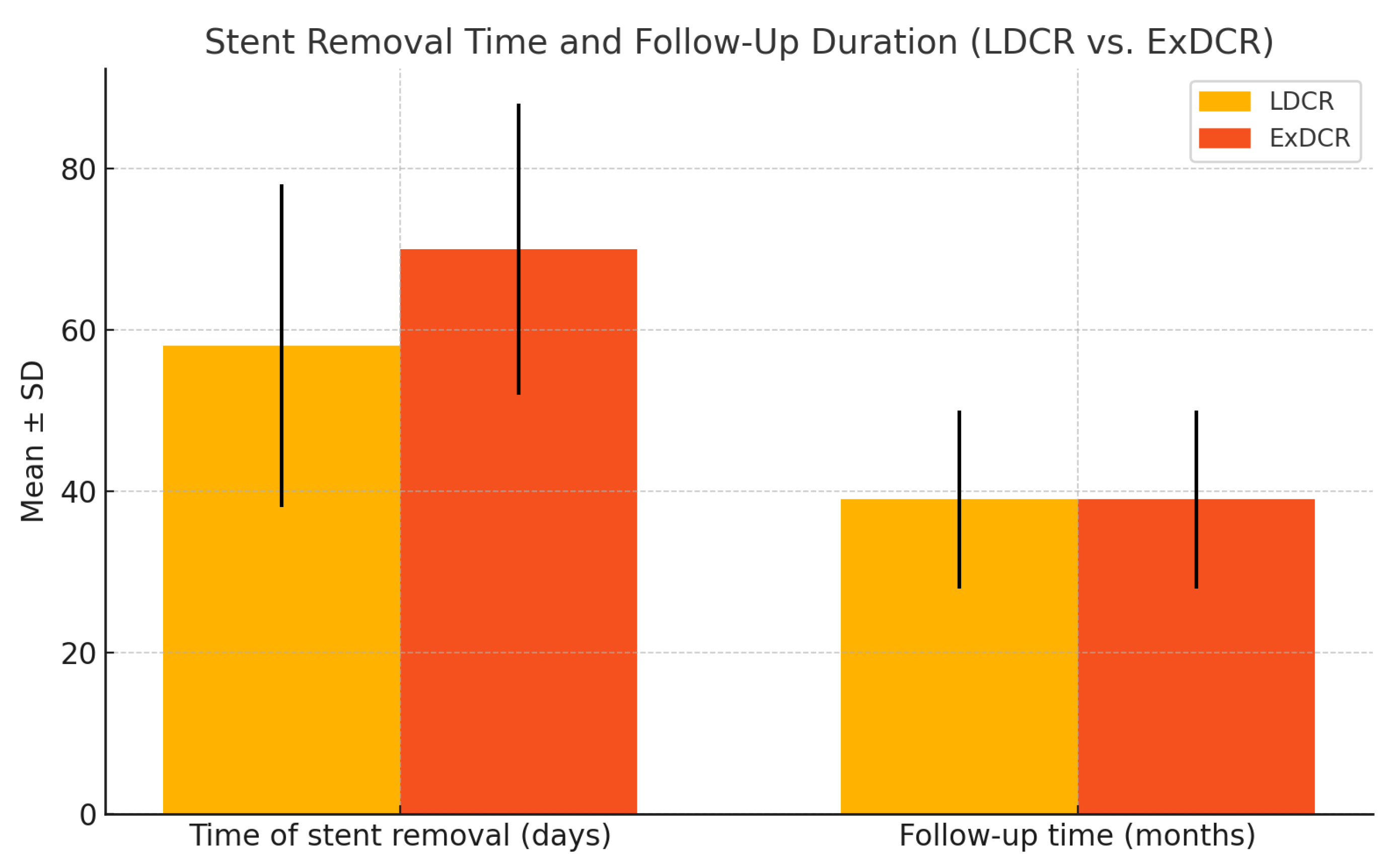
| Time of lacrimal stent removal (days) | 58 ± 20 | 70 ± 18 | p = 0.482 |
| Follow-up time (months) | 39 ± 12 | 39 ± 12 | p = 0.855 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinasz, M.; Nowak-Gospodarowicz, I.; Kicińska, A.K.; Rękas, M.; Nowak, R. Long-Term Outcomes of Revisional Powered Endoscopic Dacryocystorhinostomy (EnDCR) with Intraoperative Application of Mitomycin C in Patients After Failed Laser-Assisted (LDCR) or External Dacryocystorhinostomy (ExDCR). J. Clin. Med. 2025, 14, 3116. https://doi.org/10.3390/jcm14093116
Kinasz M, Nowak-Gospodarowicz I, Kicińska AK, Rękas M, Nowak R. Long-Term Outcomes of Revisional Powered Endoscopic Dacryocystorhinostomy (EnDCR) with Intraoperative Application of Mitomycin C in Patients After Failed Laser-Assisted (LDCR) or External Dacryocystorhinostomy (ExDCR). Journal of Clinical Medicine. 2025; 14(9):3116. https://doi.org/10.3390/jcm14093116
Chicago/Turabian StyleKinasz, Michał, Izabela Nowak-Gospodarowicz, Aleksandra Kinga Kicińska, Marek Rękas, and Rafał Nowak. 2025. "Long-Term Outcomes of Revisional Powered Endoscopic Dacryocystorhinostomy (EnDCR) with Intraoperative Application of Mitomycin C in Patients After Failed Laser-Assisted (LDCR) or External Dacryocystorhinostomy (ExDCR)" Journal of Clinical Medicine 14, no. 9: 3116. https://doi.org/10.3390/jcm14093116
APA StyleKinasz, M., Nowak-Gospodarowicz, I., Kicińska, A. K., Rękas, M., & Nowak, R. (2025). Long-Term Outcomes of Revisional Powered Endoscopic Dacryocystorhinostomy (EnDCR) with Intraoperative Application of Mitomycin C in Patients After Failed Laser-Assisted (LDCR) or External Dacryocystorhinostomy (ExDCR). Journal of Clinical Medicine, 14(9), 3116. https://doi.org/10.3390/jcm14093116






