Sleep-Disordered Breathing and Hypertension—A Systematic Review
Abstract
1. Introduction
2. Epidemiology
3. Screening and Definitions
4. Materials and Methods
4.1. Research Design
4.2. Search Strategy
4.3. Selection Criteria
4.3.1. Inclusion Criteria
- Studies that explicitly examined the relationship between sleep-disordered breathing (including sleep apnea syndrome and obstructive sleep apnea) and hypertension.
- Peer-reviewed empirical studies, including randomized controlled trials, cohort studies, and cross-sectional studies.
- Articles published in English between January 2000 and November 2024.
4.3.2. Exclusion Criteria
- Non-empirical studies, such as reviews, commentaries, or editorials.
- Articles not available in full text.
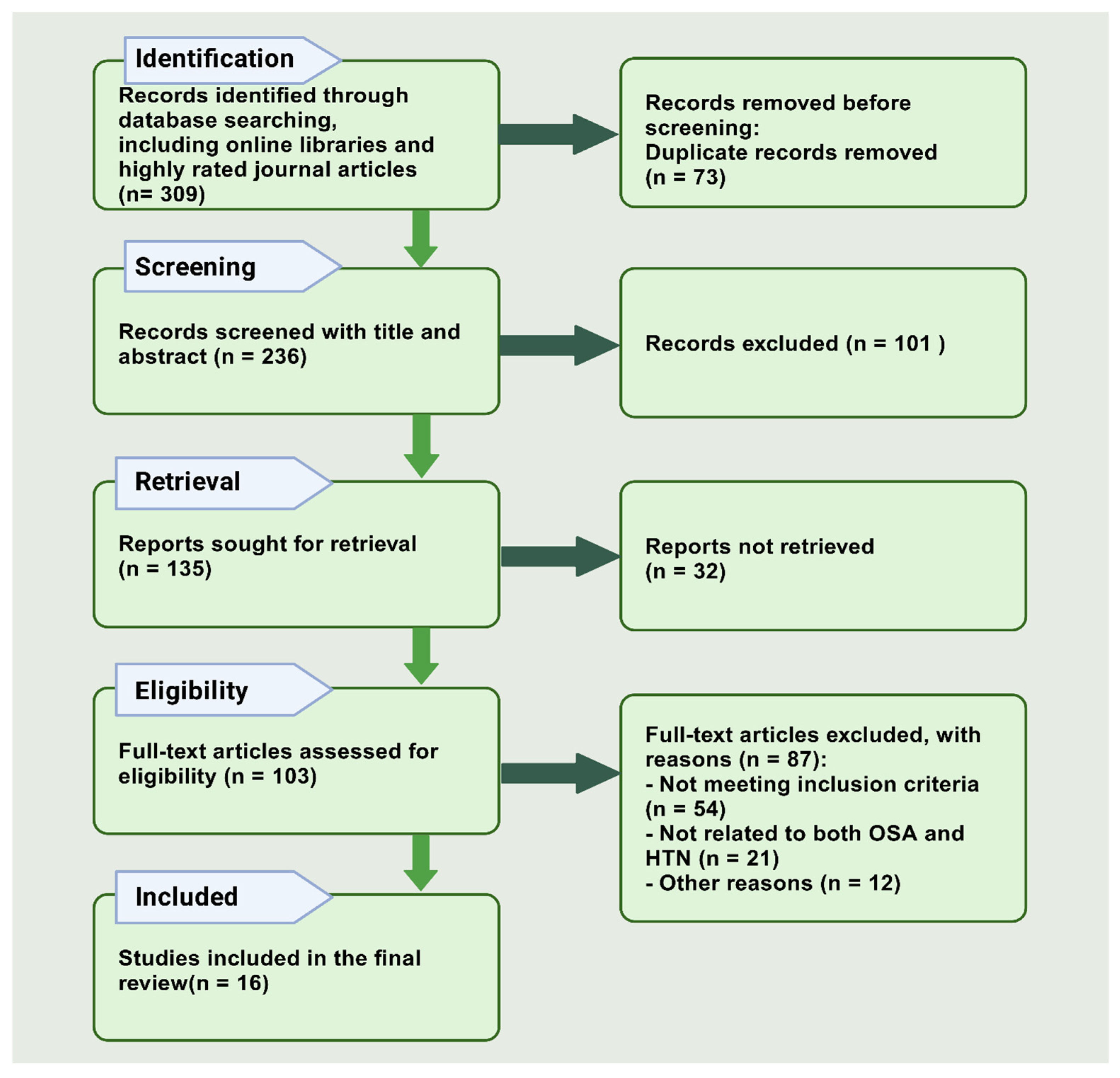
4.4. Search Process
4.5. Extraction and Management of Data
4.6. Results
5. Discussion
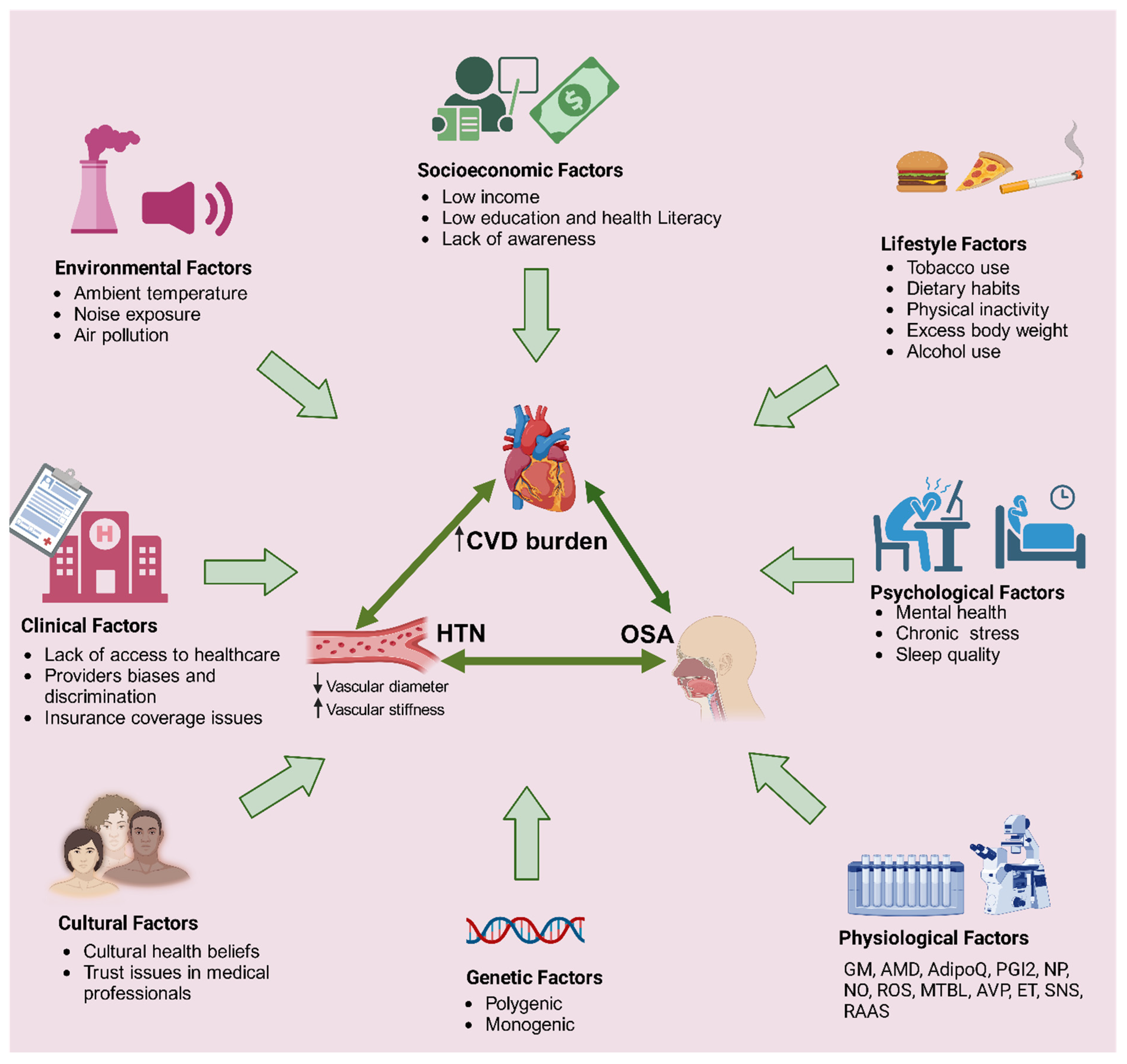
5.1. Shared Risk Factors for Both HTN and SDB
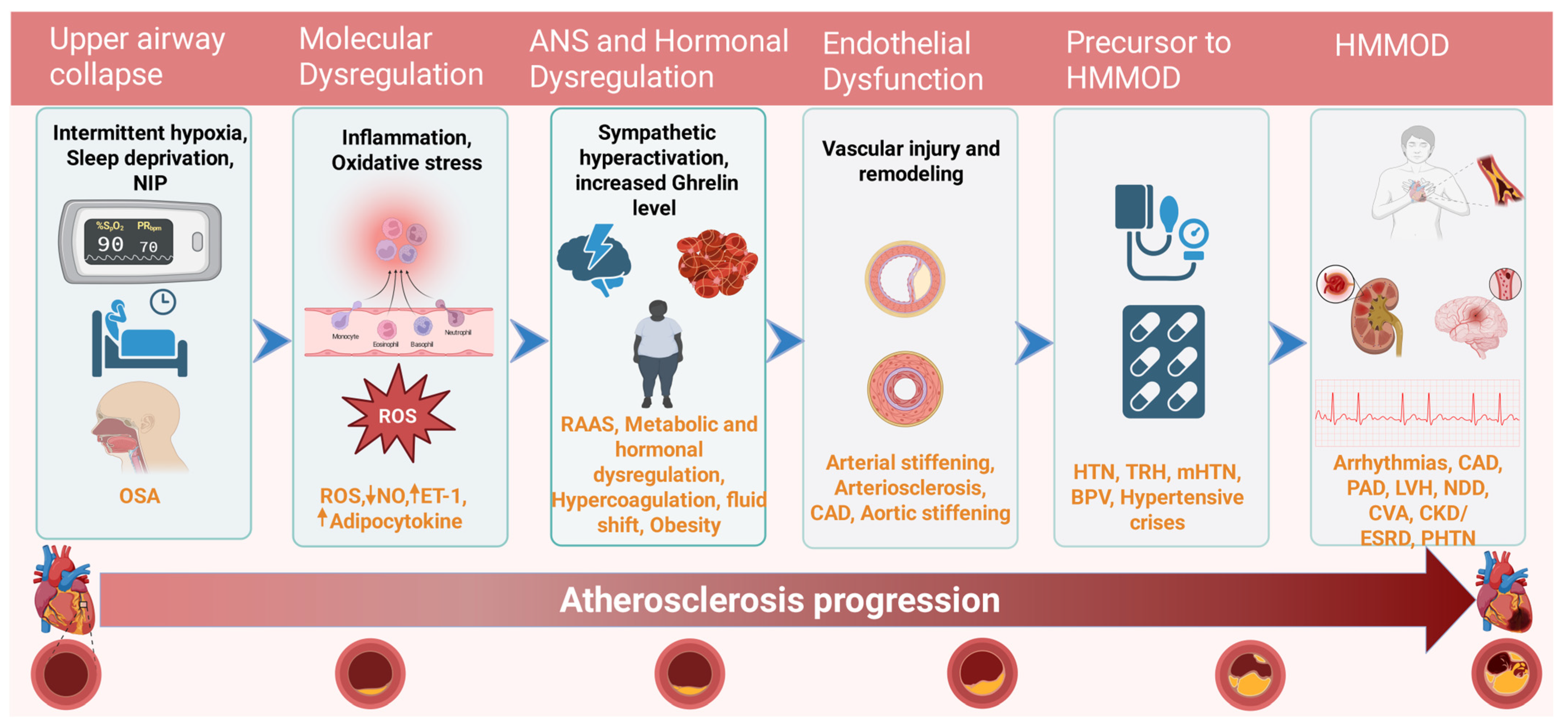
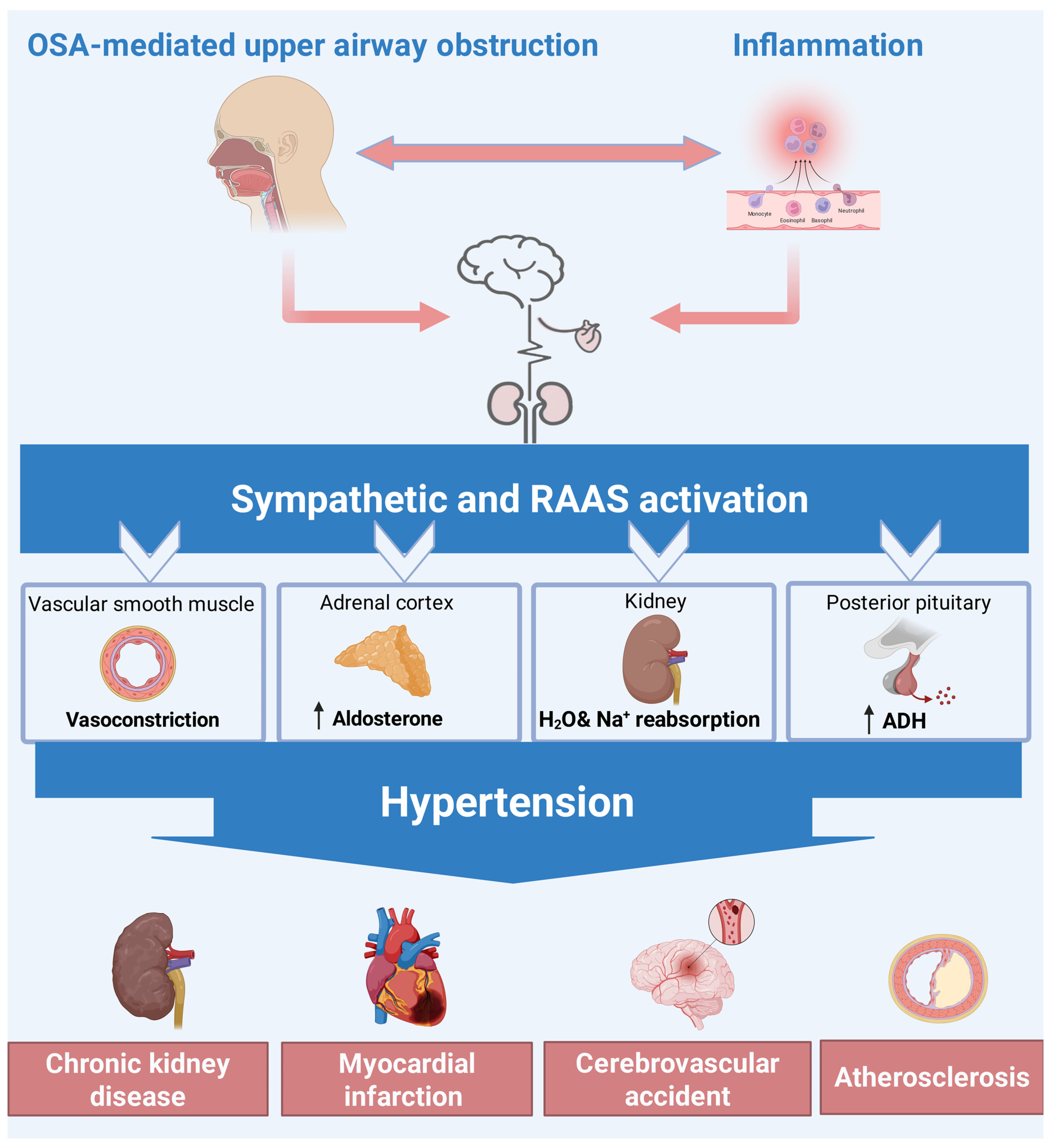
5.2. Pathophysiology Linking SDB and HTN
5.3. Clinical Features of OSA-Related Hypertension
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javaheri, S.; Javaheri, S.; Somers, V.K.; Gozal, D.; Mokhlesi, B.; Mehra, R.; McNicholas, W.T.; Zee, P.C.; Campos-Rodriguez, F.; Martinez-Garcia, M.A.; et al. Interactions of Obstructive Sleep Apnea with the Pathophysiology of Cardiovascular Disease, Part. J. Am. Coll. Cardiol. 2024, 84, 1208–1223. [Google Scholar] [CrossRef] [PubMed]
- Faverio, P.; Zanini, U.; Monzani, A.; Parati, G.; Luppi, F.; Lombardi, C.; Perger, E. Sleep-Disordered Breathing and Chronic Respiratory Infections: A Narrative Review in Adult and Pediatric Population. Int. J. Mol. Sci. 2023, 24, 5504. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Linz, D.; Redline, S.; Somers, V.K.; Simonds, A.K. Sleep Disordered Breathing and Cardiovascular Disease. J. Am. Coll. Cardiol. 2021, 78, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Schutte, A.E.; Srinivasapura Venkateshmurthy, N.; Mohan, S.; Prabhakaran, D. Hypertension in Low- and Middle-Income Countries. Circ. Res. 2021, 128, 808–826. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Brown, J.; Yazdi, F.; Jodari-Karimi, M.; Owen, J.G.; Reisin, E. Obstructive Sleep Apnea and Hypertension: Updates to a Critical Relationship. Curr. Hypertens. Rep. 2022, 24, 173–184. [Google Scholar] [CrossRef]
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; McFarlane, S.I.; Jean-Louis, G.; Myers, A.K. Obstructive sleep apnea, hypertension, resistant hypertension and cardiovascular disease. Sleep Med. Disord. Int. J. 2020, 4, 67. [Google Scholar]
- Fernandez-Mendoza, J.; He, F.; Calhoun, S.L.; Vgontzas, A.N.; Liao, D.; Bixler, E.O. Association of Pediatric Obstructive Sleep Apnea with Elevated Blood Pressure and Orthostatic Hypertension in Adolescence. JAMA Cardiol. 2021, 6, 1144. [Google Scholar] [CrossRef]
- Marin, J.M.; Agusti, A.; Villar, I.; Forner, M.; Nieto, D.; Carrizo, S.J.; Barbé, F.; Vicente, E.; Wei, Y.; Nieto, F.J.; et al. Association Between Treated and Untreated Obstructive Sleep Apnea and Risk of Hypertension. JAMA 2012, 307, 2169–2176. [Google Scholar] [CrossRef]
- Mancia, G.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; Kahan, T.; Mahfoud, F.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- Altay, S.; Fırat, S.; Peker, Y. A Narrative Review of the Association of Obstructive Sleep Apnea with Hypertension: How to Treat Both When They Coexist? J. Clin. Med. 2023, 12, 4144. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Hassen Abate, K.; Akinyemiju, T.F.; et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Li, M.; Yao, L.; Shao, L. Obstructive Sleep Apnea and Hypertension. In Secondary Hypertension: Screening, Diagnosis and Treatment; Li, N., Ed.; Springer: Singapore, 2020; pp. 461–488. ISBN 9789811505911. [Google Scholar]
- Ogunniyi, M.O.; Commodore-Mensah, Y.; Ferdinand, K.C. Race, Ethnicity, Hypertension, and Heart Disease. J. Am. Coll. Cardiol. 2021, 78, 2460–2470. [Google Scholar] [CrossRef]
- Shiina, K. Obstructive sleep apnea -related hypertension: A review of the literature and clinical management strategy. Hypertens. Res. 2024, 47, 3085–3098. [Google Scholar] [CrossRef]
- Mehra, R.; Chung, M.K.; Olshansky, B.; Dobrev, D.; Jackson, C.L.; Kundel, V.; Linz, D.; Redeker, N.S.; Redline, S.; Sanders, P.; et al. Sleep-Disordered Breathing and Cardiac Arrhythmias in Adults: Mechanistic Insights and Clinical Implications: A Scientific Statement From the American Heart Association. Circulation 2022, 146, E119–E136. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.A.; Nandkumar, S.; Addy, N.; Demko, B.G.; Freedman, N.S.; Gillespie, M.B.; Headapohl, W.; Kirsch, D.B.; Phillips, B.A.; Rosen, I.M.; et al. Study design considerations for sleep-disordered breathing devices. J. Clin. Sleep Med. 2020, 16, 441–449. [Google Scholar] [CrossRef]
- Whelton, S.P.; McEvoy, J.W.; Shaw, L.; Psaty, B.M.; Lima, J.A.C.; Budoff, M.; Nasir, K.; Szklo, M.; Blumenthal, R.S.; Blaha, M.J. Association of Normal Systolic Blood Pressure Level with Cardiovascular Disease in the Absence of Risk Factors. JAMA Cardiol. 2020, 5, 1011. [Google Scholar] [CrossRef]
- Querejeta Roca, G.; Anyaso, J.; Redline, S.; Bello, N.A. Associations Between Sleep Disorders and Hypertensive Disorders of Pregnancy and Materno-fetal Consequences. Curr. Hypertens. Rep. 2020, 22, 53. [Google Scholar] [CrossRef]
- Skomro, R.P.; Gjevre, J.; Reid, J.; McNab, B.; Ghosh, S.; Stiles, M.; Jokic, R.; Ward, H.; Cotton, D. Outcomes of home-based diagnosis and treatment of obstructive sleep apnea. Chest 2010, 138, 257–263. [Google Scholar] [CrossRef]
- Canadian Agency for Drugs and Technologies in Health (CADTH). Portable Monitoring Devices for Diagnosis of Obstructive Sleep Apnea at Home: Review of Accuracy, Cost-Effectiveness, Guidelines, and Coverage in Canada. CADTH Technol. Overv. 2010, 1, e0123. [Google Scholar]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Wickwire, E.M.; Zhang, X.; Munson, S.H.; Benjafield, A.V.; Sullivan, S.S.; Payombar, M.; Patil, S.P. The OSA patient journey: Pathways for diagnosis and treatment among commercially insured individuals in the United States. J. Clin. Sleep Med. 2024, 20, 505–514. [Google Scholar] [CrossRef]
- Kim, M.W.; Park, S.H.; Choi, M.S. Diagnostic Performance of Photoplethysmography-Based Smartwatch for Obstructive Sleep Apnea. J. Rhinol. 2022, 29, 155–162. [Google Scholar] [CrossRef]
- Ding, F.; Cotton-Clay, A.; Fava, L.; Easwar, V.; Kinsolving, A.; Kahn, P.; Rama, A.; Kushida, C. Polysomnographic validation of an under-mattress monitoring device in estimating sleep architecture and obstructive sleep apnea in adults. Sleep Med. 2022, 96, 20–27. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, C.; Shin, H. FDA-cleared home sleep apnea testing devices. npj Digit. Med. 2024, 7, 123. [Google Scholar] [CrossRef]
- Isaiah, A.; Spanier, A.J.; Grattan, L.M.; Wang, Y.; Pereira, K.D. Predictors of Behavioral Changes After Adenotonsillectomy in Pediatric Obstructive Sleep Apnea: A Secondary Analysis of a Randomized Clinical Trial. JAMA Otolaryngol. Neck Surg. 2020, 146, 900. [Google Scholar] [CrossRef]
- Pengo, M.F.; Soranna, D.; Giontella, A.; Perger, E.; Mattaliano, P.; Schwarz, E.I.; Lombardi, C.; Bilo, G.; Zambon, A.; Steier, J.; et al. Obstructive sleep apnoea treatment and blood pressure: Which phenotypes predict a response? A systematic review and meta-analysis. Eur. Respir. J. 2020, 55, 1901945. [Google Scholar] [CrossRef]
- Amin, R.; Somers, V.K.; McConnell, K.; Willging, P.; Myer, C.; Sherman, M.; McPhail, G.; Morgenthal, A.; Fenchel, M.; Bean, J.; et al. Activity-Adjusted 24-Hour Ambulatory Blood Pressure and Cardiac Remodeling in Children with Sleep Disordered Breathing. Hypertension 2008, 51, 84–91. [Google Scholar] [CrossRef]
- O’Connor, G.T.; Caffo, B.; Newman, A.B.; Quan, S.F.; Rapoport, D.M.; Redline, S.; Resnick, H.E.; Samet, J.; Shahar, E. Prospective Study of Sleep-disordered Breathing and Hypertension: The Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2009, 179, 1159–1164. [Google Scholar] [CrossRef]
- Tanigawa, T.; Tachibana, N.; Yamagishi, K.; Muraki, I.; Kudo, M.; Ohira, T.; Kitamura, A.; Sato, S.; Shimamoto, T.; Iso, H. Relationship between Sleep-Disordered Breathing and Blood Pressure Levels in Community-Based Samples of Japanese Men. Hypertens. Res. 2004, 27, 479–484. [Google Scholar] [CrossRef]
- Sánchez-de-la-Torre, M.; Khalyfa, A.; Sánchez-de-la-Torre, A.; Martinez-Alonso, M.; Martinez-García, M.Á.; Barceló, A.; Lloberes, P.; Campos-Rodriguez, F.; Capote, F.; Diaz-de-Atauri, M.J.; et al. Precision Medicine in Patients with Resistant Hypertension and Obstructive Sleep Apnea. J. Am. Coll. Cardiol. 2015, 66, 1023–1032. [Google Scholar] [CrossRef]
- Witkowski, A.; Prejbisz, A.; Florczak, E.; Kądziela, J.; Śliwiński, P.; Bieleń, P.; Michałowska, I.; Kabat, M.; Warchoł, E.; Januszewicz, M.; et al. Effects of Renal Sympathetic Denervation on Blood Pressure, Sleep Apnea Course, and Glycemic Control in Patients with Resistant Hypertension and Sleep Apnea. Hypertension 2011, 58, 559–565. [Google Scholar] [CrossRef]
- Horne, R.S.C.; Yang, J.S.C. Elevated Blood Pressure During Sleep and Wake in Children with Sleep-Disordered Breathing. Pediatrics 2011, 128, e85–e92. [Google Scholar]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective Study of the Association between Sleep-Disordered Breathing and Hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef]
- Reid, J.; Skomro, R.; Cotton, D.; Ward, H.; Olatunbosun, F.; Gjevre, J.; Guilleminault, C. Pregnant Women with Gestational Hypertension May Have a High Frequency of Sleep Disordered Breathing. Sleep 2011, 34, 1033–1038. [Google Scholar] [CrossRef]
- Li, R.; Rueschman, M.; Gottlieb, D.J.; Redline, S.; Sofer, T. A composite sleep and pulmonary phenotype predicting hypertension. EBioMedicine 2021, 68, 103433. [Google Scholar] [CrossRef]
- Minic, M.; Granton, J.T.; Ryan, C.M. Sleep Disordered Breathing in Group 1 Pulmonary Arterial Hypertension. J. Clin. Sleep Med. 2014, 10, 277–283. [Google Scholar] [CrossRef]
- Facco, F.L.; Parker, C.B.; Reddy, U.M.; Silver, R.M.; Koch, M.A.; Louis, J.M.; Basner, R.C.; Chung, J.H.; Nhan-Chang, C.-L.; Pien, G.W.; et al. Association Between Sleep-Disordered Breathing and Hypertensive Disorders of Pregnancy and Gestational Diabetes Mellitus. Obstet. Gynecol. 2017, 129, 31–41. [Google Scholar] [CrossRef]
- Ulrich, S.; Keusch, S.; Hildenbrand, F.F.; Lo Cascio, C.; Huber, L.C.; Tanner, F.C.; Speich, R.; Bloch, K.E. Effect of nocturnal oxygen and acetazolamide on exercise performance in patients with pre-capillary pulmonary hypertension and sleep-disturbed breathing: Randomized, double-blind, cross-over trial. Eur. Heart J. 2015, 36, 615–623. [Google Scholar] [CrossRef]
- Spiesshoefer, J.; Herkenrath, S.; Harre, K.; Kahles, F.; Florian, A.; Yilmaz, A.; Mohr, M.; Naughton, M.; Randerath, W.; Emdin, M.; et al. Sleep-Disordered Breathing and Nocturnal Hypoxemia in Precapillary Pulmonary Hypertension: Prevalence, Pathophysiological Determinants, and Clinical Consequences. Respiration 2021, 100, 865–876. [Google Scholar] [CrossRef]
- Eskandari, D.; Zou, D.; Grote, L.; Hoff, E.; Hedner, J. Acetazolamide Reduces Blood Pressure and Sleep-Disordered Breathing in Patients with Hypertension and Obstructive Sleep Apnea: A Randomized Controlled Trial. J. Clin. Sleep Med. 2018, 14, 309–317. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Qi, Q.; Azarbarzin, A.; Chen, H.; Shah, N.A.; Ramos, A.R.; Zee, P.C.; Cai, J.; Daviglus, M.L.; et al. Metabolomic profiles of sleep-disordered breathing are associated with hypertension and diabetes mellitus development. Nat. Commun. 2024, 15, 1845. [Google Scholar] [CrossRef]
- Kato, K.; Noda, A.; Yasuma, F.; Matsubara, Y.; Miyata, S.; Iwamoto, K.; Miyazaki, S.; Ozaki, N. Effects of sleep-disordered breathing and hypertension on cognitive function in elderly adults. Clin. Exp. Hypertens. 2020, 42, 250–256. [Google Scholar] [CrossRef]
- Li, X.; Sotres-Alvarez, D.; Gallo, L.C.; Ramos, A.R.; Aviles-Santa, L.; Perreira, K.M.; Isasi, C.R.; Zee, P.C.; Savin, K.L.; Schneiderman, N.; et al. Associations of Sleep-disordered Breathing and Insomnia with Incident Hypertension and Diabetes. The Hispanic Community Health Study/Study of Latinos. Am. J. Respir. Crit. Care Med. 2021, 203, 356–365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- André, C.; Rehel, S.; Kuhn, E.; Landeau, B.; Moulinet, I.; Touron, E.; Ourry, V.; Le Du, G.; Mézenge, F.; Tomadesso, C.; et al. Association of Sleep-Disordered Breathing with Alzheimer Disease Biomarkers in Community-Dwelling Older Adults: A Secondary Analysis of a Randomized Clinical Trial. JAMA Neurol. 2020, 77, 716. [Google Scholar] [CrossRef]
- Strenth, C.; Wani, A.; Alla, R.; Khan, S.; Schneider, F.D.; Thakur, B. Obstructive Sleep Apnea and Its Cardiac Implications in the United States: An Age-Stratified Analysis Between Young and Older Adults. J. Am. Heart Assoc. 2024, 13, e033810. [Google Scholar] [CrossRef]
- Elias, S.; Chen, Y.; Liu, X.; Slone, S.; Turkson-Ocran, R.-A.; Ogungbe, B.; Thomas, S.; Byiringiro, S.; Koirala, B.; Asano, R.; et al. Shared Decision-Making in Cardiovascular Risk Factor Management: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2024, 7, e243779. [Google Scholar] [CrossRef]
- Sands-Lincoln, M.; Grandner, M.; Whinnery, J.; Keenan, B.T.; Jackson, N.; Gurubhagavatula, I. The Association Between Obstructive Sleep Apnea and Hypertension by Race/Ethnicity in a Nationally Representative Sample. J. Clin. Hypertens. 2013, 15, 593–599. [Google Scholar] [CrossRef]
- Ioannidou, D.; Kalamaras, G.; Kotoulas, S.-C.; Pataka, A. Smoking and Obstructive Sleep Apnea: Is There An Association between These Cardiometabolic Risk Factors?—Gender Analysis. Medicina 2021, 57, 1137. [Google Scholar] [CrossRef]
- Connelly, P.J.; Currie, G.; Delles, C. Sex Differences in the Prevalence, Outcomes and Management of Hypertension. Curr. Hypertens. Rep. 2022, 24, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Won, C.H.J.; Reid, M.; Sofer, T.; Azarbarzin, A.; Purcell, S.; White, D.; Wellman, A.; Sands, S.; Redline, S. Sex differences in obstructive sleep apnea phenotypes, the multi-ethnic study of atherosclerosis. Sleep 2020, 43, zsz274. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.; Sofer, T.; Guo, N.; Wilson, J.; Redline, S. A sleep apnea prediction model developed for African Americans: The Jackson Heart Sleep Study. J. Clin. Sleep Med. 2020, 16, 1171–1178. [Google Scholar] [CrossRef]
- Yasir, M.; Pervaiz, A.; Sankari, A. Cardiovascular Outcomes in Sleep-Disordered Breathing: Are We Under-estimating? Front. Neurol. 2022, 13, 801167. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J.; et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef]
- Ogbu, I.; Menon, T.; Chahil, V.; Kahlon, A.; Devanand, D.; Kalra, D.K. Sleep Disordered Breathing and Neurocognitive Disorders. J. Clin. Med. 2024, 13, 5001. [Google Scholar] [CrossRef]
- Ogbu, I.; Hakobyan, B.; Sossou, C.; Levisman, J.; Obiagwu, C.; Danielian, A. Snoring Survivors: The impact of obstructive sleep apnoea and continuous positive airway pressure use on in-hospital mortality, length of stay and costs among patients hospitalised with acute cardiovascular disease—A retrospective analysis of 2016–2019 National Inpatient Sample Data. BMJ Open 2024, 14, e073991. [Google Scholar] [CrossRef] [PubMed]
- Budhiraja, R.; Javaheri, S.; Parthasarathy, S.; Berry, R.B.; Quan, S.F. Incidence of hypertension in obstructive sleep apnea using hypopneas defined by 3 percent oxygen desaturation or arousal but not by only 4 percent oxygen desaturation. J. Clin. Sleep Med. 2020, 16, 1753–1760. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Peng, Y.-J.; Nanduri, J. Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Investig. 2020, 130, 5042–5051. [Google Scholar] [CrossRef]
- Barhoumi, T.; Todryk, S. Role of monocytes/macrophages in renin-angiotensin system-induced hypertension and end organ damage. Front. Physiol. 2023, 14, 1199934. [Google Scholar] [CrossRef]
- Lavie, L.; Lavie, P. Molecular mechanisms of cardiovascular disease in OSAHS: The oxidative stress link. Eur. Respir. J. 2009, 33, 1467–1484. [Google Scholar] [CrossRef]
- Wenzel, U.O.; Ehmke, H.; Bode, M. Immune mechanisms in arterial hypertension. Recent advances. Cell Tissue Res. 2021, 385, 393–404. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Guazzi, M.; Testani, J.M.; Borlaug, B.A. Altered Hemodynamics and End-Organ Damage in Heart Failure: Impact on the Lung and Kidney. Circulation 2020, 142, 998–1012. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.D.; Floras, J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009, 373, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Maniaci, A.; Lavalle, S.; Parisi, F.M.; Barbanti, M.; Cocuzza, S.; Iannella, G.; Magliulo, G.; Pace, A.; Lentini, M.; Masiello, E.; et al. Impact of Obstructive Sleep Apnea and Sympathetic Nervous System on Cardiac Health: A Comprehensive Review. J. Cardiovasc. Dev. Dis. 2024, 11, 204. [Google Scholar] [CrossRef]
- Peracaula, M.; Torres, D.; Poyatos, P.; Luque, N.; Rojas, E.; Obrador, A.; Orriols, R.; Tura-Ceide, O. Endothelial Dysfunction and Cardiovascular Risk in Obstructive Sleep Apnea: A Review Article. Life 2022, 12, 537. [Google Scholar] [CrossRef]
- Kwon, Y.; Tzeng, W.S.; Seo, J.; Logan, J.G.; Tadic, M.; Lin, G.-M.; Martinez-Garcia, M.A.; Pengo, M.; Liu, X.; Cho, Y.; et al. Obstructive sleep apnea and hypertension; critical overview. Clin. Hypertens. 2024, 30, 19. [Google Scholar] [CrossRef]
- Genta-Pereira, D.C.; Furlan, S.F.; Omote, D.Q.; Giorgi, D.M.A.; Bortolotto, L.A.; Lorenzi-Filho, G.; Drager, L.F. Nondipping Blood Pressure Patterns Predict Obstructive Sleep Apnea in Patients Undergoing Ambulatory Blood Pressure Monitoring. Hypertension 2018, 72, 979–985. [Google Scholar] [CrossRef]
- Mensa Sorato, M.; Davari, M.; Kebriaeezadeh, A.; Naderi, N.; Sarrafzadegan, N.; Shibru, T.; Nikfar, S.; Arero, A.G. Cost-effectiveness of Interventional therapies for management of Treatment-resistant hypertension: Systematic review of pharmacoeconomic studies. J. Pharm. Health Serv. Res. 2020, 11, 307–319. [Google Scholar] [CrossRef]
- Walia, H.K.; Li, H.; Rueschman, M.; Bhatt, D.L.; Patel, S.R.; Quan, S.F.; Gottlieb, D.J.; Punjabi, N.M.; Redline, S.; Mehra, R. Association of Severe Obstructive Sleep Apnea and Elevated Blood Pressure Despite Antihypertensive Medication Use. J. Clin. Sleep Med. 2014, 10, 835–843. [Google Scholar] [CrossRef]
- Menon, T.; Ogbu, I.; Kalra, D.K. Sleep-Disordered Breathing and Cardiac Arrhythmias. J. Clin. Med. 2024, 13, 6635. [Google Scholar] [CrossRef] [PubMed]
- Saleeb-Mousa, J.; Nathanael, D.; Coney, A.M.; Kalla, M.; Brain, K.L.; Holmes, A.P. Mechanisms of Atrial Fibrillation in Obstructive Sleep Apnoea. Cells 2023, 12, 1661. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Olson, E.J.; Shen, W.K.; Wright, R.S.; Ballman, K.V.; Hodge, D.O.; Herges, R.M.; Howard, D.E.; Somers, V.K. Obstructive sleep apnea and the risk of sudden cardiac death: A longitudinal study of 10,701 adults. J. Am. Coll. Cardiol. 2013, 62, 610–616. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef]
- Kraiczi, H.; Hedner, J.; Peker, Y.; Grote, L. Comparison of Atenolol, Amlodipine, Enalapril, Hydrochlorothiazide, and Losartan for Antihypertensive Treatment in Patients with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2000, 161, 1423–1428. [Google Scholar] [CrossRef]
- Pépin, J.-L.; Tamisier, R.; Barone-Rochette, G.; Launois, S.H.; Lévy, P.; Baguet, J.-P. Comparison of Continuous Positive Airway Pressure and Valsartan in Hypertensive Patients with Sleep Apnea. Am. J. Respir. Crit. Care Med. 2010, 182, 954–960. [Google Scholar] [CrossRef]
- Ziegler, M.G.; Milic, M.; Dimsdale, J.E.; Mills, P.J. Sympathetic overactivity and nocturnal diuresis in obstructive sleep apnea alter the response to hypertension therapy. Clin. Hypertens. 2024, 30, 14. [Google Scholar] [CrossRef]
- Bakris, G.L.; Saxena, M.; Gupta, A.; Chalhoub, F.; Lee, J.; Stiglitz, D.; Makarova, N.; Goyal, N.; Guo, W.; Zappe, D.; et al. RNA Interference with Zilebesiran for Mild to Moderate Hypertension: The KARDIA-1 Randomized Clinical Trial. JAMA 2024, 331, 740–749. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Fitchett, D.; Ofstad, A.P.; Kraus, B.J.; Wanner, C.; Zwiener, I.; Zinman, B.; Lauer, S.; George, J.T.; Rossignol, P.; et al. Empagliflozin for Patients with Presumed Resistant Hypertension: A Post Hoc Analysis of the EMPA-REG OUTCOME Trial. Am. J. Hypertens. 2020, 33, 1092–1101. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; De Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Xie, L.; Song, S.; Li, S.; Wei, Q.; Liu, H.; Zhao, C.; Yu, F.; Tong, J. Efficacy of dapagliflozin in the treatment of HFrEF with obstructive sleep apnea syndrome (DAHOS study): Study protocol for a multicentric, prospective, randomized controlled clinical trial. Trials 2023, 24, 318. [Google Scholar] [CrossRef]
- Riaz, M.; Certal, V.; Nigam, G.; Abdullatif, J.; Zaghi, S.; Kushida, C.A.; Camacho, M. Nasal Expiratory Positive Airway Pressure Devices (Provent) for OSA: A Systematic Review and Meta-Analysis. Sleep Disord. 2015, 2015, 734798. [Google Scholar] [CrossRef] [PubMed]
- Dedhia, R.C.; Bliwise, D.L.; Quyyumi, A.A.; Thaler, E.R.; Boon, M.S.; Huntley, C.T.; Seay, E.G.; Tangutur, A.; Strollo, P.J.; Gurel, N.; et al. Hypoglossal Nerve Stimulation and Cardiovascular Outcomes for Patients with Obstructive Sleep Apnea: A Randomized Clinical Trial. JAMA Otolaryngol. Neck Surg. 2024, 150, 39. [Google Scholar] [CrossRef] [PubMed]
- Strollo, P.J.; Soose, R.J.; Maurer, J.T.; De Vries, N.; Cornelius, J.; Froymovich, O.; Hanson, R.D.; Padhya, T.A.; Steward, D.L.; Gillespie, M.B.; et al. Upper-Airway Stimulation for Obstructive Sleep Apnea. N. Engl. J. Med. 2014, 370, 139–149. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Kandzari, D.E.; O’Neill, W.W.; D’Agostino, R.; Flack, J.M.; Katzen, B.T.; Leon, M.B.; Liu, M.; Mauri, L.; Negoita, M.; et al. A Controlled Trial of Renal Denervation for Resistant Hypertension. N. Engl. J. Med. 2014, 370, 1393–1401. [Google Scholar] [CrossRef]
- Kario, K.; Bhatt, D.L.; Kandzari, D.E.; Brar, S.; Flack, J.M.; Gilbert, C.; Oparil, S.; Robbins, M.; Townsend, R.R.; Bakris, G. Impact of Renal Denervation on Patients with Obstructive Sleep Apnea and Resistant Hypertension—Insights From the SYMPLICITY HTN-3 Trial. Circ. J. 2016, 80, 1404–1412. [Google Scholar] [CrossRef]
- Azizi, M.; Saxena, M.; Wang, Y.; Jenkins, J.S.; Devireddy, C.; Rader, F.; Fisher, N.D.L.; Schmieder, R.E.; Mahfoud, F.; Lindsey, J.; et al. Endovascular Ultrasound Renal Denervation to Treat Hypertension: The RADIANCE II Randomized Clinical Trial. JAMA 2023, 329, 651. [Google Scholar] [CrossRef]
- Azizi, M.; Sanghvi, K.; Saxena, M.; Gosse, P.; Reilly, J.P.; Levy, T.; Rump, L.C.; Persu, A.; Basile, J.; Bloch, M.J.; et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): A randomised, multicentre, single-blind, sham-controlled trial. Lancet 2021, 397, 2476–2486. [Google Scholar] [CrossRef]
- Malhotra, A.; Grunstein, R.R.; Fietze, I.; Weaver, T.E.; Redline, S.; Azarbarzin, A.; Sands, S.A.; Schwab, R.J.; Dunn, J.P.; Chakladar, S.; et al. Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity. N. Engl. J. Med. 2024, 391, 1193–1205. [Google Scholar] [CrossRef]
- Schiavon, C.A.; Bersch-Ferreira, A.C.; Santucci, E.V.; Oliveira, J.D.; Torreglosa, C.R.; Bueno, P.T.; Frayha, J.C.; Santos, R.N.; Damiani, L.P.; Noujaim, P.M.; et al. Effects of Bariatric Surgery in Obese Patients with Hypertension: The GATEWAY Randomized Trial (Gastric Bypass to Treat Obese Patients with Steady Hypertension). Circulation 2018, 137, 1132–1142. [Google Scholar] [CrossRef]
- Kennedy, C.; Hayes, P.; Cicero, A.F.G.; Dobner, S.; Le Roux, C.W.; McEvoy, J.W.; Zgaga, L.; Hennessy, M. Semaglutide and blood pressure: An individual patient data meta-analysis. Eur. Heart J. 2024, 45, 4124–4134. [Google Scholar] [CrossRef]
- Van Ryswyk, E.; Anderson, C.S.; Barbe, F.; Loffler, K.A.; Lorenzi-Filho, G.; Luo, Y.; Quan, W.; Wang, J.; Zheng, D.; McEvoy, R.D. Effect of Continuous Positive Airway Pressure on Blood Pressure in Obstructive Sleep Apnea with Cardiovascular Disease. Am. J. Respir. Crit. Care Med. 2019, 199, 1433–1435. [Google Scholar] [CrossRef]
- Martínez-García, M.-A.; Capote, F.; Campos-Rodríguez, F.; Lloberes, P.; Díaz De Atauri, M.J.; Somoza, M.; Masa, J.F.; González, M.; Sacristán, L.; Barbé, F.; et al. Effect of CPAP on Blood Pressure in Patients with Obstructive Sleep Apnea and Resistant Hypertension: The HIPARCO Randomized Clinical Trial. JAMA 2013, 310, 2407. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; Pengo, M.F. Clinical Phenotype of Resistant Hypertension Responders to Continuous Positive Airway Pressure Treatment: Results From the HIPARCO Randomized Clinical Trial. Hypertension 2021, 78, 559–561. [Google Scholar] [CrossRef]
- Sun, L.; Chang, Y.-F.; Wang, Y.-F.; Xie, Q.-X.; Ran, X.-Z.; Hu, C.-Y.; Luo, B.; Ning, B. Effect of Continuous Positive Airway Pressure on Blood Pressure in Patients with Resistant Hypertension and Obstructive Sleep Apnea: An Updated Meta-analysis. Curr. Hypertens. Rep. 2024, 26, 201–211. [Google Scholar] [CrossRef]
- Ou, Y.-H.; Tan, A.; Lee, C.-H. Management of hypertension in obstructive sleep apnea. Am. J. Prev. Cardiol. 2023, 13, 100475. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.X.; Lin, Y.N.; Zhang, L.Y.; Li, S.Q.; Zhang, L.; Yan, Y.R.; Lu, F.Y.; Li, N.; Li, Q.Y. The Role of Aldosterone in OSA and OSA-Related Hypertension. Front. Endocrinol. 2022, 12, 801689. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, K.; Pimenta, E.; Thomas, S.J.; Cofield, S.S.; Oparil, S.; Harding, S.M.; Calhoun, D.A. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: A preliminary report. J. Hum. Hypertens. 2010, 24, 532–537. [Google Scholar] [CrossRef]
- Morgan, E.S.; Tami, Y.; Hu, K.; Brambatti, M.; Mullick, A.E.; Geary, R.S.; Bakris, G.L.; Tsimikas, S. Antisense Inhibition of Angiotensinogen with IONIS-AGT-LRx: Results of Phase 1 and Phase 2 Studies. JACC Basic Transl. Sci. 2021, 6, 485–496. [Google Scholar] [CrossRef]
- Tanriover, C.; Ucku, D.; Akyol, M.; Cevik, E.; Kanbay, A.; Sridhar, V.S.; Cherney, D.Z.I.; Kanbay, M. Potential Use of SGLT-2 Inhibitors in Obstructive Sleep Apnea: A new treatment on the horizon. Sleep Breath. 2023, 27, 77–89. [Google Scholar] [CrossRef]
- Neeland, I.J.; Eliasson, B.; Kasai, T.; Marx, N.; Zinman, B.; Inzucchi, S.E.; Wanner, C.; Zwiener, I.; Wojeck, B.S.; Yaggi, H.K.; et al. The Impact of Empagliflozin on Obstructive Sleep Apnea and Cardiovascular and Renal Outcomes: An Exploratory Analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2020, 43, 3007–3015. [Google Scholar] [CrossRef]
- Wojeck, B.S.; Inzucchi, S.E.; Neeland, I.J.; Mancuso, J.P.; Frederich, R.; Masiukiewicz, U.; Cater, N.B.; McGuire, D.K.; Cannon, C.P.; Yaggi, H.K. Ertugliflozin and incident obstructive sleep apnea: An analysis from the VERTIS CV trial. Sleep Breath. Schlaf Atm. 2023, 27, 669–672. [Google Scholar] [CrossRef]
- Armentaro, G.; Pelaia, C.; Condoleo, V.; Severini, G.; Crudo, G.; De Marco, M.; Pastura, C.A.; Tallarico, V.; Pezzella, R.; Aiello, D.; et al. Effect of SGLT2-Inhibitors on Polygraphic Parameters in Elderly Patients Affected by Heart Failure, Type 2 Diabetes Mellitus, and Sleep Apnea. Biomedicines 2024, 12, 937. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Sagris, M.; Patoulias, D.; Koufakis, T.; Theofilis, P.; Klisic, A.; Fragakis, N.; El Tanani, M.; Rizzo, M. Mitigating Increased Cardiovascular Risk in Patients with Obstructive Sleep Apnea Using GLP-1 Receptor Agonists and SGLT2 Inhibitors: Hype or Hope? Biomedicines 2024, 12, 2503. [Google Scholar] [CrossRef]
- Monda, V.M.; Gentile, S.; Porcellati, F.; Satta, E.; Fucili, A.; Monesi, M.; Strollo, F. Heart Failure with Preserved Ejection Fraction and Obstructive Sleep Apnea: A Novel Paradigm for Additional Cardiovascular Benefit of SGLT2 Inhibitors in Subjects with or without Type 2 Diabetes. Adv. Ther. 2022, 39, 4837–4846. [Google Scholar] [CrossRef]
- Mazzotti, D.R.; Waitman, L.R.; Miller, J.; Sundar, K.M.; Stewart, N.H.; Gozal, D.; Song, X.; Greater Plains Collaborative. Positive Airway Pressure, Mortality, and Cardiovascular Risk in Older Adults with Sleep Apnea. JAMA Netw. Open 2024, 7, e2432468. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.F.; Jerrentrup, A.; Ploch, T.; Grote, L.; Penzel, T.; Sullivan, C.E.; Peter, J.H. Effect of Nasal Continuous Positive Airway Pressure Treatment on Blood Pressure in Patients with Obstructive Sleep Apnea. Circulation 2003, 107, 68–73. [Google Scholar] [CrossRef]
- Pepperell, J.C.; Ramdassingh-Dow, S.; Crosthwaite, N.; Mullins, R.; Jenkinson, C.; Stradling, J.R.; Davies, R.J. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: A randomised parallel trial. Lancet 2002, 359, 204–210. [Google Scholar] [CrossRef]
- Sundman, J.; Nerfeldt, P.; Fehrm, J.; Bring, J.; Browaldh, N.; Friberg, D. Effectiveness of Tonsillectomy vs. Modified Uvulopalatopharyngoplasty in Patients with Tonsillar Hypertrophy and Obstructive Sleep Apnea: The TEAMUP Randomized Clinical Trial. JAMA Otolaryngol. Neck Surg. 2022, 148, 1173. [Google Scholar] [CrossRef]
- Punjabi, N.M.; Caffo, B.S.; Goodwin, J.L.; Gottlieb, D.J.; Newman, A.B.; O’Connor, G.T.; Rapoport, D.M.; Redline, S.; Resnick, H.E.; Robbins, J.A.; et al. Sleep-Disordered Breathing and Mortality: A Prospective Cohort Study. PLoS Med. 2009, 6, e1000132. [Google Scholar] [CrossRef]
- Sutherland, K.; Cistulli, P. Mandibular advancement splints for the treatment of sleep apnea syndrome. Swiss Med. Wkly. 2011, 141, w13276. [Google Scholar] [CrossRef] [PubMed]
| ABPM Parameter | Hypertension Threshold | Non-Dipping Criterion |
|---|---|---|
| 24 h Mean BP | ≥130/80 mmHg | |
| Daytime (awake) Mean BP | ≥135/85 mmHg | |
| Nighttime (sleep) Mean BP | ≥120/70 mmHg | |
| Nocturnal BP Drop | Normal dip: 10–20% fall Non-dipper: <10% fall Extreme dipper: >20% fall Reverse dipper: nighttime BP > daytime |
| PSG Parameter | Definition and Measurement | OSA Diagnostic Threshold/Severity |
|---|---|---|
| Apnea-Hypopnea Index (AHI) | Number of apneas + hypopneas per hour of sleep (events/hour). An apnea is a ≥10 s breathing cessation; a hypopnea is a ≥30–50% airflow reduction with desaturation ≥3% or arousal | Primary metric for OSA diagnosis. AHI ≥ 5/h indicates OSA (with symptoms). Severity is classified as: Mild OSA if AHI 5–14; moderate OSA if AHI 15–29; severe OSA if AHI ≥ 30. |
| Oxygen Desaturation Index (ODI) | Number of oxygen desaturations ≥3% (or ≥4%) per hour of sleep (events/hour), reflecting frequency of oxygen drops. ODI is often reported with a 4% criterion in sleep studies. | Correlates with AHI and OSA severity. ODI is a surrogate for hypoxic burden. For example, ODI ≥ 5/h is abnormal and roughly corresponds to AHI ≥ 5. An ODI ≥ 15/h suggests at least moderate OSA, and ODI ≥ 30/h correlates with severe OSA. (ODI values show ~84–94% accuracy in predicting corresponding AHI severity cut-offs.) Higher ODI indicates more frequent or deeper desaturations, which are linked to cardiovascular risk. |
| Arousal Index | Number of EEG arousals per hour of sleep. An arousal is an abrupt shift in EEG to wake or lighter stage (≥3 s), often following an apnea/hypopnea. | Reflects sleep fragmentation. OSA causes frequent arousals from sleep due to respiratory events. A normal adult arousal index is on the order of <10–15/h (though it increases with age). OSA patients often have markedly elevated arousal indices proportional to AHI (e.g., an AHI of 30/h typically produces dozens of arousals per hour). A high arousal index contributes to non-restorative sleep and daytime sleepiness. |
| Sleep Architecture | Distribution of sleep stages (N1–N3 non-REM stages and REM sleep) and sleep continuity measures (total sleep time, sleep efficiency). PSG reports the percentage of time spent in each stage and sleep efficiency (% of time in bed asleep) | OSA-related alterations: OSA disrupts normal architecture. Normal adult sleep: ~5% stage N1, ~50% N2, ~20% N3 (slow-wave sleep), ~20–25% REM, with sleep efficiency >85%. In OSA, there is often an increase in light sleep (N1, N2) and a reduction in deep N3 and REM sleep due to recurrent arousals. Severe OSA can significantly reduce slow-wave and REM sleep percentages. Sleep efficiency may be reduced (<80%) because frequent awakenings and arousals fragment the sleep. These disruptions in architecture improve with effective OSA treatment (REM and N3 rebound with CPAP therapy) |
| Author | Year | Study Design | Sample Size | Population Characteristics | SDB and Hypertension Measures | Key Findings |
|---|---|---|---|---|---|---|
| Amin et al. [30]. | 2008 | Comparative observational study | 140 | Children with SDB; otherwise, healthy | Apnea-hypopnea index (AHI), 24-h ambulatory blood pressure | SDB is independently associated with increased morning BP surge, BP load, and 24-h ambulatory BP. It is also associated with left ventricular remodeling, highlighting increased cardiovascular morbidity. |
| O’Connor et al. [31]. | 2009 | Prospective cohort study | 2470 | Adults aged 40+ without baseline hypertension | AHI, incident hypertension over 5 years | The relationship between AHI and incident hypertension was attenuated after adjusting for BMI. The association was not statistically significant, but a modest association cannot be excluded. |
| Tanigawa et al. [32]. | 2004 | Cross-sectional study | 1424 | Japanese men aged 40–69 in rural and urban communities | 3% Oxygen Desaturation Index (ODI), SBP/DBP | 3% ODI level is positively associated with higher SBP and DBP. The association was more evident among overweight individuals, suggesting a role of SDB in HTN development among Japanese men. |
| Sánchez-de-la-Torre et al. [33]. | 2015 | Experimental study | 100 (subsample) | Adults with resistant hypertension and obstructive sleep apnea (OSA) | BP response to CPAP treatment, miRNA profiles | A pre-CPAP treatment cluster of 3 plasma miRNAs predicted BP responses to CPAP in patients with Resistant hypertension and OSA. |
| Witkowski et al. [34]. | 2011 | Interventional study | 10 | Patients with resistant hypertension and sleep apnea | BP measurements, AHI, polysomnography | Renal sympathetic denervation significantly lowered BP and improved sleep apnea severity. |
| Horne et al. [35]. | 2011 | Comparative observational study | 141 | Children with varying severities of SDB and nonsmoking controls | BP during sleep and wake, categorized by sleep states | SDB, regardless of severity, was associated with elevated BP during both sleep and wake states compared to controls. |
| Peppard et al. [36]. | 2000 | Prospective, Population-Based | 709 | Participants from the Wisconsin Sleep Cohort Study, general population sample | AHI measured via polysomnography; hypertension defined as BP ≥ 140/90 mm Hg or use of medications | A dose-response association between SDB and hypertension was observed, independent of confounding factors. |
| Reid et al. [37]. | 2011 | Cross-Sectional | 60 | Pregnant women with gestational hypertension (n = 34) and healthy pregnant women (n = 26) | Frequency of SDB assessed via polysomnography; respiratory disturbance index ≥ 5 | Women with gestational hypertension had a higher frequency of SDB compared to healthy women; the impact of SDB and obesity on this condition is unclear. |
| Li et al. [38]. | 2020 | Prospective, Population-Based | 11,623 | U.S. Hispanic/Latino participants from the Hispanic Community Health Study/Study of Latinos | AHI ≥ 5 for SDB; insomnia assessed with Women’s Health Initiative Insomnia Rating Scale ≥ 9; incident hypertension and diabetes | SDB was associated with higher odds of incident hypertension and diabetes. Insomnia was associated with incident hypertension, with a stronger association among men. |
| Minic et al. [39]. | 2014 | Retrospective, Cross-Sectional | 52 | Patients with WHO group 1 pulmonary arterial hypertension (PAH) | Prevalence of SDB assessed via PSG; Epworth Sleepiness Scale (ESS) for subjective sleepiness | High prevalence of SDB in PAH patients. Older age and higher ESS scores were predictive of SDB. |
| Facco et al. [40]. | 2017 | Prospective Cohort | 3705 | Nulliparous women, assessed early and mid-pregnancy | AHI ≥ 5 used to define SDB; hypertensive disorders of pregnancy (HDP), including preeclampsia and gestational diabetes mellitus (GDM) measured | SDB in pregnancy is independently associated with an increased risk of preeclampsia, hypertensive disorders, and GDM. The relationship between AHI and these outcomes is exposure–response dependent. |
| Ulrich et al. [41]. | 2013 | Randomized, Double-Blind, Cross-Over | 23 | Patients with pre-capillary pulmonary hypertension (PH) and SDB | Nocturnal oxygen therapy (NOT) and acetazolamide effects on 6-min walk distance (MWD), SDB, and hemodynamics | NOT improved 6 MWD and SDB along with hemodynamic improvements in patients with pre-capillary PH. No significant improvement in quality of life or exercise performance was observed with acetazolamide. |
| Spiesshoefer, et al. [42]. | 2021 | Prospective, Cross-Sectional | 71 | Patients with pre-capillary pulmonary hypertension (PH) and 35 matched controls | Prevalence and severity of OSA and nocturnal hypoxemia; Assessed via overnight cardiorespiratory polygraphy, lung function, HCVR, and cardiac MRI | High prevalence of OSA in PH patients, with significant associations between SDB severity and reduced 6 MWD, NT-proBNP levels, and right ventricular function. The clinical impact of these findings warrants further investigation. |
| Eskandari et al. [43]. | 2018 | Randomized Controlled Trial (Three-way Crossover) | 13 | Male patients with hypertension and moderate to severe OSA; mean age: 64 ± 7 years; BMI: 29 ± 4 kg/m2; AHI: 37 ± 23 events/h | Blood pressure (office and 24-h), arterial stiffness, polygraphic sleep study data, AHI, venous bicarbonate concentration | AZT alone and AZT + CPAP reduced blood pressure and AHI. CPAP alone did not significantly affect blood pressure. AZT is a potential drug therapy for OSA with hypertension. |
| Zhang et al. [44]. | 2024 | Observational Study with PCA and Metabolomic Analysis | 3299 (Discovery) + 1522 (Validation) | Hispanic Community Health Study/Study of Latinos; OSA characterized by age, gender, respiratory event frequency, and sleep quality | SDB-related measures, metabolite risk scores (MRS), incident hypertension and diabetes | SDB is associated with distinct metabolomic profiles. SDB-related metabolite signatures are linked with 6-year incident hypertension and diabetes, suggesting potential biomarkers for SDB risk stratification. |
| Kato et al. [45]. | 2019 | Observational Study | 52 | Elderly adults with SDB; mean age: 69.6 ± 4.0 years; no impairment in daily living activities | Apnea/hypopnea index (AHI), minimum oxygen saturation (SpO2), hypertension (measured via questionnaire and BP value) | Nocturnal hypoxia (SpO2 < 90%) and hypertension negatively impacted cognitive function. HTN was the most significant factor for cognitive decline in tasks like WCST and N-back task. |
| Treatment | Mechanism | Benefits | Limitations | Selected RCT and Meta-Analysis (MA) in Patients with OSA and HTN | |
|---|---|---|---|---|---|
| Pharmacologic Therapies | Antihypertensives ACE inhibitors, ARBs, calcium channel blockers, beta-blockers | Reduction in vasoconstriction and sympathetic overactivity | Reduces BP, prevents cardiovascular events. | Limited efficacy in OSA due to persistent airway obstruction; potential negative effects on sleep quality due to frequent awakening from nocturnal diuresis. | RCT 2000: Atenolol and Hydrochlorothiazide were more effective than amlodipine, losartan, and enalapril in lowering blood pressure in OSA patients with HTN [76]. VALSAS trial: Valsartan induced a fourfold decrease in mean 24-h BP in OSA patients by inhibiting angiotensin II and preventing vasoconstriction [77]. |
| Diuretics and Sympatholytic | Lowers arterial pressure and volume particularly in OSA-mediated fluid retention and sympathetic overdrive | Reduces preload and lowers BP, particularly effective in patients with fluid retention. | Ineffective as standalone treatment; does not address airway obstruction or intermittent hypoxia. | RCT 2024: in OSA patients with HTN, Guanfacine lowered 24-h blood pressure more than HCTZ, but HCTZ still showed a significant effect [78]. | |
| RNA Interference (RNAi) Precision medicine | Targets gene expression to reduce angiotensin II production, lowering BP. | Promising for OSA-related HTN and TRH; potential shift in HTN management. | Novel therapy with limited clinical experience; requires more long-term data. | RCT: in OSA patients with TRH, 3 identified miRNAs predict BP response to CPAP [33]. KARDIA-1 trial: RNA interference with single subcutaneous dose of zilebesiran significantly lowers BP up to 6 months [79]. | |
| SGLT2-Inhibitors | Promotes natriuresis and glycosuria, reducing plasma volume and BP. | Cardioprotective, addresses metabolic and cardiovascular issues; benefits patients with comorbid diabetes or HF. | Not a primary treatment for OSA; efficacy may vary between patients. | EMPA-REG OUTCOME trial: empagliflozin leads to a significant reduction in BP for Patients with Presumed TRH not specific to OSA-related HTN [80]. CANVAS trial: canagliflozin leads to significant BP lowering, not specific to OSA-related HTN [81]. DAHOS trial: in HFrEF with OSA patients, dapagliflozin significantly reduced blood pressure and apnea-hypopnea index (AHI) [82]. | |
| Non-CPAP Therapies | Oral/Nasal Appliances | Devices like soft-palate lifts, tongue-retaining devices, and mandibular advancement appliances reposition anatomical structures to alleviate airway obstruction. | Reduces apneic episodes, improves BP control, alternative for CPAP intolerance. | Variable efficacy; not as effective as CPAP therapy. | MA: Provent is a disposable nasal device that reduces AHI and improves oxygen saturation in patients with OSA. The data on BP benefits are limited. It is currently discontinued [83]. |
| Expansion Sphincter Pharyngoplasty, Hypoglossal Nerve Stimulation | Surgical and minimally invasive procedures to stabilize and enlarge the airway or stimulate upper airway muscles. | Reduces OSA severity and improves BP in some patients intolerant to standard treatments. | Invasive, costly, with potential complications; reserved for specific cases. | CARDIOSA-12 trial: Hypoglossal Nerve Stimulation (HGNS) therapy did not improve BP and other CV measures [84]. STAR trial: HGNS reduces AHI and a number of oxygen desaturations [85]. 22 April 2025 2:23:00 PM | |
| Uvulopalatopharyngoplasty (UPPP), Renal Denervation | UPPP removes excess throat tissue to enlarge the airway; renal denervation impairs renal sympathetic nerves to control BP. | UPPP reduces apneic episodes; renal denervation shows potential in reducing BP in TRH patients. | Mixed BP results; UPPP may not address core OSA pathophysiology; renal denervation is invasive and a last resort. | SYMPLICITY HTN-3: Renal denervation reduced BP in TRH patients; in a post hoc analysis, OSA patients appeared to be responsive to renal denervation therapy [86,87]. RADIANCE trials: renal denervation showed a significant BP reduction in TRH; but this was not specific to OSA-related HTN [86,87,88,89]. | |
| Lifestyle Interventions | Weight Loss | Reduces OSA severity, lowers BP, and improves overall cardiovascular health through lifestyle changes, medications, or bariatric surgery. | Effective in reducing both OSA and HTN severity; bariatric surgery is an option for severe obesity. | Requires long-term commitment; bariatric surgery carries risks and is suitable for selected patients only. | SURMOUNT-OSA: in patients with moderate-to-severe OSA plus Obesity, Tirzepatide reduced AHI, BMI, and SBP [90]. GATEWAY: Bariatric surgery reduced BP in obese patients with HTN [91]. MA: A decline in body weight in patients on GLP-1 RA resulted in a decrease in SBP [92]. 22 April 2025 2:23:00 PM |
| Gold Standard Therapy | CPAP Therapy | Provides continuous airflow to prevent upper airway collapse during sleep, reducing apnea, hypoxia, and sympathetic activity. | Reduces both nocturnal and daytime BP; prevents cardiovascular events such as heart attack and stroke. | Tolerance issues in some patients; adherence is critical for effectiveness. | SAVE trial: Early CPAP adherence is associated with modest reductions in BP and significant cardiovascular benefits in OSA patients [93]. HIPARCO trial: Optimal adherence to CPAP restores BP dipping pattern and significantly reduces daytime and nighttime blood pressure in patients with OSA and TRH [94,95]. MA: CPAP treatment significantly reduced BP in OSA patients with TRH [96]. 22 April 2025 2:23:00 PM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battisha, A.; Kahlon, A.; Kalra, D.K. Sleep-Disordered Breathing and Hypertension—A Systematic Review. J. Clin. Med. 2025, 14, 3115. https://doi.org/10.3390/jcm14093115
Battisha A, Kahlon A, Kalra DK. Sleep-Disordered Breathing and Hypertension—A Systematic Review. Journal of Clinical Medicine. 2025; 14(9):3115. https://doi.org/10.3390/jcm14093115
Chicago/Turabian StyleBattisha, Ayman, Amrit Kahlon, and Dinesh K. Kalra. 2025. "Sleep-Disordered Breathing and Hypertension—A Systematic Review" Journal of Clinical Medicine 14, no. 9: 3115. https://doi.org/10.3390/jcm14093115
APA StyleBattisha, A., Kahlon, A., & Kalra, D. K. (2025). Sleep-Disordered Breathing and Hypertension—A Systematic Review. Journal of Clinical Medicine, 14(9), 3115. https://doi.org/10.3390/jcm14093115







