Paraspinal Muscle Fat Infiltration as a Key Predictor of Symptomatic Intravertebral Vacuum Cleft: A Machine Learning Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
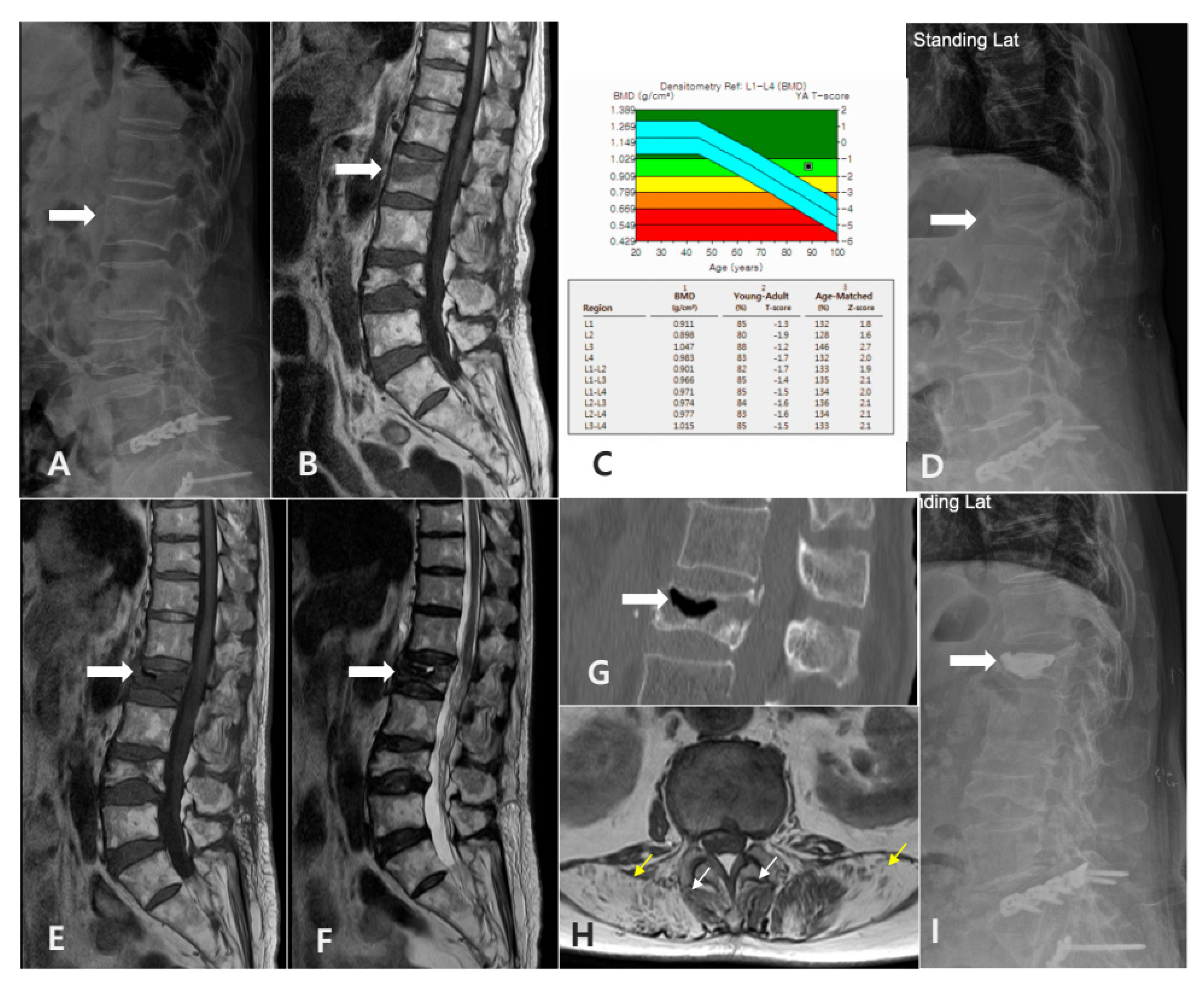
2.2. Definition and Diagnostic Criteria of SIVC
- Clinical Criteria:
- -
- Initial acute pain following a VCF.
- -
- Period of initial improvement with conservative treatment.
- -
- Development of recurrent pain (VAS > 3).
- -
- Location-specific pain corresponding to the level of vacuum cleft.
- Radiographic Criteria:
- -
- Radiographic evidence of intravertebral vacuum cleft (IVC) on plain thoracolumbar radiographs.
- -
- Absence of other significant pathology at the affected and adjacent levels, such as new fractures.
2.3. Data Collection
2.4. Machine Learning Models
2.5. Evaluation Matrix
3. Results
3.1. Patient Characteristics
3.2. Performances of Machine Learning Models
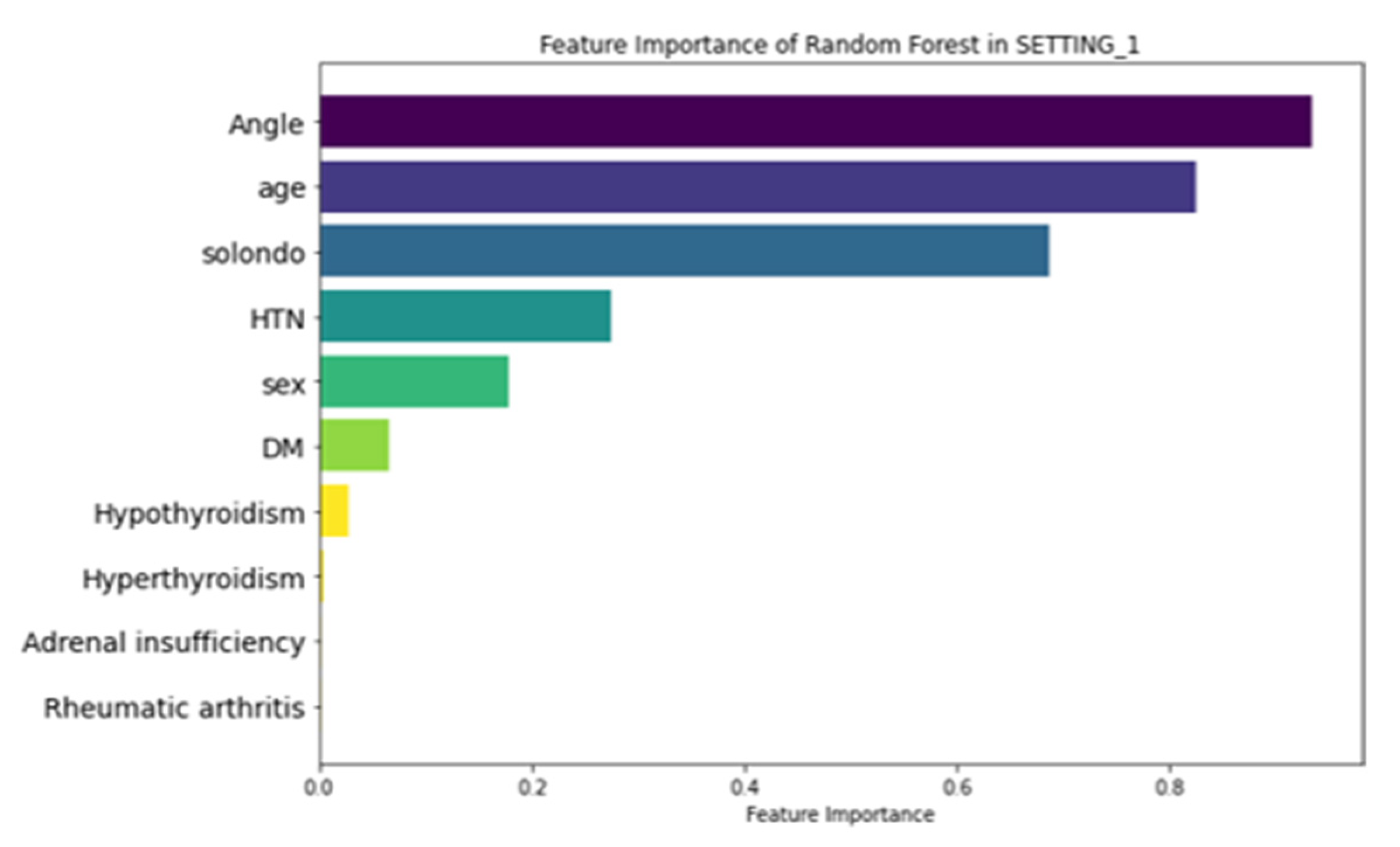
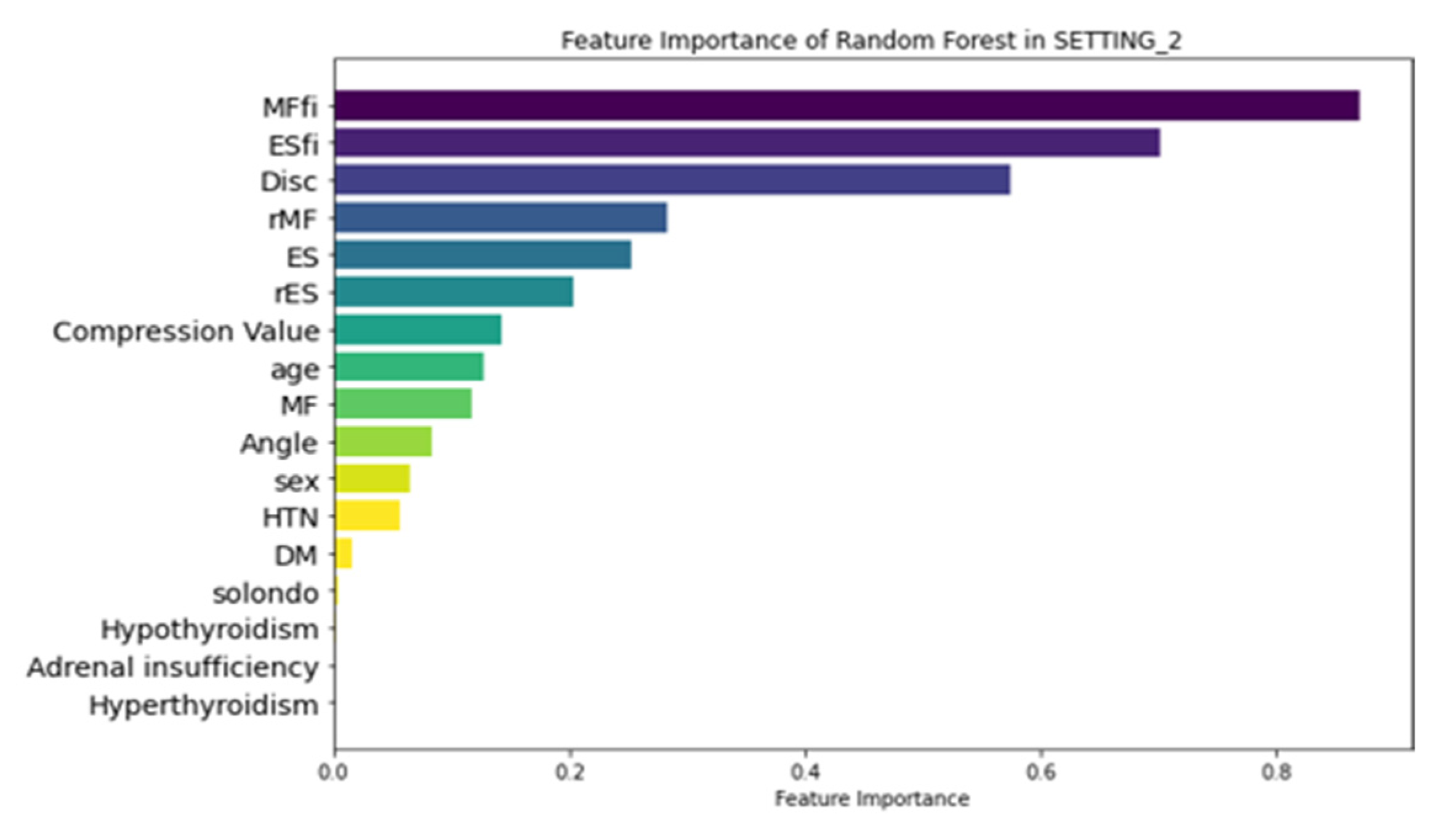
3.3. Feature Importance
3.4. LIME Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SIVC | Symptomatic intravertebral vacuum cleft |
| VCF | Vertebral compression fracture |
| EMR | Electronic medical records |
| ML | Machine learning |
| TLJ | Thoracolumbar junction |
| MF | Multifidus |
| ES | Erector spinae |
| MFfi | MF fat infiltration |
| ESfi | ES fat infiltration |
| CSA | Cross-sectional area |
| rMF | Relative CSA of multifidus per CSA of endplate |
| rES | Relative CSA of erector spinae per CSA of endplate |
| LR | Logistic regression |
| RF | Random forest |
| XGBoost | Extreme gradient boosting |
| MLP | Multi-layer perceptron |
| AUROC | Area under the receiver operating characteristic curve |
| BMD | Bone mineral density |
| BMI | Body mass index |
| PR | Plain radiograph |
References
- Watanabe, K.; Lenke, L.G.; Bridwell, K.H.; Kim, Y.J.; Koester, L.; Hensley, M. Proximal junctional vertebral fracture in adults after spinal deformity surgery using pedicle screw constructs: Analysis of morphological features. Spine 2010, 35, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Park, W.M.; Choi, D.K.; Kim, K.; Kim, Y.J.; Kim, Y.H. Biomechanical effects of fusion levels on the risk of proximal junctional failure and kyphosis in lumbar spinal fusion surgery. Clin. Biomech. 2015, 30, 1162–1169. [Google Scholar] [CrossRef]
- Silverman, S.L. The clinical consequences of vertebral compression fracture. Bone 1992, 13 (Suppl. 2), S27–S31. [Google Scholar] [CrossRef] [PubMed]
- Heggeness, M.H. Spine fracture with neurological deficit in osteoporosis. Osteoporos. Int. 1993, 3, 215–221. [Google Scholar] [CrossRef]
- Baba, H.; Maezawa, Y.; Kamitani, K.; Furusawa, N.; Imura, S.; Tomita, K. Osteoporotic vertebral collapse with late neurological complications. Paraplegia 1995, 33, 281–289. [Google Scholar] [CrossRef]
- Sarli, M.; Perez Manghi, F.C.; Gallo, R.; Zanchetta, J.R. The vacuum cleft sign: An uncommon radiological sign. Osteoporos. Int. 2005, 16, 1210–1214. [Google Scholar] [CrossRef]
- Jang, J.S.; Kim, D.Y.; Lee, S.H. Efficacy of percutaneous vertebroplasty in the treatment of intravertebral pseudarthrosis associated with noninfected avascular necrosis of the vertebral body. Spine 2003, 28, 1588–1592. [Google Scholar]
- Osterhouse, M.D.; Kettner, N.W. Delayed posttraumatic vertebral collapse with intravertebral vacuum cleft. J. Manip. Physiol. Ther. 2002, 25, 270–275. [Google Scholar] [CrossRef]
- He, D.; Yu, W.; Chen, Z.; Li, L.; Zhu, K.; Fan, S. Pathogenesis of the intravertebral vacuum of Kummell’s disease. Exp. Ther. Med. 2016, 12, 879–882. [Google Scholar] [CrossRef]
- Adamska, O.; Modzelewski, K.; Stolarczyk, A.; Kseniuk, J. Is Kummell’s Disease a Misdiagnosed and/or an Underreported Complication of Osteoporotic Vertebral Compression Fractures? A Pattern of the Condition and Available Treatment Modalities. J. Clin. Med. 2021, 10, 2584. [Google Scholar] [CrossRef]
- Nickell, L.T.; Schucany, W.G.; Opatowsky, M.J. Kummell disease. Bayl. Univ. Med. Cent. Proc. 2013, 26, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, P.R.; Nagar, A.M.; Prasad, S.S.; Shah, J.R.; Patkar, D.P. Avascular necrosis of spine: A rare appearance. Spine 2004, 29, E119–E122. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Hasegawa, Y.; Toda, K.; Nakahara, S. Pathogenesis and diagnosis of delayed vertebral collapse resulting from osteoporotic spinal fracture. Spine J. 2002, 2, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Swartz, K.; Fee, D. Kummell’s disease: A case report and literature review. Spine 2008, 33, E152–E155. [Google Scholar] [CrossRef]
- Lee, D.G.; Bae, J.H. Fatty infiltration of the multifidus muscle independently increases osteoporotic vertebral compression fracture risk. BMC Musculoskelet. Disord. 2023, 24, 508. [Google Scholar] [CrossRef]
- Kim, J.Y.; Chae, S.U.; Kim, G.D.; Cha, M.S. Changes of paraspinal muscles in postmenopausal osteoporotic spinal compression fractures: Magnetic resonance imaging study. J. Bone Metab. 2013, 20, 75–81. [Google Scholar] [CrossRef]
- Jeon, I.; Kim, S.W.; Yu, D. Paraspinal muscle fatty degeneration as a predictor of progressive vertebral collapse in osteoporotic vertebral compression fractures. Spine J. 2022, 22, 313–320. [Google Scholar] [CrossRef]
- Martin, B.I.; Bono, C.M. Artificial intelligence and spine: Rise of the machines. Spine J. 2021, 21, 1604–1605. [Google Scholar] [CrossRef]
- Yabu, A.; Hoshino, M.; Tabuchi, H.; Takahashi, S.; Masumoto, H.; Akada, M.; Morita, S.; Maeno, T.; Iwamae, M.; Inose, H.; et al. Using artificial intelligence to diagnose fresh osteoporotic vertebral fractures on magnetic resonance images. Spine J. 2021, 21, 1652–1658. [Google Scholar] [CrossRef]
- Wang, Y.; Mehrabi, S.; Sohn, S.; Atkinson, E.J.; Amin, S.; Liu, H. Natural language processing of radiology reports for identification of skeletal site-specific fractures. BMC Med. Inform. Decis. Mak. 2019, 19 (Suppl. 3), 73. [Google Scholar] [CrossRef]
- Wright, R.E. Logistic regression. In Reading and Understanding Multivariate Statistics; American Psychological Association: Washington, DC, USA, 1995; pp. 217–244. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016. [Google Scholar] [CrossRef]
- Pal, S.K.; Mitra, S. Multilayer perceptron, fuzzy sets, and classification. IEEE Trans. Neural Netw. 1992, 3, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.I.; Maus, T.P.; Wald, J.T.; Thielen, K.R.; Bobra, S.; Luetmer, P.H. Intravertebral clefts opacified during vertebroplasty: Pathogenesis, technical implications, and prognostic significance. Am. J. Neuroradiol. 2002, 23, 1642–1646. [Google Scholar] [PubMed]
- Gu, Y.; Wang, C.; Hu, J.; Chen, Y.; Yu, W.; Wang, Z.; Wang, X.; Yuan, W. Association Between the Cervical Extensor Musculature and the Demographic Features, Symptoms, and Sagittal Balance in Patients with Multilevel Cervical Spondylotic Myelopathy. World Neurosurg. 2023, 169, e40–e50. [Google Scholar] [CrossRef]
- Suo, M.; Zhang, J.; Sun, T.; Wang, J.; Liu, X.; Huang, H.; Li, Z. The association between morphological characteristics of paraspinal muscle and spinal disorders. Ann. Med. 2023, 55, 2258922. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, M.; Serrano Sosa, M.; Cattell, R.; Fan, W.; Li, M.; Chen, J.; Gao, M.; Zhou, Q.; Li, S.; et al. Fatty infiltration of paraspinal muscles is associated with bone mineral density of the lumbar spine. Arch. Osteoporos. 2019, 14, 99. [Google Scholar] [CrossRef]
- Tekin, Z.N.; Karatekin, B.D. Relationship between the Properties of Paraspinal Muscles and Bone Mineral Density in Osteoporotic Patients. J. Coll. Physicians Surg. Pak. 2022, 32, 1137–1142. [Google Scholar] [CrossRef]
- You, K.H.; Cho, M.; Lee, J.H. Effect of Muscularity and Fatty Infiltration of Paraspinal Muscles on Outcome of Lumbar Interbody Fusion. J. Korean Med. Sci. 2023, 38, e151. [Google Scholar] [CrossRef]
- Han, G.; Zou, D.; Liu, Z.; Zhang, B.; Gong, C.; Zhou, S.; Li, W.; Sun, Z.; Li, W. Fat infiltration of paraspinal muscles as an independent risk for bone nonunion after posterior lumbar interbody fusion. BMC Musculoskelet. Disord. 2022, 23, 232. [Google Scholar] [CrossRef]
- Moore, R.J. The vertebral end-plate: What do we know? Eur. Spine J. 2000, 9, 92–96. [Google Scholar] [CrossRef]
- Ollila, L.; Oura, P.; Karppinen, J.; Niinimäki, J.; Junno, J.A. Association between vertebral cross-sectional area and lumbar disc displacement—A population-based study. Eur. Spine J. 2024, 33, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, H.; Dong, S.; Tang, Z.R.; Chen, L.; Cai, X.; Hu, Z.; Yin, C. Establishment and validation of a nomogram and web calculator for the risk of new vertebral compression fractures and cement leakage after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fractures. Eur. Spine J. 2022, 31, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- Hiwatashi, A.; Westesson, P.L. Patients with osteoporosis on steroid medication tend to sustain subsequent fractures. Am. J. Neuroradiol. 2007, 28, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; Disoteo, O.E.; De Tullio, A.; De Geronimo, V.; Giagulli, V.A.; Monzani, F.; Jirillo, E.; Cozzi, R.; Guastamacchia, E.; De Pergola, G.; et al. Sarcopenia and Diabetes: A Detrimental Liaison of Advancing Age. Nutrients 2023, 16, 63. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Y.; Shi, Y.; Wang, X.; Liao, Z.; Wei, P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front. Endocrinol. 2020, 11, 568. [Google Scholar] [CrossRef]
- Karakousis, N.D.; Biliou, S.; Pyrgioti, E.E.; Georgakopoulos, P.N.; Liakopoulos, V.; Papanas, N. Frailty, sarcopenia and diabetic kidney disease: Where do we stand? Int. Urol. Nephrol. 2023, 55, 1173–1181. [Google Scholar] [CrossRef]
- Murray, C.E.; Coleman, C.M. Impact of Diabetes Mellitus on Bone Health. Int. J. Mol. Sci. 2019, 20, 4873. [Google Scholar] [CrossRef]
- Henderson, S.; Ibe, I.; Cahill, S.; Chung, Y.H.; Lee, F.Y. Bone Quality and Fracture-Healing in Type-1 and Type-2 Diabetes Mellitus. J. Bone Jt. Surg. 2019, 101, 1399–1410. [Google Scholar] [CrossRef]
- McCarthy, J.; Davis, A. Diagnosis and Management of Vertebral Compression Fractures. Am. Fam. Physician 2016, 94, 44–50. [Google Scholar]
- Cheng, Y.; Cheng, X.; Wu, H. Risk factors of new vertebral compression fracture after percutaneous vertebroplasty or percutaneous kyphoplasty. Front. Endocrinol. 2022, 13, 964578. [Google Scholar] [CrossRef]
- Nielsen, B.R.; Abdulla, J.; Andersen, H.E.; Schwarz, P.; Suetta, C. Sarcopenia and osteoporosis in older people: A systematic review and meta-analysis. Eur. Geriatr. Med. 2018, 9, 419–434. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.; Park, S.H.; Park, J.; Chang, B.S.; Lee, C.K.; Yeom, J.S. Prevalence of Frailty in Patients with Osteoporotic Vertebral Compression Fracture and Its Association with Numbers of Fractures. Yonsei Med. J. 2018, 59, 317–324. [Google Scholar] [CrossRef]



| Data Source | Characteristics | Number of Patients (%) | Description | p Value | |
|---|---|---|---|---|---|
| VCF Patients Without IVC | VCF Patients with IVC | ||||
| EMR | Age | 72.44 ± 1.15 | 79.85 ± 3.35 | Age at diagnosis | <0.05 * |
| Sex | F: 464 (71%) M: 190 (29%) | F: 27 (67%) M: 13 (33%) | Sex of patients | 0.7746 | |
| DM | P: 76 (12%) N: 578 (88%) | P: 4 (10%) N: 36 (90%) | Diabetes status | 0.9549 | |
| HTN | P: 259 (40%) N: 395 (60%) | P: 13 (67%) N: 27 (33%) | Hypertension status | 0.4676 | |
| Adrenal insufficiency | P: 7 (1%) N: 647 (99%) | P: 1 (3%) N: 39 (97%) | Adrenal insufficiency status | 0.9527 | |
| Hyperthyroidism | P: 1 (1%) N: 653 (99%) | P: 0 (0%) N: 40 (100%) | Hyperthyroidism status | 1.0 | |
| Hypothyroidism | P: 10 (2%) N: 644 (98%) | P: 0 (0%) N: 40 (100%) | Hypothyroidism status | 0.9169 | |
| Steroid | P: 23 (4%) N: 631 (96%) | P: 1 (3%) N: 39 (97%) | Continuously taking oral steroids = for more than 3 months | 1.0 | |
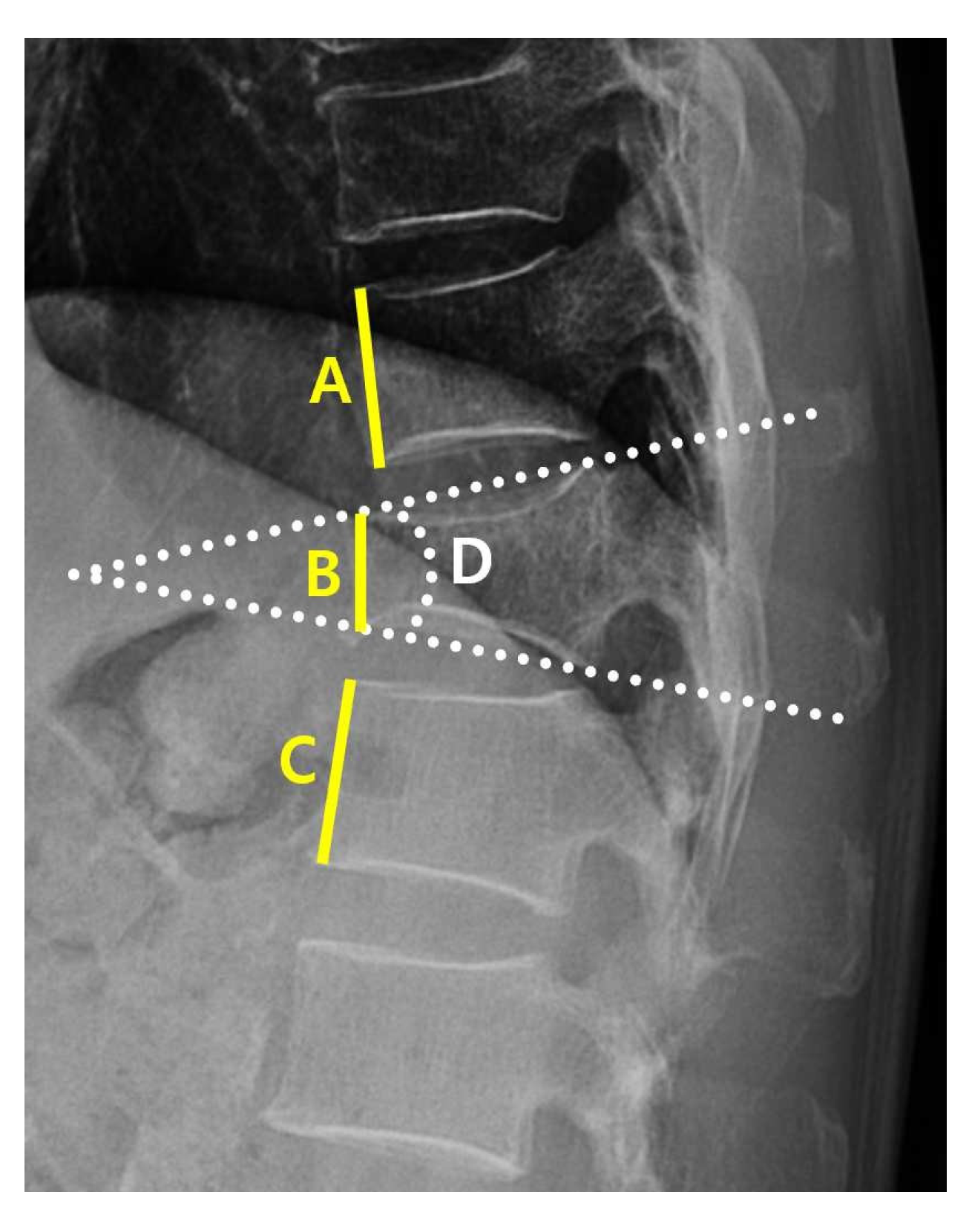
| PR (lateral view) | Kyphotic angle (°) | 19.89 ± 0.49 | 25.01 ± 4.21 | Kyphotic angulation at VCF | <0.05 * |
| Compression Value | 34.41 ± 1.27 | 48.31 ± 6.50 | Compression ratio at VCF | <0.05 * | |
| MRI (axial view) | Disc (cm/m2) | 12.51 ± 0.21 | 15.14 ± 0.93 | CSA of endplate at VCF | <0.05 * |
| MF (cm/m2) | 4.13 ± 0.14 | 2.95 ± 0.31 | CSA of MF at VCF | <0.05 * | |
| MFfi (%) | 16.39 ± 0.53 | 37.29 ± 4.58 | Fatty infiltration percentage of MF | <0.05 * | |
| rMF | 0.34 ± 0.01 | 0.20 ± 0.02 | CSA of relative MF at VCF | <0.05 * | |
| ES (cm/m2) | 20.37 ± 0.66 | 12.12 ± 1.43 | CSA of ES at VCF | <0.05 * | |
| ESfi (%) | 10.25 ± 0.51 | 29.03 ± 4.85 | Fatty infiltration percentage of ES | <0.05 * | |
| rES | 1.66 ± 0.05 | 0.83 ± 0.11 | CSA of relative ES at VCF | <0.05 * | |
| Datasets | Models | Accuracy | Specificity | Sensitivity | Precision | F1-Score | AUROC |
|---|---|---|---|---|---|---|---|
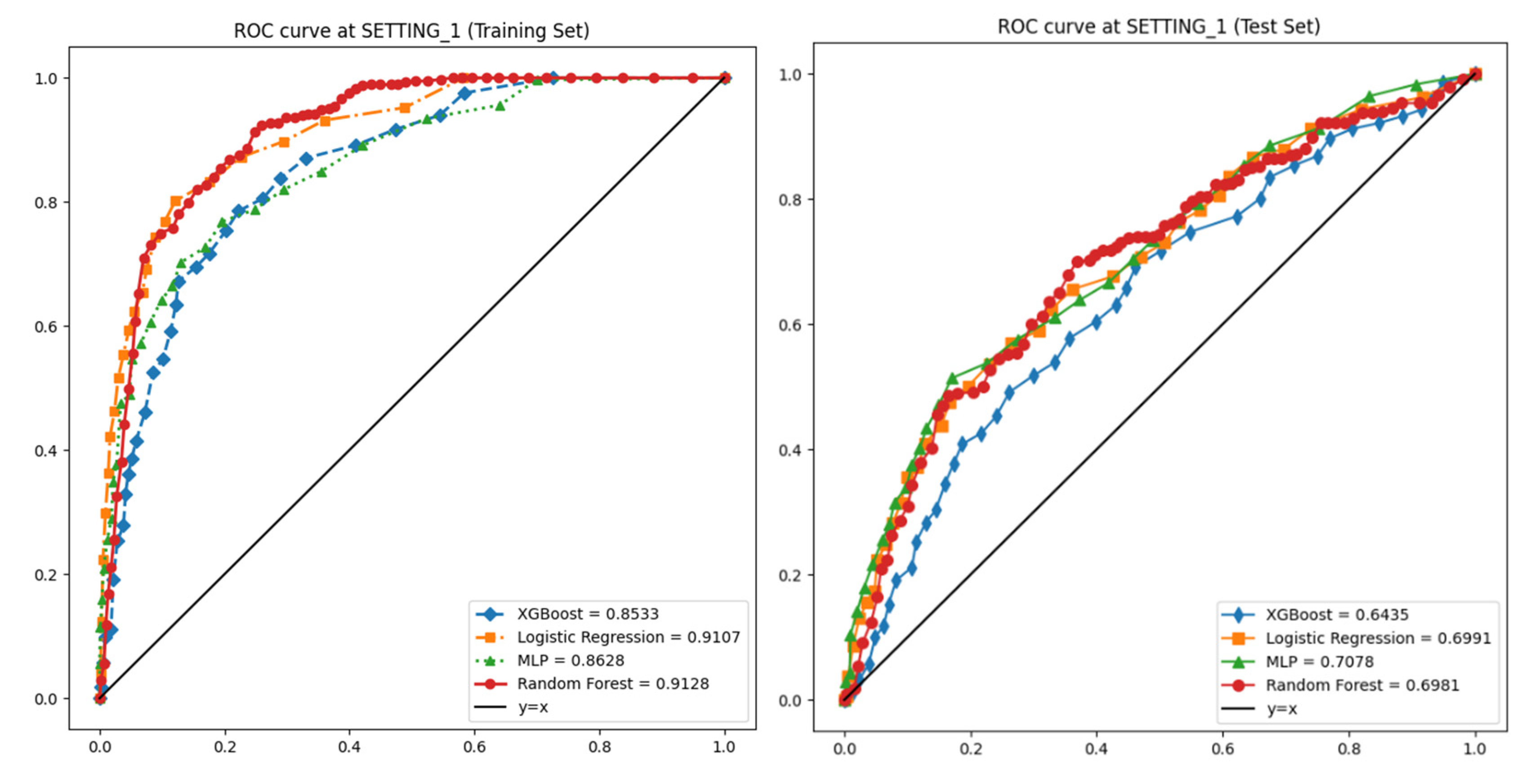
| SETTING_1 | Logistic Regression | 0.923 | 0.981 | 0.57 | 0.527 | 0.548 | 0.911 |
| Random Forest | 0.94 | 0.977 | 0.461 | 0.365 | 0.407 | 0.913 | |
| XGBoost | 0.935 | 0.969 | 0.397 | 0.296 | 0.339 | 0.853 | |
| Multi-Layer Perceptron | 0.891 | 0.991 | 0.488 | 0.553 | 0.519 | 0.863 | |
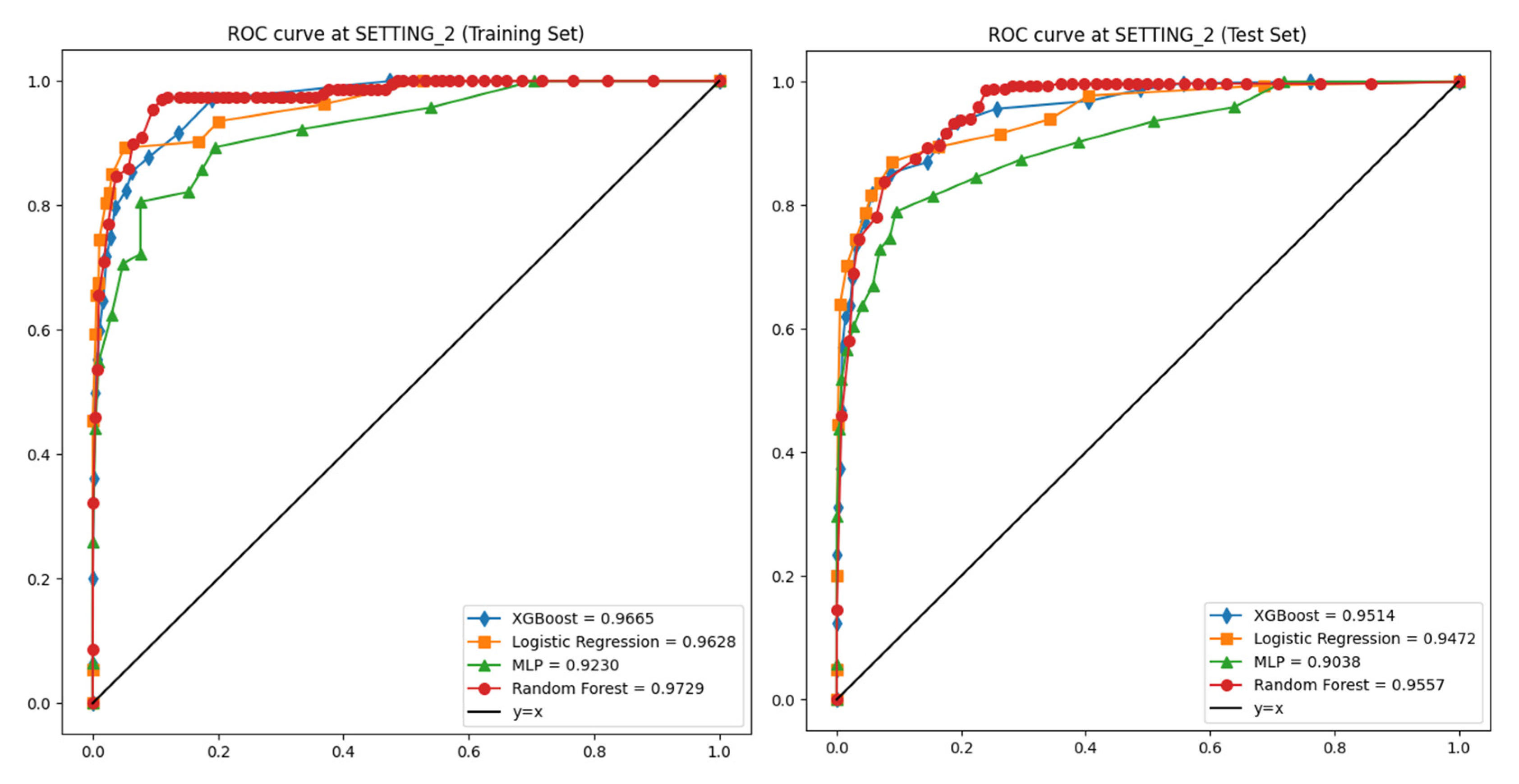
| SETTING_2 | Logistic Regression | 0.973 | 0.992 | 0.898 | 0.903 | 0.901 | 0.963 |
| Random Forest | 0.99 | 0.992 | 0.906 | 0.93 | 0.918 | 0.973 | |
| XGBoost | 0.993 | 0.996 | 0.917 | 0.888 | 0.902 | 0.967 | |
| Multi-Layer Perceptron | 0.972 | 0.975 | 0.921 | 0.892 | 0.706 | 0.923 |
| Datasets | Models | Accuracy | Specificity | Sensitivity | Precision | F1-Score | AUROC |
|---|---|---|---|---|---|---|---|
| SETTING_1 | Logistic Regression | 0.757 | 0.957 | 0.143 | 0.527 | 0.223 | 0.699 |
| Random Forest | 0.871 | 0.958 | 0.185 | 0.365 | 0.244 | 0.698 | |
| XGBoost | 0.856 | 0.949 | 0.154 | 0.296 | 0.192 | 0.643 | |
| Multi-Layer Perceptron | 0.76 | 0.961 | 0.151 | 0.553 | 0.232 | 0.708 | |
| SETTING_2 | Logistic Regression | 0.951 | 0.987 | 0.606 | 0.825 | 0.693 | 0.947 |
| Random Forest | 0.966 | 0.982 | 0.737 | 0.731 | 0.731 | 0.956 | |
| XGBoost | 0.962 | 0.987 | 0.661 | 0.796 | 0.72 | 0.951 | |
| Multi-Layer Perceptron | 0.961 | 0.978 | 0.748 | 0.716 | 0.723 | 0.904 |
| Fold | Training Accuracy | Validation Accuracy | Training AUC | Validation AUC |
|---|---|---|---|---|
| 1 | 0.973 | 0.968 | 0.981 | 0.962 |
| 2 | 0.978 | 0.959 | 0.984 | 0.953 |
| 3 | 0.975 | 0.964 | 0.983 | 0.959 |
| 4 | 0.982 | 0.971 | 0.987 | 0.964 |
| 5 | 0.979 | 0.967 | 0.985 | 0.961 |
| Mean | 0.977 ± 0.003 | 0.966 ± 0.004 | 0.984 ± 0.002 | 0.960 ± 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.; Soh, J.; Kim, Y.-H.; Lee, J.C.; Lee, J.-S.; Park, H.-Y.; Lee, J.-H.; Lee, J.; Shin, Y. Paraspinal Muscle Fat Infiltration as a Key Predictor of Symptomatic Intravertebral Vacuum Cleft: A Machine Learning Approach. J. Clin. Med. 2025, 14, 3109. https://doi.org/10.3390/jcm14093109
Ahn J, Soh J, Kim Y-H, Lee JC, Lee J-S, Park H-Y, Lee J-H, Lee J, Shin Y. Paraspinal Muscle Fat Infiltration as a Key Predictor of Symptomatic Intravertebral Vacuum Cleft: A Machine Learning Approach. Journal of Clinical Medicine. 2025; 14(9):3109. https://doi.org/10.3390/jcm14093109
Chicago/Turabian StyleAhn, Joonghyun, Jaewan Soh, Young-Hoon Kim, Jae Chul Lee, Jun-Seok Lee, Hyung-Youl Park, Jeong-Han Lee, June Lee, and Youjin Shin. 2025. "Paraspinal Muscle Fat Infiltration as a Key Predictor of Symptomatic Intravertebral Vacuum Cleft: A Machine Learning Approach" Journal of Clinical Medicine 14, no. 9: 3109. https://doi.org/10.3390/jcm14093109
APA StyleAhn, J., Soh, J., Kim, Y.-H., Lee, J. C., Lee, J.-S., Park, H.-Y., Lee, J.-H., Lee, J., & Shin, Y. (2025). Paraspinal Muscle Fat Infiltration as a Key Predictor of Symptomatic Intravertebral Vacuum Cleft: A Machine Learning Approach. Journal of Clinical Medicine, 14(9), 3109. https://doi.org/10.3390/jcm14093109






