Inclusion of Speckle Tracking Echocardiography Analysis in the Management of Intrauterine Growth Restrictions—Literature Review and Case Reports
Abstract
1. Introduction
2. Materials and Methods
3. Results
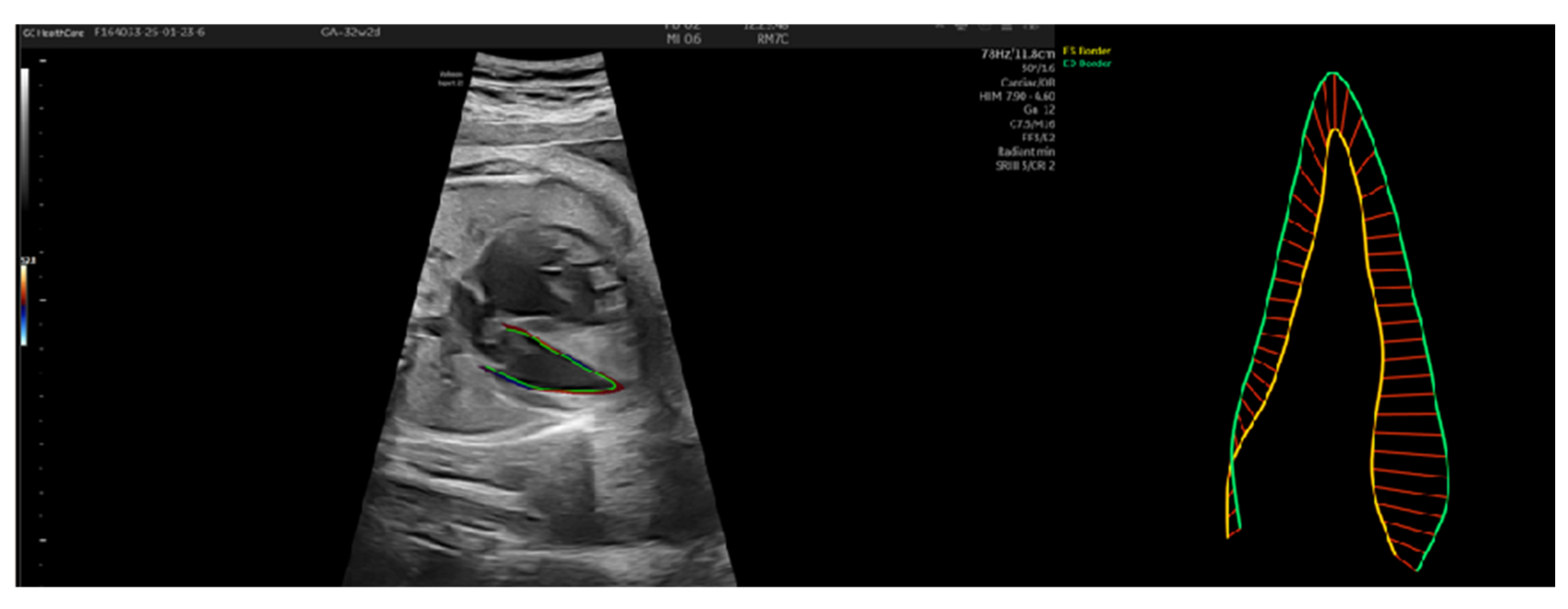
3.1. Patient Number 1
3.2. Patient Number 2
4. Discussion
4.1. The Fetal Heart in IUGR
4.2. 2D vs. 3D in Fetal Heart Speckle Tracking
4.3. Speckle Tracking in IUGR
4.4. Current Status and Perspectives in IUGR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IUGR | Intrauterine growth restriction |
| LV | Left ventricle |
| RV | Right ventricle |
| BPD | Biparietal diameter |
| HC | Head circumference |
| AC | Abdominal circumference |
| FL | Femur length |
| AFI | Amniotic fluid index |
| IVS | Interventricular sept |
| UA | Umbilical artery |
| IR | Resistance index |
| IP | Pulsatility index |
| MCA | Medial cerebral artery |
| DV | Ductus venosus |
| Ut A | Uterine artery |
| GLS | Global longitudinal strain |
| EF | Ejection fraction |
| LV EDA | LV end-diastolic area: Cross-sectional area of the LV at end-diastole. |
| LV ESA | LV end-systolic area: Cross-sectional area of the LV at end-systole. |
| LV ESL | LV end-systolic length: Length of LV cavity during systole. |
| ESD bas | End-systolic diameter (basal) |
| LV ESD | Left Ventricular End-Systolic Diameter |
| LV EDL | Left Ventricular End-Diastolic Length |
| LV EDD | Left Ventricular End-Diastolic Diameter |
| LV EDV | Left Ventricular End-Diastolic Volume |
| LV ESV | Left Ventricular End-Systolic Volume |
| MAPSE | Mitral annulus Plane Systolic Excursion |
| FAC | Fractional area change |
References
- Rao, S.V.; Venkateswarlu, B. Role of doppler sonography in clinically suspected iugr pregnencies: A descriptive study. Med. Sci. 2016, 6, 65–66. [Google Scholar]
- Rautray, P.; Swain, B. Ultrasonography with doppler assessment of the fetus with Intrauterine Growth Restriction (IUGR). Indian J. Community Fam. Med. 2016, 2, 29. [Google Scholar] [CrossRef]
- Das, P.K.; Das, S.; Chakrabarti, S. Role of doppler ultrasound in prediction of perinatal outcome in IUGR. J. Évid. Based Med. Healthc. 2017, 4, 4423–4426. [Google Scholar] [CrossRef] [PubMed]
- Botsis, D.; Vrachnis, N.; Christodoulakos, G. Doppler Assessment of the Intrauterine Growth-Restricted Fetus. Ann. N. Y. Acad. Sci. 2006, 1092, 297–303. [Google Scholar] [CrossRef]
- Pasupuleti, B.; Narra, R.; Jukuri, N.; Kamaraju, S.K.; Sushmitha, S. Colour doppler evaluation of pregnancy induced hyper-tension and iugr. Int. J. Biol. Med. Res. 2015, 6, 4793–4797. [Google Scholar]
- Hssan, H.G.E.M.A.; El Wahed, M.A.A.; Aziz, M.M.A. Interventricular Septal Thickness and Doppler Indices as Multiparametric Assessment of High-Risk Pregnancy and Their Relation to Fetal Outcome. Egypt. J. Hosp. Med. 2022, 87, 1876–1882. [Google Scholar] [CrossRef]
- Machlitt, A.; Wauer, R.R.; Chaoui, R. Longitudinal observation of deterioration of Doppler parameters, computerized cardiotocogram and clinical course in a fetus with growth restriction. J. Perinat. Med. 2001, 29, 71–76. [Google Scholar] [CrossRef]
- Morissens, M.; Rodriguez, J.C.; Azerad, M.; Besse-Hammer, T.; Efira, A. Added value of speckle tracking in the evaluation of cardiac function in patients with sickle cell disease. Br. J. Haematol. 2018, 185, 151–153. [Google Scholar] [CrossRef]
- Amer, S.M.; El Nemr, S.B.; El Amrosy, D.M.; Elrasol, O.A. Speckle tracking echocardiography of right and left ventricles in children of atrial septal defect and ventricular septal defect. Tanta Med. J. 2024, 52, 352–356. [Google Scholar] [CrossRef]
- Nichting, T.J.; de Vet, C.M.; van der Ven, M.; van der Woude, D.A.; van Sloun, R.J.; Oei, S.G.; van Oostrum, N.H.; van Laar, J.O.H. Angle Independency of Fetal Speckle-Tracking Echocardiography: A Commentary Letter. J. Am. Soc. Echocardiogr. 2022, 35, 783–785. [Google Scholar] [CrossRef]
- Day, T.G.; Charakida, M.; Simpson, J.M. Using speckle-tracking echocardiography to assess fetal myocardial deformation: Are we there yet? Ultrasound Obstet. Gynecol. 2019, 54, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Hooton, C.; Zimmerman, M.B.; Reinking, B.; Gupta, U. Fetal left and right ventricular strain parameters using speckle tracking in congenital heart diseases. Int. J. Cardiovasc. Imaging 2023, 40, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Kasliwal, R.R. How do I do it? Speckle-tracking echocardiography. Indian Heart J. 2013, 65, 117–123. [Google Scholar] [CrossRef]
- García-Otero, L.; Gómez, O.; Rodriguez-López, M.; Torres, X.; Soveral, I.; Sepúlveda-Martínez, Á.; Guirado, L.; Valenzuela-Alcaraz, B.; López, M.; Martínez, J.M.; et al. Nomograms of Fetal Cardiac Dimensions at 18–41 Weeks of Gestation. Fetal Diagn. Ther. 2020, 47, 387–398. [Google Scholar] [CrossRef]
- Akazawa, Y.; Yasukochi, S.; Takei, K.; Takigiku, K.; Inamura, N.; Takagi, K.; Pooh, R.K.; Yoshimatsu, J.; Kamei, Y.; Tamaru, S.; et al. Normal values and distribution of ventricular global longitudinal strain in 513 healthy fetuses measured by two-dimensional speckle-tracking echocardiography: A multi-institutional cohort study. Heart Vessel. 2024, 40, 414–425. [Google Scholar] [CrossRef]
- Girsen, A.; Ala-Kopsala, M.; Mäkikallio, K.; Vuolteenaho, O.; Räsänen, J. Cardiovascular hemodynamics and umbilical artery N-terminal peptide of proB-type natriuretic peptide in human fetuses with growth restriction. Ultrasound Obstet. Gynecol. 2007, 29, 296–303. [Google Scholar] [CrossRef]
- Änghagen, O.; Engvall, J.; Gottvall, T.; Nelson, N.; Nylander, E.; Bang, P. Developmental Differences in Left Ventricular Strain in IUGR vs. Control Children the First Three Months of Life. Pediatr. Cardiol. 2022, 43, 1286–1297. [Google Scholar] [CrossRef]
- Sapoval, J.; Singh, V.; Carter, R.E. Ultrasound Biophysical Profile (Archived). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Lalor, J.G.; Fawole, B.; Alfirevic, Z.; Devane, D. Biophysical profile for fetal assessment in high risk pregnancies. Cochrane Database Syst. Rev. 2008, 2012, CD000038. [Google Scholar] [CrossRef]
- Baschat, A.A.; Galan, H.L.; Lee, W.; DeVore, G.R.; Mari, G.; Hobbins, J.; Vintzileos, A.; Platt, L.D.; Manning, F.A. The role of the fetal biophysical profile in the management of fetal growth restriction. Am. J. Obstet. Gynecol. 2022, 226, 475–486. [Google Scholar] [CrossRef]
- Huntley, E.S.; Hernandez-Andrade, E.; Soto, E.; DeVore, G.; Sibai, B.M. Novel Speckle Tracking Analysis Showed Excellent Reproducibility for Size and Shape of the Fetal Heart and Good Reproducibility for Strain and Fractional Shortening. Fetal Diagn. Ther. 2021, 48, 541–550. [Google Scholar] [CrossRef]
- Mazzeo, S.; Scalia, M.; Zamagni, G.; Bussolaro, S.; Ricci, G.; Greco, P.; Stampalija, T. EP01.01: Speckle-tracking echocardiography: Fetal cardiac parameters in fetuses with ventricular chamber disproportion and neonatal outcome. Ultrasound Obstet. Gynecol. 2023, 62, 93. [Google Scholar] [CrossRef]
- Gores, G.; Raith, W.; Ravekes, W.; Koestenberger, M. Relevance of longitudinal systolic heart function in fetuses with intrauterine growth restriction. Ultrasound Obstet. Gynecol. 2014, 43, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Tsyv’Yan, P.B.; Bashmakova, N.V.; Artem’Eva, O.G.; Panacheva, N.M.; Orekhova, V.K.; Protsenko, Y.L. Contractile Cardiac Activity in Fetuses with Intrauterine Growth Retardation: Correlations between Regional Nonuniformity, Relaxation, and Afterload. Hum. Physiol. 2004, 30, 319–323. [Google Scholar] [CrossRef]
- Nichting, T.J.; de Vet, C.M.; van der Ven, M.; van der Woude, D.A.A.; Regis, M.; van Sloun, R.J.G.; Oei, S.G.; van Laar, J.O.E.H.; van Oostrum, N.H.M. The impact of angles of insonation on left and right ventricular global longitudinal strain estimation in fetal speckle tracking echocardiography. PLoS ONE 2023, 18, e0287003. [Google Scholar] [CrossRef]
- DeVore, G.R.; Klas, B.; Satou, G.; Sklansky, M. Evaluation of Fetal Left Ventricular Size and Function Using Speckle-Tracking and the Simpson Rule. J. Ultrasound Med. 2018, 38, 1209–1221. [Google Scholar] [CrossRef]
- DeVore, G.R.; Cuneo, B.; Klas, B.; Satou, G.; Sklansky, M. Comprehensive Evaluation of Fetal Cardiac Ventricular Widths and Ratios Using a 24-Segment Speckle Tracking Technique. J. Ultrasound Med. 2018, 38, 1039–1047. [Google Scholar] [CrossRef]
- Willruth, A.; Geipel, A.; Merz, W.; Gembruch, U. Speckle tracking—Ein neues Ultraschallverfahren zur Beurteilung der fetalen Myokardfunktion. Z. Geburtshilfe Neonatol. 2012, 216, 114–121. [Google Scholar] [CrossRef]
- Ta-Shma, A.; Perles, Z.; Gavri, S.; Golender, J.; Tarshansky, S.; Shlichter, C.; Bar Tov, H.; Rein, A.J. Analysis of Segmental and Global Function of the Fetal Heart Using Novel Automatic Functional Imaging. J. Am. Soc. Echocardiogr. 2008, 21, 146–150. [Google Scholar] [CrossRef]
- Im, D.; Kim, Y.; Park, J.; Ahn, K.; Won, H.; Kwon, J.; Nam, G.; Seong, W.; Lee, K.; Hong, S. EP01.31: Evaluation of fetal heart function using fetal speckle-tracking echocardiography: Korean multicentre prospective study. Ultrasound Obstet. Gynecol. 2023, 62, 100. [Google Scholar] [CrossRef]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef]
- Barker, P.C.; Houle, H.; Li, J.S.; Miller, S.; Herlong, J.R.; Camitta, M.G. Global Longitudinal Cardiac Strain and Strain Rate for Assessment of Fetal Cardiac Function: Novel Experience with Velocity Vector Imaging. Echocardiography 2009, 26, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Haeger, C.; Hammer, K.; Braun, J.; Oelmeier, K.; Köster, H.A.; Möllers, M.; Koch, R.; Steinhard, J.; Klockenbusch, W.; Schmitz, R. Importance of frame rate for the measurement of strain and synchrony in fetuses using speckle tracking echocardiography. J. Perinat. Med. 2021, 50, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Maffessanti, F.; Nesser, H.-J.; Weinert, L.; Steringer-Mascherbauer, R.; Niel, J.; Gorissen, W.; Sugeng, L.; Lang, R.M.; Mor-Avi, V. Quantitative Evaluation of Regional Left Ventricular Function Using Three-Dimensional Speckle Tracking Echocardiography in Patients With and Without Heart Disease. Am. J. Cardiol. 2009, 104, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- de Isla, L.P.; Vivas, D.; Zamorano, J. Three-dimensional speckle tracking. Curr. Cardiovasc. Imaging Rep. 2008, 1, 25–29. [Google Scholar] [CrossRef]
- Piveta, R.B.; Aguiar, M.O.D.; da Silva, L.L.M.; Vieira, M.L.C. My Approach to 3-Dimensional Left Ventricular Strain. ABC Imagem Cardiovasc. 2024, 37, e20240028. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, B.; Wu, T.; Li, Y.; Qi, X.; Tian, Y.; Chen, J.; Luo, H. Value of two-dimensional speckle-tracking echocardiography in evaluation of cardiac function in small fetuses. Quant. Imaging Med. Surg. 2023, 14, 8155–8166. [Google Scholar] [CrossRef]
- Sharma, B.; Verma, A.; Meena, C.; Gurjar, A.; Chakraborty, A.; Srivastav, A. Assessment of the Cardiac Function in Intrauterine Growth-Restricted Fetuses and Appropriate for Gestational Age Fetuses. J. Obstet. Gynecol. India 2019, 69, 313–316. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, C. OC17.01: Clinical value of 2D speckle tracking for the evaluation of ventricular global longitudinal strain in low-risk fetuses and SGA fetuses. Ultrasound Obstet. Gynecol. 2023, 62, 38–39. [Google Scholar] [CrossRef]
- Bhorat, I.E.; Bagratee, J.S.; Pillay, M.; Reddy, T. Determination of the myocardial performance index in deteriorating grades of intrauterine growth restriction and its link to adverse outcomes. Prenat. Diagn. 2014, 35, 266–273. [Google Scholar] [CrossRef]
- Salomon, L.; Alfirevic, Z.; Da Silva Costa, F.; Deter, R.; Figueras, F.; Ghi, T.; Glanc, P.; Khalil, A.; Lee, W.; Napolitano, R.; et al. ISUOG Practice Guidelines: Ultrasound assessment of fetal biometry and growth. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2019, 53, 715–723. [Google Scholar] [CrossRef]
- Melamed, N.; Baschat, A.; Yinon, Y.; Athanasiadis, A.; Mecacci, F.; Figueras, F.; Berghella, V.; Nazareth, A.; Tahlak, M.; McIntyre, H.D.; et al. FIGO (International Federation of Gynecology and Obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynecol. Obstet. 2021, 152 (Suppl. 1), 3–57. [Google Scholar] [CrossRef]
- Lees, C.; Stampalija, T.; Baschat, A.A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
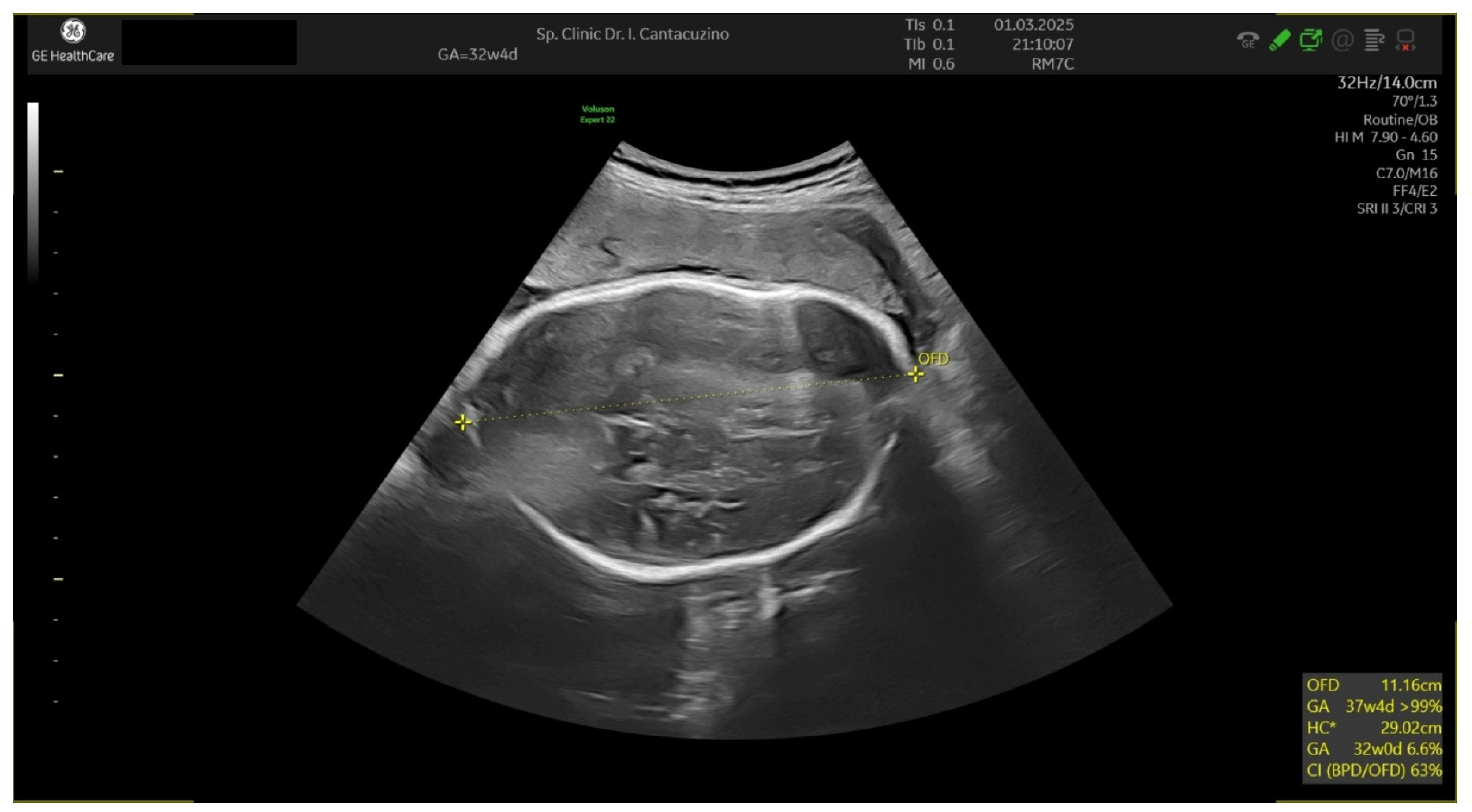
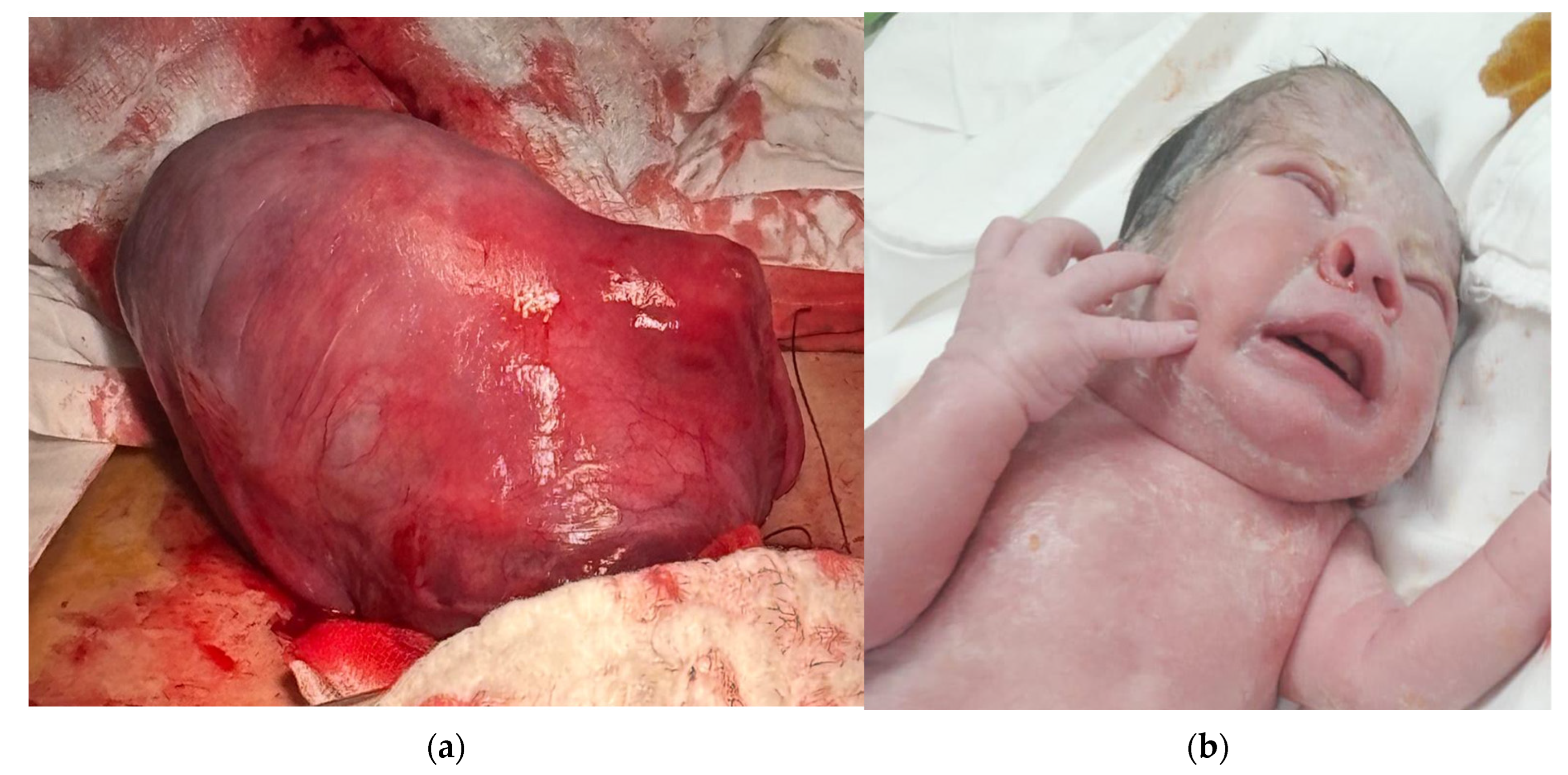
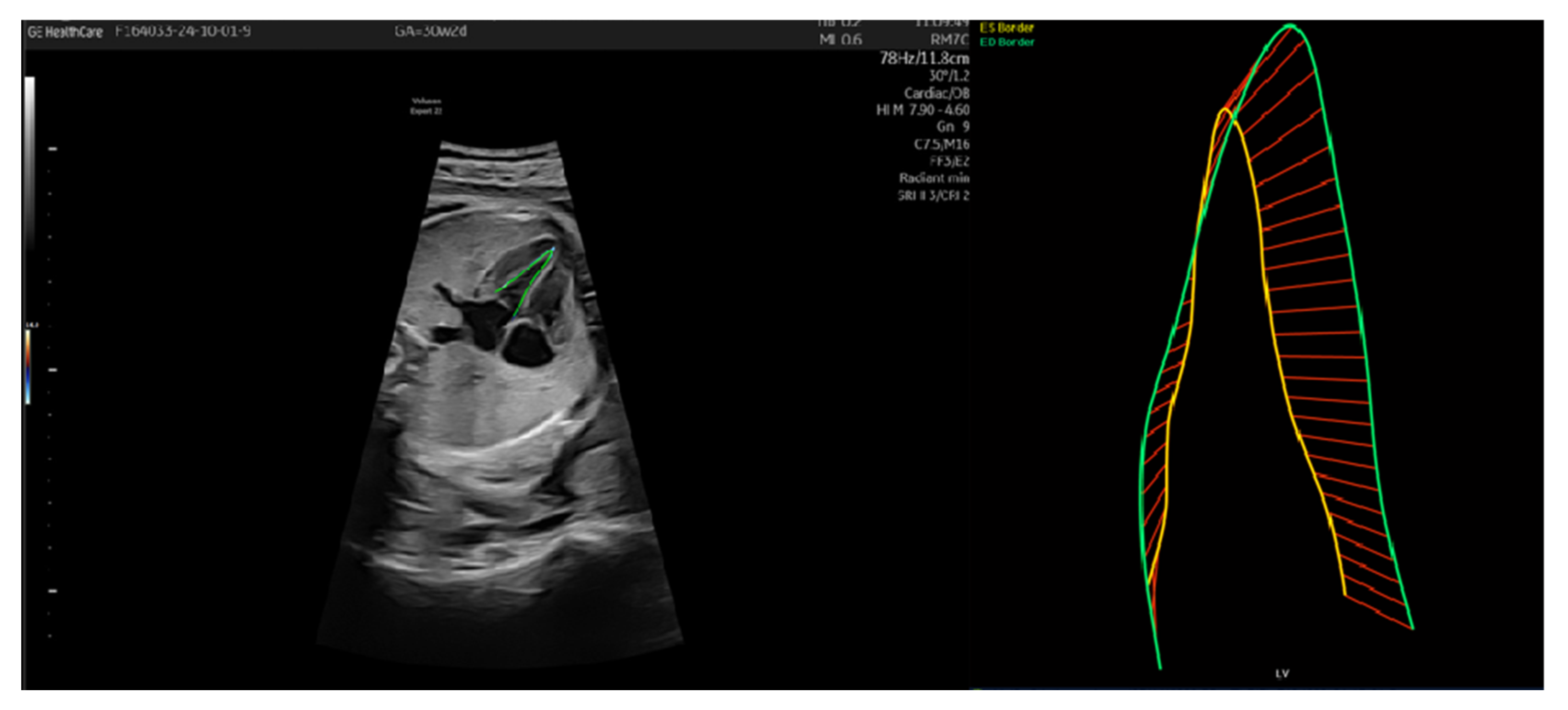
| Gestational Age (Weeks) | 26 Weeks | 28 Weeks | 30 Weeks |
|---|---|---|---|
| EGF | 737 | 994 | 1093 |
| Hadlock % | 4.1 | 2.2 | <1 |
| BPD | 6.18 | 6.66 | 6.9 |
| HC | 23.31 | 25.11 | 25.96 |
| AC | 19.85 | 23.83 | 25.94 |
| FL | 4.52 | 4.98 | 5.29 |
| AFI modified | no | no | no |
| Barcelona index | no | Barcelona stage I | Barcelona stage I |
| IVS thickness (mm) | 1.41 | 2.56 | 2.45 |
| Patient Number 1 | 26 Weeks | 28 Weeks | 30 Weeks | 36 Weeks |
|---|---|---|---|---|
| UA IP | 1.02 | 1.25 | 1.26 | 1.30 |
| UA IR | 0.66 | 0.72 | 0.72 | 0.74 |
| reverse flow UA | no | no | no | No |
| MCA IP | 1.72 | 1.65 | 1.91 | 1.64 |
| MCA IR | 0.81 | 0.77 | 0.82 | 0.75 |
| DV IP | 0.61 | no | no | No |
| right Ut A IP | 0.77 | 0.96 | 0.83 | 0.79 |
| left Ut A IP | 1.12 | 0.87 | 1.19 | 1.1 |
| LV endo GLS % | −21.48 | −28.27 | −23.71 | −11.5 |
| EF % | 62.53 | 70.93 | 72.76 | 69.1 |
| LV EDA cm2 | 0.9 | 1.25 | 1.32 | 1.39 |
| LV ESA cm2 | 0.49 | 0.53 | 0.58 | 0.71 |
| LV ESL cm | 1.35 | 1.57 | 1.64 | 1.9 |
| ESD bas cm | 0.53 | 0.7 | 0.69 | 0.7 |
| LV ESD mid cm | 0.39 | 0.42 | 0.35 | 0.3 |
| LV EDL cm | 1.72 | 2.16 | 2.13 | 2.1 |
| LV EDD bas cm | 0.73 | 0.71 | 0.89 | 1.0 |
| LV EDD mid cm | 0.58 | 0.67 | 0.73 | 0.7 |
| LV EDV ml | 0.44 | 0.68 | 0.74 | 0.8 |
| LV ESV ml | 0.16 | 0.2 | 0.2 | 0.27 |
| MAPSE l (lateral mitral annulus) cm | 0.37 | 0.53 | 0.39 | 0 |
| MAPSE s (septal mitral annulus) cm | 0.39 | 0.64 | 0.6 | 0.4 |
| FAC (fractional area change) % | 45.95 | 57.31 | 56.09 | 49.3 |
| basal inf/sept peak value % (longitudinal strain) | 232 | 218 | 77 | 208 |
| mid inf/sept peak value | 232 | 205 | 90 | 390 |
| apical septal peak value | 155 | 256 | 192 | 364 |
| apical lateral peak value | 144 | 307 | 154 | 364 |
| mid ant/lat peak value | 221 | 205 | 179 | 364 |
| basal ant/lats peak value | 232 | 218 | 102 | 364 |
| basal inf/sept longitudinal displacement (mm) | 232 | 205 | 269 | - |
| mid inf/sept longitudinal displacement | 122 | 141 | 269 | - |
| apical septal longitudinal displacement | 88 | 26 | 1 | - |
| apical lateral longitudinal displacement | 11 | 461 | 90 | - |
| mid ant/lat longitudinal displacement | 111 | 320 | 282 | - |
| basal ant/lat longitudinal displacement | 232 | 192 | 269 | - |
| Gestational Age (Weeks) | 27 Weeks | 29 Weeks | 32 Weeks 2 Days | 32 Weeks 4 Days |
|---|---|---|---|---|
| EGF | 967 | 1186 | 1548 | 1560 |
| Hadlock % | 15 | 12 | 4.1 | 3.1 |
| BPD | 5.96 | 6.43 | 6.76 | 7.08 |
| HC | 25.49 | 26.8 | 28.7 | 29.35 |
| AC | 22.2 | 23.83 | 25.94 | 25.95 |
| FL | 4.99 | 5.37 | 5.97 | 5.87 |
| AFI modified | no | no | no | no |
| Barcelona index | no | no | Barcelona stage I | Barcelona stage I |
| IVS thickness (mm) | 1.91 | 2.14 | 4.03 | 4 |
| Patient Number 2 | 27 Weeks | 29 Weeks | 32 Weeks 2 Days | 32 Weeks 4 Days |
|---|---|---|---|---|
| UA IP | 1.98 | 1.81 | 1.77 | 1.2 |
| UA IR | 0.72 | 0.86 | 0.82 | 0.83 |
| reverse flow UA | no | no | no | no |
| MCA IP | 1.94 | 2.22 | 1.64 | 1.98 |
| MCA IR | 0.86 | 0.88 | 0.8 | 0.83 |
| DV IP | no | no | no | 0.83 |
| right Ut A IP | no | no | no | 0.95 |
| left Ut A IP | no | no | no | 1.72 |
| LV endo GLS % | 14.01 | −19.01 | −13.27 | −10.14 |
| EF % | −32.93 | 64.4 | 63.8 | 49.57 |
| LV EDA cm2 | 0.91 | 1.4 | 1.95 | 1.32 |
| LV ESA cm2 | 1.13 | 0.76 | 1.08 | 0.83 |
| LV ESL cm | 1.59 | 1.31 | 2.27 | 1.85 |
| ESD bas cm | 0.97 | 0.84 | 0.88 | 0.87 |
| LV ESD mid cm | 0.73 | 0.61 | 0.53 | 0.48 |
| LV EDL cm | 1.42 | 1.54 | 2.59 | 2.03 |
| LV EDD bas cm | 0.93 | 1.1 | 0.95 | 0.81 |
| LV EDD mid cm | 0.73 | 1 | 0.9 | 0.78 |
| LV EDV mL | 0.51 | 1.08 | 1.37 | 0.75 |
| LV ESV mL | 0.68 | 0.38 | 0.5 | 0.38 |
| MAPSE l (lateral mitral annulus) cm | 0.13 | 0.25 | 0.41 | 0.21 |
| MAPSE s (septal mitral annulus) cm | 0.22 | 0.27 | 0.23 | 0.14 |
| FAC (fractional area change) % | −24.7 | 45.72 | 44.36 | 36.99 |
| basal inf/sept peak value % (longitudinal strain) | 381 | 160 | 218 | 231 |
| mid inf/sept peak value | 417 | 135 | 141 | 179 |
| apical septal peak value | 417 | 160 | 218 | 154 |
| apical lateral peak value | 417 | 147 | 218 | 141 |
| mid ant/lat peak value | 417 | 147 | 128 | 179 |
| basal ant/lats peak value | 417 | 197 | 192 | 269 |
| basal inf/sept longitudinal displacement (mm) | 12 | 197 | 103 | 179 |
| mid inf/sept longitudinal displacement | 37 | 209 | 269 | 320 |
| apical septal longitudinal displacement | 37 | 221 | 26 | 333 |
| apical lateral longitudinal displacement | 307 | 197 | 77 | 77 |
| mid ant/lat longitudinal displacement | 320 | 147 | 116 | 141 |
| basal ant/lat longitudinal displacement | 357 | 160 | 128 | 154 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neacșu, A.V.; Nenciu, A.-E.; Nastasia, Ș.; Crețu, O.-E.; Dîrlău, A.-A.; Ceaușu, I. Inclusion of Speckle Tracking Echocardiography Analysis in the Management of Intrauterine Growth Restrictions—Literature Review and Case Reports. J. Clin. Med. 2025, 14, 3099. https://doi.org/10.3390/jcm14093099
Neacșu AV, Nenciu A-E, Nastasia Ș, Crețu O-E, Dîrlău A-A, Ceaușu I. Inclusion of Speckle Tracking Echocardiography Analysis in the Management of Intrauterine Growth Restrictions—Literature Review and Case Reports. Journal of Clinical Medicine. 2025; 14(9):3099. https://doi.org/10.3390/jcm14093099
Chicago/Turabian StyleNeacșu, Adrian Valeriu, Adina-Elena Nenciu, Șerban Nastasia, Oana-Eliza Crețu, Alina-Alexandra Dîrlău, and Iuliana Ceaușu. 2025. "Inclusion of Speckle Tracking Echocardiography Analysis in the Management of Intrauterine Growth Restrictions—Literature Review and Case Reports" Journal of Clinical Medicine 14, no. 9: 3099. https://doi.org/10.3390/jcm14093099
APA StyleNeacșu, A. V., Nenciu, A.-E., Nastasia, Ș., Crețu, O.-E., Dîrlău, A.-A., & Ceaușu, I. (2025). Inclusion of Speckle Tracking Echocardiography Analysis in the Management of Intrauterine Growth Restrictions—Literature Review and Case Reports. Journal of Clinical Medicine, 14(9), 3099. https://doi.org/10.3390/jcm14093099








