Potential Utility of Combined Presepsin and LDH Tracking for Predicting Therapeutic Efficacy of Steroid Pulse Therapy in Acute Exacerbation of Interstitial Lung Diseases: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Collection
2.3. Evaluation of Respiratory Status Before and After Steroid Pulse Therapy
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics and Clinical Characteristics
3.2. Comparison of Presepsin Levels Before and After Steroid Pulse Therapy
3.3. Clinical Course of Presepsin, CRP, LDH, and SP-D Levels Before and After Steroid Pulse Therapy
3.4. Creation of Post-/Pre-Presepsin, CRP, LDH, and SP-D Ratios
3.5. Comparison of Post-/Pre-Presepsin, CRP, LDH, and SP-D Ratios in Survival and Non-Survival Groups
3.6. Creation and Performance of Post-/Pre-Presepsin–LDH Index as a Prognostic Marker for Non-Survival
3.7. Clinical Course After Steroid Pulse Therapy Based on the Post-/Pre-Presepsin–LDH Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leuschner, G.; Behr, J. Acute exacerbation in interstitial lung disease. Front. Med. 2017, 4, 176. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, C.D.; Batra, K.; Torrealba, J.R.; Terada, L.S. Characteristics and evaluation of acute exacerbations in chronic interstitial lung diseases. Respir. Med. 2021, 183, 106400. [Google Scholar] [CrossRef]
- Kishaba, T. Acute exacerbation of idiopathic pulmonary fibrosis. Medicina 2019, 55, 70. [Google Scholar] [CrossRef]
- Collard, H.R.; Ryerson, C.J.; Corte, T.J.; Jenkins, G.; Kondoh, Y.; Lederer, D.J.; Lee, J.S.; Maher, T.M.; Wells, A.U.; Antoniou, K.M.; et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group report. Am. J. Respir. Crit. Care Med. 2016, 194, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kondoh, Y.; Brown, K.K.; Johkoh, T.; Kataoka, K.; Fukuoka, J.; Kimura, T.; Matsuda, T.; Yokoyama, T.; Fukihara, J.; et al. Acute exacerbations of fibrotic interstitial lung diseases. Respirology 2020, 25, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Raghu, G. Treatment of acute exacerbations of interstitial lung diseases with corticosteroids: Evidence? Respirology 2024, 29, 747–750. [Google Scholar] [CrossRef]
- Bando, M.; Homma, S.; Date, H.; Kishi, K.; Yamauchi, H.; Sakamoto, S.; Miyamoto, A.; Goto, Y.; Nakayama, T.; Azuma, A.; et al. Ministry of Health, Labour and Welfare; the Study Group on Diffuse Pulmonary Disorders; Scientific Research/Research on Intractable Diseases; and Japanese Respiratory Society. Japanese guidelines for the treatment of idiopathic pulmonary fibrosis 2023:Revised edition. Respir. Investig. 2024, 62, 402–418. [Google Scholar]
- Farrand, E.; Vittinghoff, E.; Ley, B.; Butte, A.J.; Collard, H.R. Corticosteroid use is not associated with improved outcomes in acute exacerbation of IPF. Respirology 2020, 25, 629–635, Erratum in Respirology 2022, 27, 905. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Marchioni, A.; Tonelli, R.; Ball, L.; Fantini, R.; Castaniere, I.; Cerri, S.; Luppi, F.; Malerba, M.; Pelosi, P.; Clini, E. Acute exacerbation of idiopathic pulmonary fibrosis: Lessons learned from acute respiratory distress syndrome? Crit. Care 2018, 22, 80. [Google Scholar] [CrossRef]
- Chen, T.; He, X.; Zhou, N.; Song, J.; Weng, D.; Li, H. C-type lectin Mincle initiates IL-17-mediated inflammation in acute exacerbations of idiopathic pulmonary fibrosis. Biomed. Pharmacother. 2023, 159, 114253. [Google Scholar]
- Desgeorges, T.; Caratti, G.; Mounier, R.; Tuckermann, J.; Chazaud, B. Glucocorticoids shape macrophage phenotype for tissue repair. Front. Immunol. 2019, 10, 1591. [Google Scholar] [CrossRef] [PubMed]
- Ehrchen, J.M.; Roth, J.; Barczyk-Kahlert, K. More than suppression: Glucocorticoid action on monocytes and macrophages. Front. Immunol. 2019, 10, 2028. [Google Scholar] [CrossRef] [PubMed]
- Tu, G.W.; Shi, Y.; Zheng, Y.J.; Ju, M.J.; He, H.Y.; Ma, G.G.; Hao, G.W.; Luo, Z. Glucocorticoid attenuates acute lung injury through induction of type 2 macrophage. J. Transl. Med. 2017, 15, 181. [Google Scholar] [CrossRef]
- Piccioni, A.; Santoro, M.C.; de Cunzo, T.; Tullo, G.; Cicchinelli, S.; Saviano, A.; Valletta, F.; Pascale, M.M.; Candelli, M.; Covino, M.; et al. Presepsin as early marker of sepsis in emergency department: A narrative review. Medicina 2021, 57, 770. [Google Scholar] [CrossRef]
- Landmann, R.; Müller, B.; Zimmerli, W. CD14, new aspects of ligand and signal diversity. Microbes Infect. 2000, 2, 295–304. [Google Scholar] [CrossRef]
- Ikegame, A.; Kondo, A.; Kitaguchi, K.; Sasa, K.; Miyoshi, M. Presepsin production in monocyte/macrophage-mediated phagocytosis of neutrophil extracellular traps. Sci. Rep. 2022, 12, 5978. [Google Scholar] [CrossRef]
- Tanimura, S.; Fujieda, Y.; Kono, M.; Shibata, Y.; Hisada, R.; Sugawara, E.; Nakamura, H.; Ohmura, K.; Shimamura, S.; Mitani, A.; et al. Clinical significance of plasma presepsin levels in patients with systemic lupus erythematosus. Mod. Rheumatol. 2018, 28, 865–871. [Google Scholar] [CrossRef]
- Pruitt, W.C.; Jacobs, M. Breathing lessons: Basics of oxygen therapy. Nursing 2003, 33, 43–45. [Google Scholar] [CrossRef]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef]
- Muller, M.P.; Tomlinson, G.; Marrie, T.J.; Tang, P.; McGeer, A.; Low, D.E.; Detsky, A.S.; Gold, W.L. Can routine laboratory tests discriminate between severe acute respiratory syndrome and other causes of community-acquired pneumonia? Clin. Infect. Dis. 2005, 40, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kumar, P.; Badagabettu, S.N.; Lewis, M.G.; Adiga, M.; Padur, A.A. Determination of Spearman correlation coefficient (r) to evaluate the linear association of dermal collagen and elastic fibers in the perspectives of skin injury. Dermatol. Res. Pract. 2018, 2018, 4512840. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Shozushima, T.; Takahashi, G.; Matsumoto, N.; Kojika, M.; Okamura, Y.; Endo, S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J. Infect. Chemother. 2011, 17, 764–769. [Google Scholar] [CrossRef]
- Ugajin, M.; Matsuura, Y.; Matsuura, K.; Matsuura, H. Impact of initial plasma presepsin level for clinical outcome in hospitalized patients with pneumonia. J. Thorac. Dis. 2019, 11, 1387–1396. [Google Scholar] [CrossRef]
- Roca, O.; Caralt, B.; Messika, J.; Samper, M.; Sztrymf, B.; Hernández, G.; García-de-Acilu, M.; Frat, J.P.; Masclans, J.R.; Ricard, J.D. An Index Combining Respiratory Rate and Oxygenation to Predict Outcome of Nasal High-Flow Therapy. Am. J. Respir. Crit. Care Med. 2019, 199, 1368–1376. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Wu, C.C.; Lan, H.M.; Han, S.T.; Chaou, C.H.; Yeh, C.F.; Liu, S.H.; Li, C.H.; Blaney, G.N.; Liu, Z.Y.; Chen, K.F. Comparison of diagnostic accuracy in sepsis between presepsin, procalcitonin, and C-reactive protein: A systematic review and meta-analysis. Ann. Intensive Care 2017, 7, 91. [Google Scholar] [CrossRef]
- Kondo, A.; Morinishi, T.; Yamaguchi, Y.; Ikegame, A. Identification of organs of origin of macrophages that produce presepsin via neutrophil extracellular trap phagocytosis. Sci. Rep. 2024, 14, 16386. [Google Scholar] [CrossRef]
- Ba, C.; Wang, H.; Jiang, C.; Shi, X.; Jin, J.; Fang, Q. Clinical manifestations and prognostic factors analysis of patients hospitalised with acute exacerbation of idiopathic pulmonary fibrosis and other interstitial lung diseases. BMJ Open Respir. Res. 2024, 11, e001997. [Google Scholar] [CrossRef]
- Kishaba, T. Evaluation and management of Idiopathic Pulmonary Fibrosis. Respir. Investig. 2019, 57, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Kishaba, T.; Nei, Y.; Momose, M.; Nagano, H.; Yamashiro, S. Clinical Characteristics Based on the New Criteria of Acute Exacerbation in Patients with Idiopathic Pulmonary Fibrosis. Eurasian J. Med. 2018, 50, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, M.; Inoue, Y.; Nishioka, M.; Ikegame, A.; Nakao, T.; Kishi, S.; Doi, T.; Nagai, K. Clinical evaluation of presepsin considering renal function. PLoS ONE 2019, 14, e0215791. [Google Scholar] [CrossRef] [PubMed]

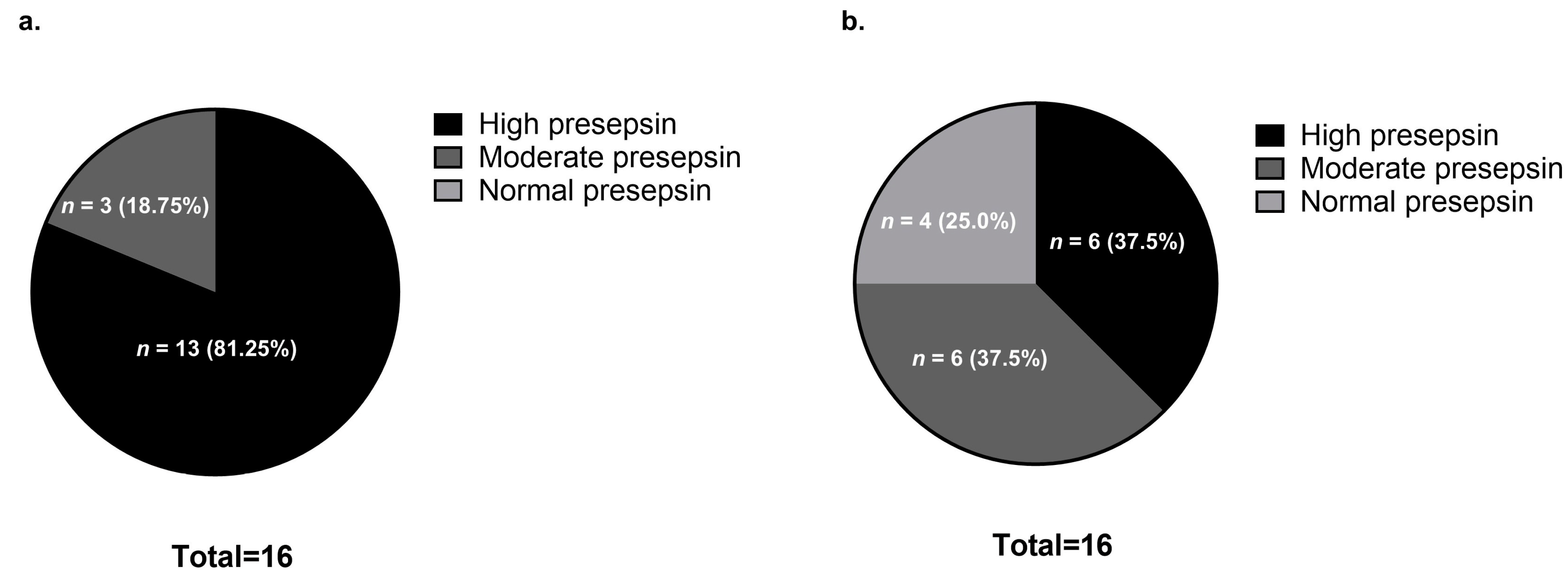
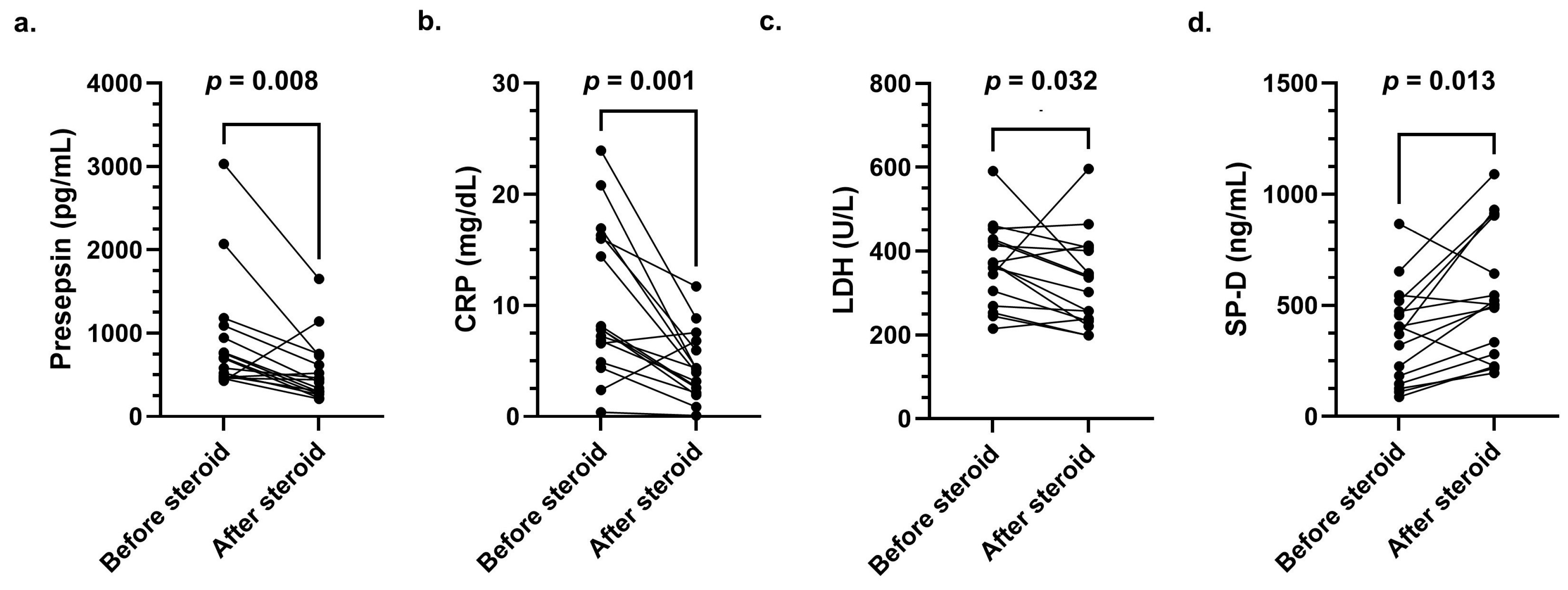
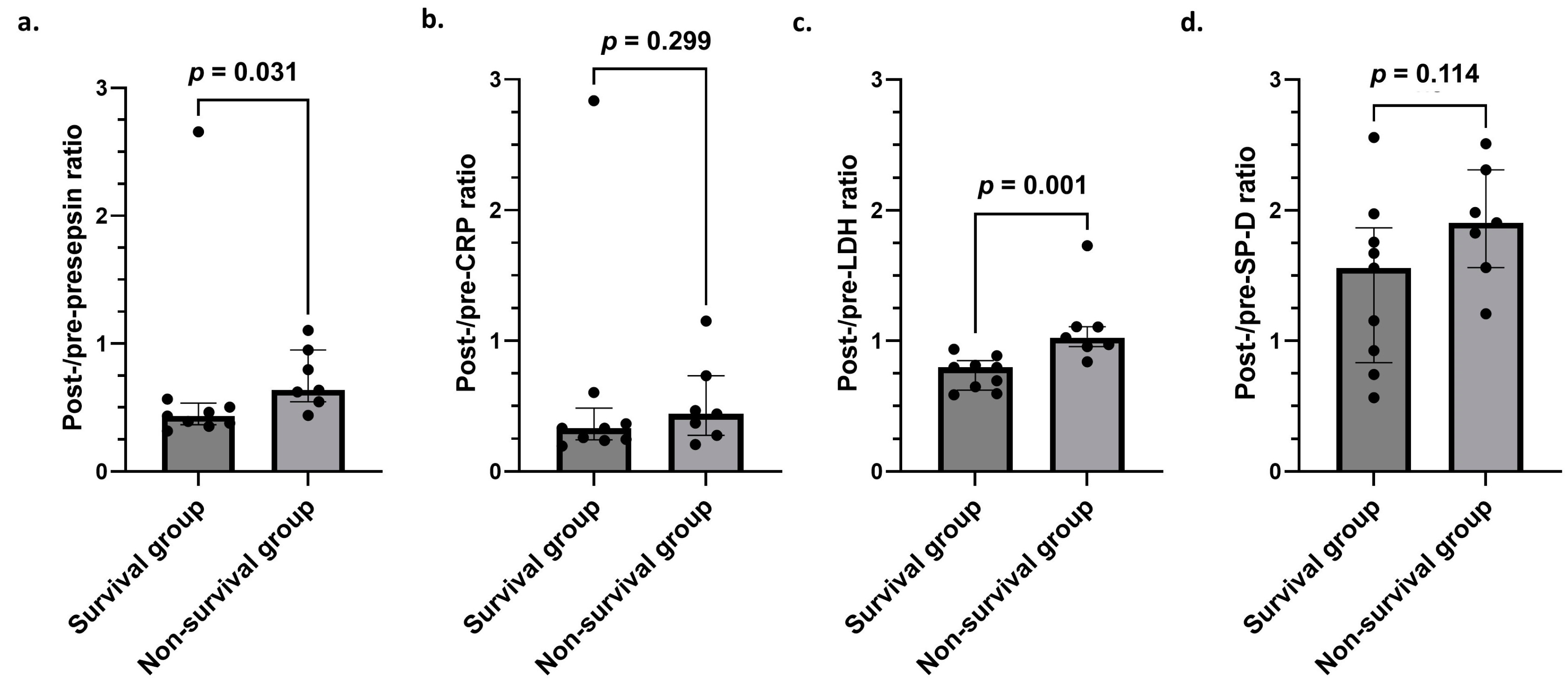
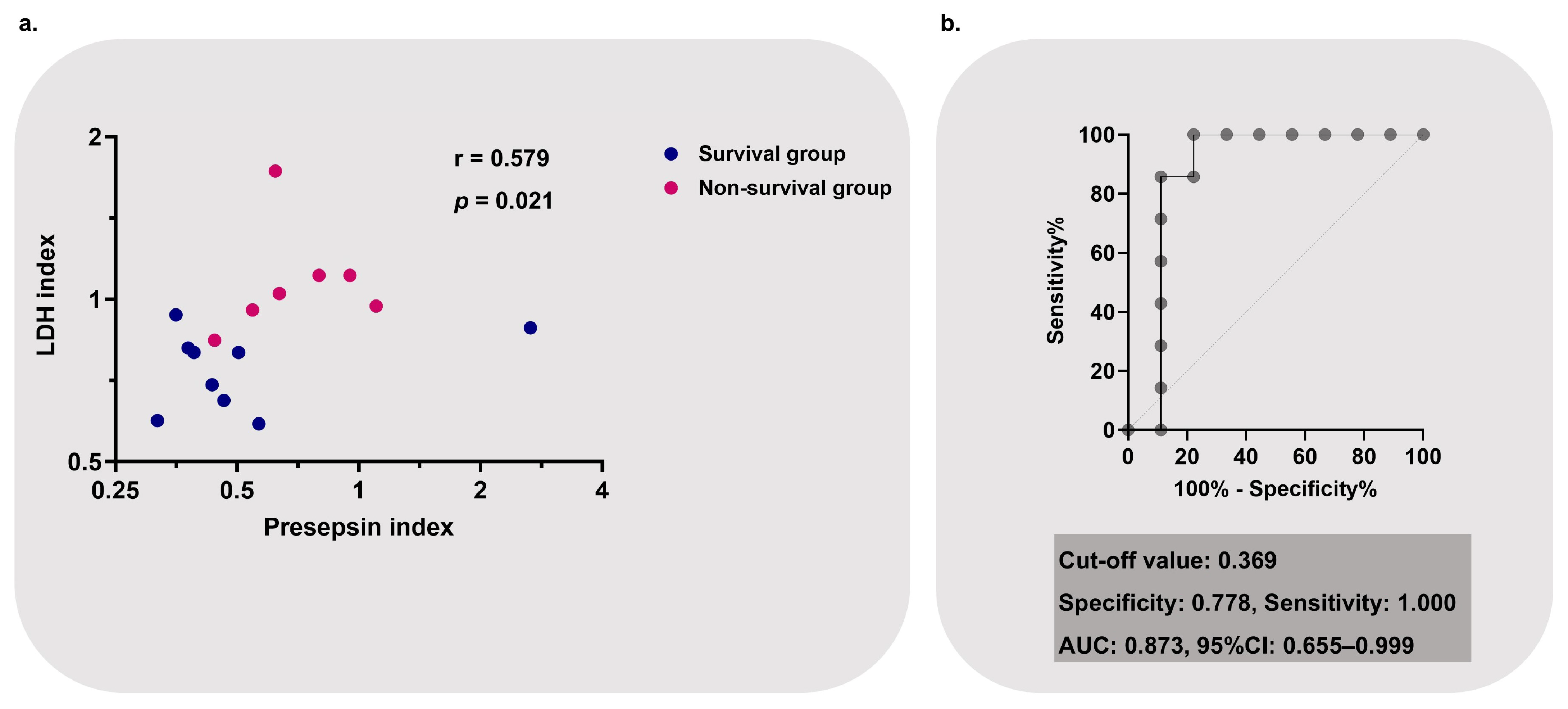
| Variable | Survival Group (n = 9) | Non-Survival Group (n = 7) | p-Value |
|---|---|---|---|
| Age (years) | 71 (66–78) | 72 (71–80) | 0.458 |
| Male sex (%) | 6 (66.7%) | 7 (100.0%) | 0.212 |
| Smoking history (%) | 6 (66.7%) | 4 (57.1%) | >0.999 |
| Diabetes mellitus (%) | 3 (33.3%) | 3 (42.9%) | >0.999 |
| Hypertension (%) | 7 (80.0%) | 4 (57.1%) | 0.596 |
| Malignancy (%) | 1 (11.1%) | 2 (28.6%) | 0.550 |
| AE-IPF (%) | 5 (55.6%) | 4 (57.1%) | >0.999 |
| AE-NSIP (%) | 1 (10.0%) | 0 (0.0%) | >0.999 |
| AE-CHP (%) | 0 (0.0%) | 0 (0.0%) | >0.999 |
| RP-CTD-ILD (%) | 3 (33.3%) | 3 (42.9%) | >0.999 |
| NT-proBNP | 504 (273–1567) | 2682 (428–2975) | 0.606 |
| Baseline treatment | |||
| Prednisolone | 1 (11.1%) | 3 (42.9%) | 0.262 |
| Immunosuppressant | 1 (11.1%) | 1 (14.3%) | >0.999 |
| Nintedanib | 2 (22.2%) | 3 (42.9%) | 0.596 |
| Pirfenidone | 0 (0.0%) | 1 (14.3%) | 0.438 |
| Variable | Post-/Pre-Presepsin–LDH Index < 0.369 (n = 8) | Post-/Pre-Presepsin–LDH Index ≥ 0.369 (n = 8) | p-Value |
|---|---|---|---|
| After steroid pulse therapy (day 4) | |||
| Laboratory data | |||
| CRP (mg/dL) | 2.64 (1.67–6.17) | 4.34 (3.77–5.17) | 0.270 |
| SP-D (ng/mL) | 511 (228–638) | 497 (321–708) | >0.999 |
| D-dimer (μg/mL) | 2.93 (1.65–7.58) | 3.16 (2.38–6.96) | 0.798 |
| qSOFA (score) | |||
| 0 | 5 (62.5%) | 1 (12.5%) | 0.119 |
| 1 | 3 (37.5%) | 7 (87.5%) | 0.119 |
| 2 | 0 (0.0%) | 0 (0.0%) | >0.999 |
| 3 | 0 (0.0%) | 0 (0.0%) | >0.999 |
| Respiratory condition | |||
| SpO2/FIO2 | 339 (283–394) | 204 (116–303) | 0.031 |
| Respiratory rate (breaths/minute) | 18 (18–20) | 24 (19–26) | 0.309 |
| ROX index | 18.85 (14.93–20.24) | 8.19 (4.67–16.44) | 0.040 |
| Oxygen device | |||
| Conventional oxygen therapy (%) | 8 (100.0%) | 3 (37.5%) | 0.026 |
| High-flow oxygen therapy (%) | 0 (0.0%) | 5 (62.5%) | 0.026 |
| Prognosis | |||
| Additional treatment requirement | |||
| Second steroid pulse therapy | 1 (12.5%) | 4 (50.0%) | 0.282 |
| Tacrolimus | 2 (25.0%) | 3 (37.5%) | >0.999 |
| Mortality | |||
| 30-day mortality | 0 (0.0%) | 4 (50.0%) | 0.077 |
| 60-day mortality | 0 (0.0%) | 5 (62.5%) | 0.026 |
| 90-day mortality | 1 (12.5%) | 6 (75.0%) | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeshita, Y.; To, Y.; To, M.; Furusho, N.; Kurosawa, Y.; Kinouchi, T.; Abe, M.; Terada, J.; Tada, Y.; Sakao, S. Potential Utility of Combined Presepsin and LDH Tracking for Predicting Therapeutic Efficacy of Steroid Pulse Therapy in Acute Exacerbation of Interstitial Lung Diseases: A Pilot Study. J. Clin. Med. 2025, 14, 3068. https://doi.org/10.3390/jcm14093068
Takeshita Y, To Y, To M, Furusho N, Kurosawa Y, Kinouchi T, Abe M, Terada J, Tada Y, Sakao S. Potential Utility of Combined Presepsin and LDH Tracking for Predicting Therapeutic Efficacy of Steroid Pulse Therapy in Acute Exacerbation of Interstitial Lung Diseases: A Pilot Study. Journal of Clinical Medicine. 2025; 14(9):3068. https://doi.org/10.3390/jcm14093068
Chicago/Turabian StyleTakeshita, Yuichiro, Yasuo To, Masako To, Naho Furusho, Yusuke Kurosawa, Toru Kinouchi, Mitsuhiro Abe, Jiro Terada, Yuji Tada, and Seiichiro Sakao. 2025. "Potential Utility of Combined Presepsin and LDH Tracking for Predicting Therapeutic Efficacy of Steroid Pulse Therapy in Acute Exacerbation of Interstitial Lung Diseases: A Pilot Study" Journal of Clinical Medicine 14, no. 9: 3068. https://doi.org/10.3390/jcm14093068
APA StyleTakeshita, Y., To, Y., To, M., Furusho, N., Kurosawa, Y., Kinouchi, T., Abe, M., Terada, J., Tada, Y., & Sakao, S. (2025). Potential Utility of Combined Presepsin and LDH Tracking for Predicting Therapeutic Efficacy of Steroid Pulse Therapy in Acute Exacerbation of Interstitial Lung Diseases: A Pilot Study. Journal of Clinical Medicine, 14(9), 3068. https://doi.org/10.3390/jcm14093068






