Living Donor Liver Transplantation Versus Deceased Donor Liver Transplantation for Hepatocellular Carcinoma and HCV Patients: An Initial Umbrella Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Umbrella Review Methods
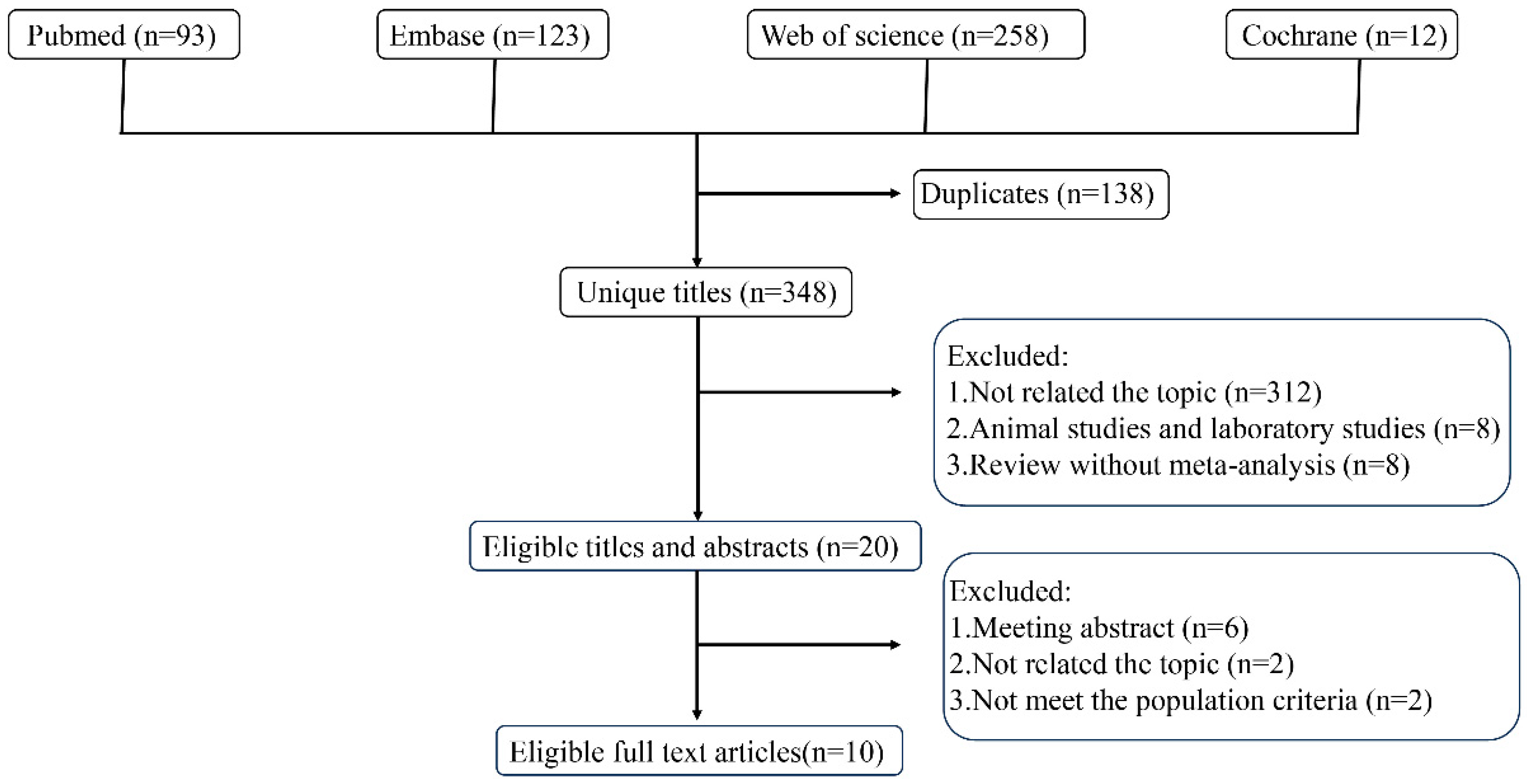
2.2. Literature Search
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Quality Assessment of Methods and Evidence
2.6. Data Analysis
3. Results
3.1. Characteristics of Included Meta-Analyses
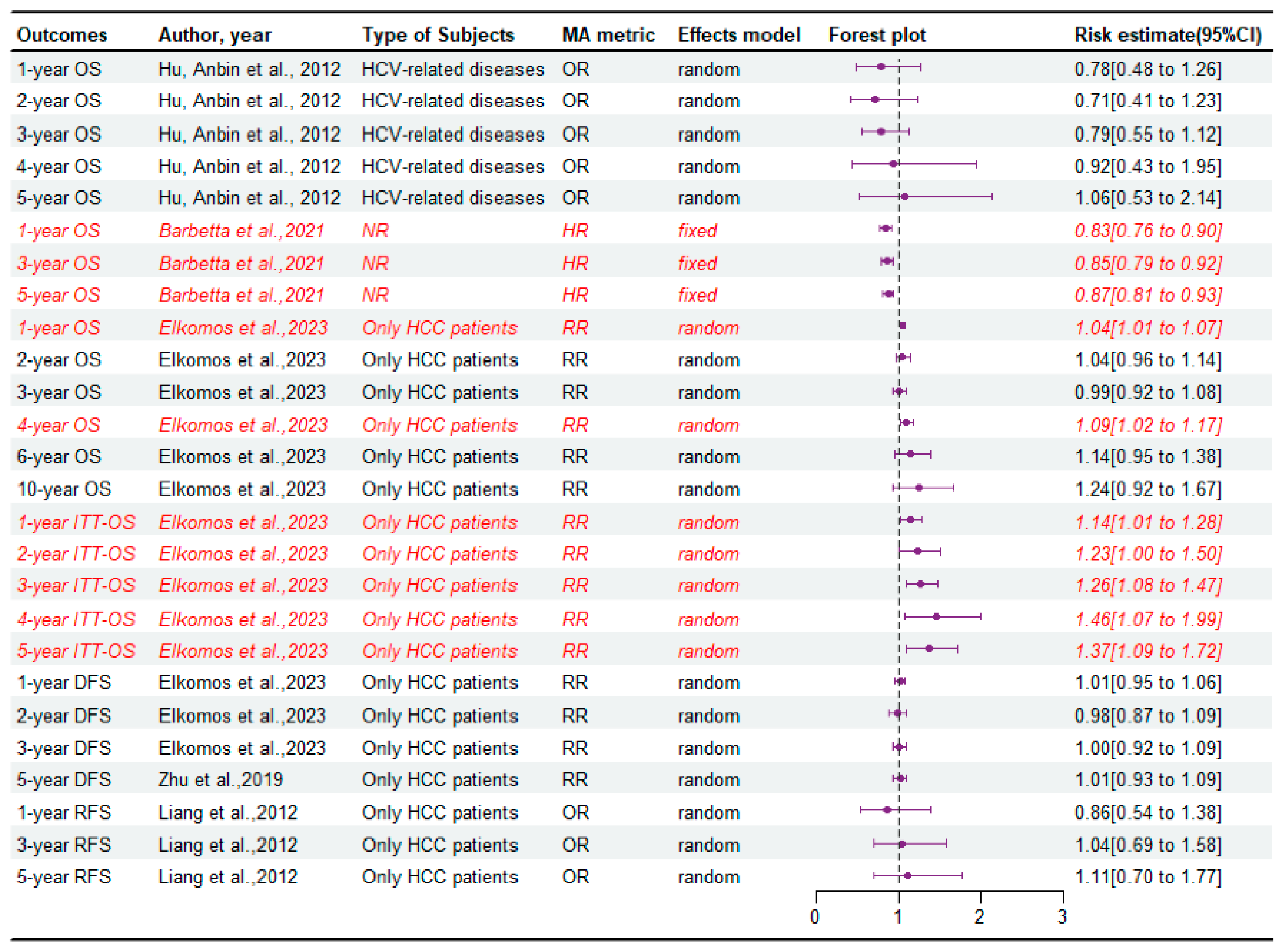
3.2. The Survival of Recipient Patients
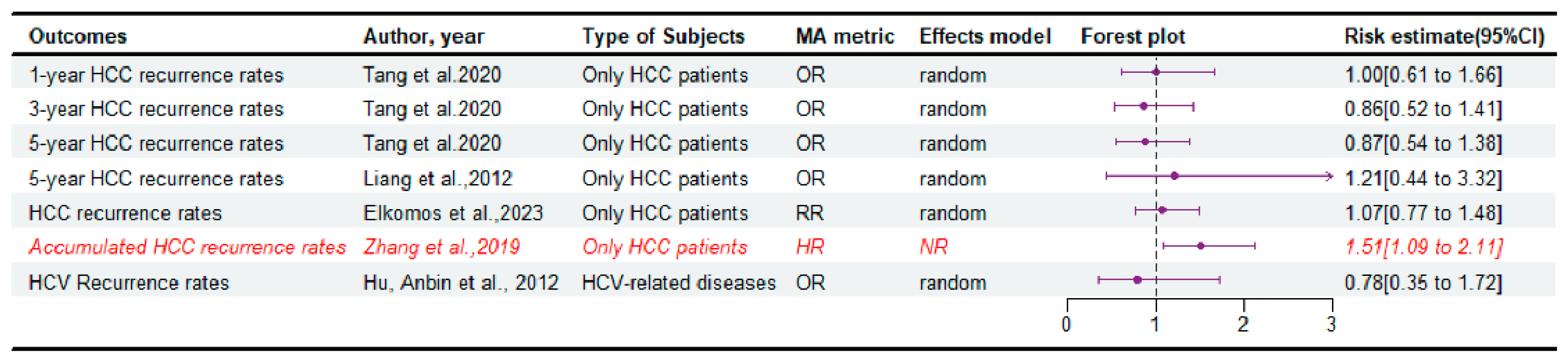
3.3. The Disease Relapse
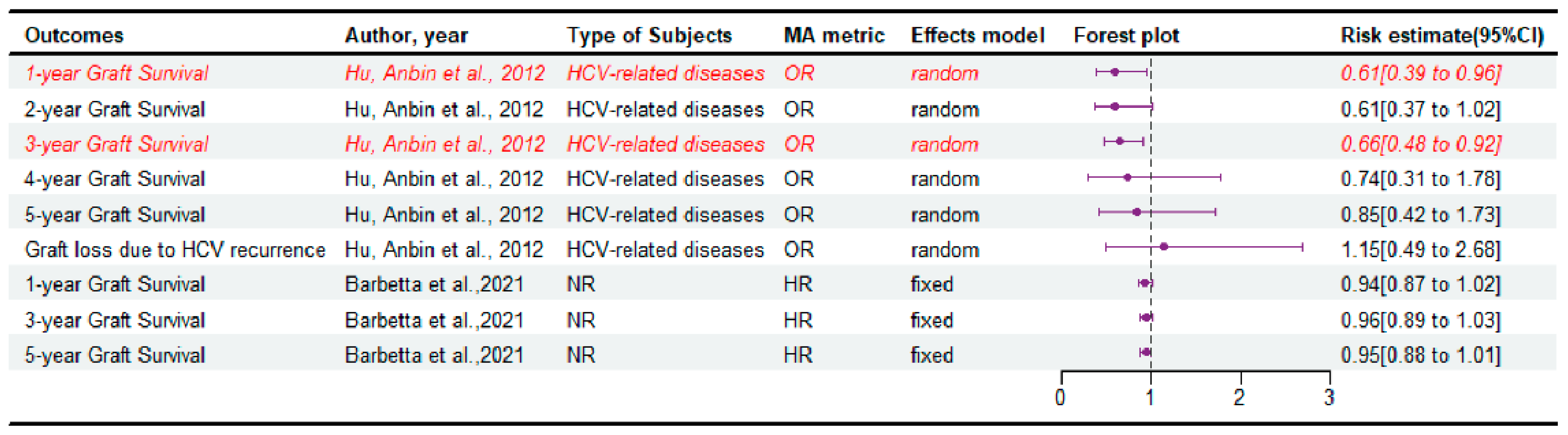
3.4. The Survival of Graft
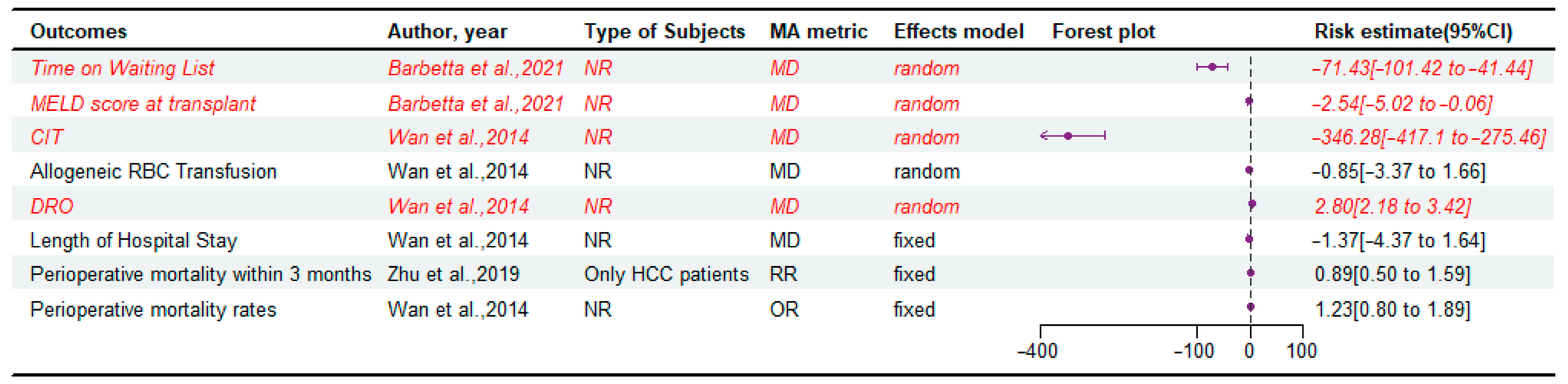
3.5. Perioperative Outcomes
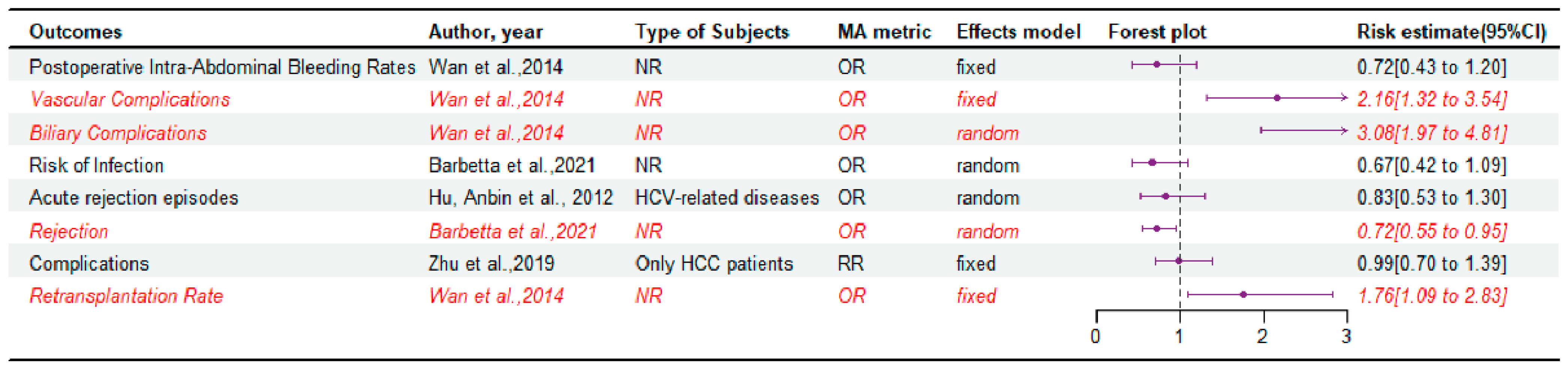
3.6. Postoperative Complications and Retransplantation Rate
3.7. Heterogeneity
3.8. Publication Bias
3.9. Outcome of Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LDLT | Living donor liver transplantation |
| DDLT | Deceased donor liver transplantation |
| LT | Liver transplantation |
| OPTN | Organ Procurement and Transplantation Network |
| SRTR | Scientific Registry of Transplant Recipients |
| ICU | Intensive Care Unit |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| HCC | Hepatocellular carcinoma |
| DRO | Duration of the recipient operation |
| CIT | Cold ischemia time |
| RBC | Red blood cell |
| MELD | Model for end-stage liver disease |
| PELD | Pediatric End-Stage Liver Disease |
| HCV | Hepatitis C virus |
References
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Starzl, T.E.; Marchioro, T.L.; Vonkaulla, K.N.; Hermann, G.; Brittain, R.S.; Waddell, W.R. Homotransplantation of the Liver in Humans. Surg. Gynecol. Obstet. 1963, 117, 659–676. [Google Scholar] [PubMed]
- Kwong, A.J.; Ebel, N.H.; Kim, W.R.; Lake, J.R.; Smith, J.M.; Schladt, D.P.; Schnellinger, E.M.; Handarova, D.; Weiss, S.; Cafarella, M.; et al. OPTN/SRTR 2021 Annual Data Report: Liver. Am. J. Transplant. 2023, 23, S178–S263. [Google Scholar] [CrossRef] [PubMed]
- Hibi, T.; Wei Chieh, A.K.; Chi-Yan Chan, A.; Bhangui, P. Current status of liver transplantation in Asia. Int. J. Surg. 2020, 82s, 4–8. [Google Scholar] [CrossRef]
- Strong, R.W.; Lynch, S.V.; Ong, T.H.; Matsunami, H.; Koido, Y.; Balderson, G.A. Successful liver transplantation from a living donor to her son. N. Engl. J. Med. 1990, 322, 1505–1507. [Google Scholar] [CrossRef]
- Barbetta, A.; Aljehani, M.; Kim, M.; Tien, C.; Ahearn, A.; Schilperoort, H.; Sher, L.; Emamaullee, J. Meta-analysis and meta-regression of outcomes for adult living donor liver transplantation versus deceased donor liver transplantation. Am. J. Transplant. 2021, 21, 2399–2412. [Google Scholar] [CrossRef]
- Tran, L.; Humar, A. Current status of adult liver transplantation: Utilization of living donor versus deceased donor graft. Curr. Opin. Organ. Transpl. 2021, 26, 133–138. [Google Scholar] [CrossRef]
- Liang, W.; Wu, L.; Ling, X.; Schroder, P.M.; Ju, W.; Wang, D.; Shang, Y.; Kong, Y.; Guo, Z.; He, X. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: A meta-analysis. Liver Transplant. 2012, 18, 1226–1236. [Google Scholar] [CrossRef]
- Song, W.; Chen, C.; Huang, Y.; Gu, G. Living donor liver transplantation for pediatric patients with metabolic disease vs. deceased donation. Asian J. Surg. 2021, 44, 629–635. [Google Scholar] [CrossRef]
- Kim, J.M.; Joh, J.W.; Kim, H.J.; Kim, S.H.; Rha, M.; Sinn, D.H.; Choi, G.S.; Kwon, C.H.; Cho, Y.Y.; Suh, J.M.; et al. Early Enteral Feeding After Living Donor Liver Transplantation Prevents Infectious Complications: A Prospective Pilot Study. Medicine 2015, 94, e1771. [Google Scholar] [CrossRef]
- Park, M.S.; Lee, K.W.; Suh, S.W.; You, T.; Choi, Y.; Kim, H.; Hong, G.; Yi, N.J.; Kwon, C.H.; Joh, J.W.; et al. Living-donor liver transplantation associated with higher incidence of hepatocellular carcinoma recurrence than deceased-donor liver transplantation. Transplantation 2014, 97, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, L.N.; Queiroz, R.M.T.; Paz, C.; Lyra, A.C. Better Living Donor Liver Transplantation Patient Survival Compared to Deceased Donor—A Systematic Review and Meta-Analysis. Arq. De Gastroenterol. 2022, 59, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Elkomos, B.E.; Abdo, M.; Mamdouh, R.; Abdelaal, A. Can living donor liver transplantation provide similar outcomes to deceased-donor liver transplantation for hepatocellular carcinoma? A systematic review and meta-analysis. Hepatol. Int. 2023, 17, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.C.; Sandhu, L.; Dixon, P.R.; Greig, P.D.; Grant, D.R.; McGilvray, I.D. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: A systematic review and meta-analysis. Clin. Transplant. 2013, 27, 140–147. [Google Scholar] [CrossRef]
- Tang, W.; Qiu, J.G.; Cai, Y.; Cheng, L.; Du, C.Y. Increased Surgical Complications but Improved Overall Survival with Adult Living Donor Compared to Deceased Donor Liver Transplantation: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2020, 2020, 1320830. [Google Scholar] [CrossRef]
- Wan, P.; Yu, X.; Xia, Q. Operative outcomes of adult living donor liver transplantation and deceased donor liver transplantation: A systematic review and meta-analysis. Liver Transplant. 2014, 20, 425–436. [Google Scholar] [CrossRef]
- Zhang, H.M.; Shi, Y.X.; Sun, L.Y.; Zhu, Z.J. Hepatocellular carcinoma recurrence in living and deceased donor liver transplantation: A systematic review and meta-analysis. Chin. Med. J. 2019, 132, 1599–1609. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, J.; Li, H.; Chen, X.; Zeng, Y. Living or deceased organ donors in liver transplantation for hepatocellular carcinoma: A systematic review and meta-analysis. HPB 2019, 21, 133–147. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Z.; Chen, B.; Li, J.; Yuan, X.; Li, J.; Wang, W.; Dai, T.; Chen, H.; Wang, Y.; et al. Dietary sugar consumption and health: Umbrella review. BMJ 2023, 381, e071609. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Liang, W.; Zheng, Z.; Guo, Z.; He, X. Living donor vs. deceased donor liver transplantation for patients with hepatitis C virus-related diseases. J. Hepatol. 2012, 57, 1228–1243. [Google Scholar] [CrossRef]
- Abu-Gazala, S.; Olthoff, K.M. Current Status of Living Donor Liver Transplantation in the United States. Annu. Rev. Med. 2019, 70, 225–238. [Google Scholar] [CrossRef]
- Bhangui, P.; Vibert, E.; Majno, P.; Salloum, C.; Andreani, P.; Zocrato, J.; Ichai, P.; Saliba, F.; Adam, R.; Castaing, D.; et al. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: Living versus deceased donor transplantation. Hepatology 2011, 53, 1570–1579. [Google Scholar] [CrossRef]
- Sandhu, L.; Sandroussi, C.; Guba, M.; Selzner, M.; Ghanekar, A.; Cattral, M.S.; McGilvray, I.D.; Levy, G.; Greig, P.D.; Renner, E.L.; et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: Comparable survival and recurrence. Liver Transplant. 2012, 18, 315–322. [Google Scholar] [CrossRef]
- Abt, P.L.; Mange, K.C.; Olthoff, K.M.; Markmann, J.F.; Reddy, K.R.; Shaked, A. Allograft survival following adult-to-adult living donor liver transplantation. Am. J. Transplant. 2004, 4, 1302–1307. [Google Scholar] [CrossRef]
- Berg, C.L.; Gillespie, B.W.; Merion, R.M.; Brown, R.S., Jr.; Abecassis, M.M.; Trotter, J.F.; Fisher, R.A.; Freise, C.E.; Ghobrial, R.M.; Shaked, A.; et al. Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology 2007, 133, 1806–1813. [Google Scholar] [CrossRef]
- Goldaracena, N.; Gorgen, A.; Doyle, A.; Hansen, B.E.; Tomiyama, K.; Zhang, W.; Ghanekar, A.; Lilly, L.; Cattral, M.; Galvin, Z.; et al. Live donor liver transplantation for patients with hepatocellular carcinoma offers increased survival vs. deceased donation. J. Hepatol. 2019, 70, 666–673. [Google Scholar] [CrossRef]
- Humar, A.; Ganesh, S.; Jorgensen, D.; Tevar, A.; Ganoza, A.; Molinari, M.; Hughes, C. Adult Living Donor Versus Deceased Donor Liver Transplant (LDLT Versus DDLT) at a Single Center: Time to Change Our Paradigm for Liver Transplant. Ann. Surg. 2019, 270, 444–451. [Google Scholar] [CrossRef]
- Lai, Q.; Sapisochin, G.; Gorgen, A.; Vitale, A.; Halazun, K.J.; Iesari, S.; Schaefer, B.; Bhangui, P.; Mennini, G.; Wong, T.C.L.; et al. Evaluation of the Intention-to-Treat Benefit of Living Donation in Patients With Hepatocellular Carcinoma Awaiting a Liver Transplant. JAMA Surg. 2021, 156, e213112. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.C.L.; Ng, K.K.C.; Fung, J.Y.Y.; Chan, A.A.C.; Cheung, T.T.; Chok, K.S.H.; Dai, J.W.C.; Lo, C.M. Long-Term Survival Outcome Between Living Donor and Deceased Donor Liver Transplant for Hepatocellular Carcinoma: Intention-to-Treat and Propensity Score Matching Analyses. Ann. Surg. Oncol. 2019, 26, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Abu-Gazala, S.; Olthoff, K.M. Status of Adult Living Donor Liver Transplantation in the United States: Results from the Adult-To-Adult Living Donor Liver Transplantation Cohort Study. Gastroenterol. Clin. N. Am. 2018, 47, 297–311. [Google Scholar] [CrossRef]
- Kim, P.T.; Testa, G. Living donor liver transplantation in the USA. Hepatobiliary Surg. Nutr. 2016, 5, 133–140. [Google Scholar] [CrossRef]
- Olthoff, K.M.; Smith, A.R.; Abecassis, M.; Baker, T.; Emond, J.C.; Berg, C.L.; Beil, C.A.; Burton, J.R., Jr.; Fisher, R.A.; Freise, C.E.; et al. Defining long-term outcomes with living donor liver transplantation in North America. Ann. Surg. 2015, 262, 465–475. [Google Scholar] [CrossRef]
- Miller, C.M.; Quintini, C.; Dhawan, A.; Durand, F.; Heimbach, J.K.; Kim-Schluger, H.L.; Kyrana, E.; Lee, S.G.; Lerut, J.; Lo, C.M.; et al. The International Liver Transplantation Society Living Donor Liver Transplant Recipient Guideline. Transplantation 2017, 101, 938–944. [Google Scholar] [CrossRef]
- Rela, M.; Rammohan, A. Why are there so many liver transplants from living donors in Asia and so few in Europe and the US? J. Hepatol. 2021, 75, 975–980. [Google Scholar] [CrossRef]
- Lo, C.M.; Fan, S.T.; Liu, C.L.; Chan, S.C.; Ng, I.O.; Wong, J. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br. J. Surg. 2007, 94, 78–86. [Google Scholar] [CrossRef]
- Vakili, K.; Pomposelli, J.J.; Cheah, Y.L.; Akoad, M.; Lewis, W.D.; Khettry, U.; Gordon, F.; Khwaja, K.; Jenkins, R.; Pomfret, E.A. Living donor liver transplantation for hepatocellular carcinoma: Increased recurrence but improved survival. Liver Transplant. 2009, 15, 1861–1866. [Google Scholar] [CrossRef]
- Fisher, R.A.; Kulik, L.M.; Freise, C.E.; Lok, A.S.; Shearon, T.H.; Brown, R.S., Jr.; Ghobrial, R.M.; Fair, J.H.; Olthoff, K.M.; Kam, I.; et al. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am. J. Transplant. 2007, 7, 1601–1608. [Google Scholar] [CrossRef]
- Ninomiya, M.; Shirabe, K.; Facciuto, M.E.; Schwartz, M.E.; Florman, S.S.; Yoshizumi, T.; Harimoto, N.; Ikegami, T.; Uchiyama, H.; Maehara, Y. Comparative study of living and deceased donor liver transplantation as a treatment for hepatocellular carcinoma. J. Am. Coll. Surg. 2015, 220, 297–304.e3. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.M.; Fisher, R.A.; Rodrigo, D.R.; Brown, R.S., Jr.; Freise, C.E.; Shaked, A.; Everhart, J.E.; Everson, G.T.; Hong, J.C.; Hayashi, P.H.; et al. Outcomes of living and deceased donor liver transplant recipients with hepatocellular carcinoma: Results of the A2ALL cohort. Am. J. Transplant. 2012, 12, 2997–3007. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, M.L.; Stravitz, R.T.; Contos, M.J.; Mills, A.S.; Sterling, R.K.; Luketic, V.A.; Sanyal, A.J.; Cotterell, A.; Maluf, D.; Posner, M.P.; et al. Histologic recurrence of chronic hepatitis C virus in patients after living donor and deceased donor liver transplantation. Liver Transplant. 2004, 10, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Orrego, M.; Rodriguez-Luna, H.; Balan, V.; Byrne, T.; Chopra, K.; Douglas, D.D.; Harrison, E.; Moss, A.; Reddy, K.S.; et al. Living donor liver transplantation for hepatitis C-related cirrhosis: No difference in histological recurrence when compared to deceased donor liver transplantation recipients. Liver Transplant. 2006, 12, 560–565. [Google Scholar] [CrossRef]
- Terrault, N.A.; Shiffman, M.L.; Lok, A.S.; Saab, S.; Tong, L.; Brown, R.S., Jr.; Everson, G.T.; Reddy, K.R.; Fair, J.H.; Kulik, L.M.; et al. Outcomes in hepatitis C virus-infected recipients of living donor vs. deceased donor liver transplantation. Liver Transplant. 2007, 13, 122–129. [Google Scholar] [CrossRef]
- Terrault, N.A.; Stravitz, R.T.; Lok, A.S.; Everson, G.T.; Brown, R.S., Jr.; Kulik, L.M.; Olthoff, K.M.; Saab, S.; Adeyi, O.; Argo, C.K.; et al. Hepatitis C disease severity in living versus deceased donor liver transplant recipients: An extended observation study. Hepatology 2014, 59, 1311–1319. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, K.W.; Song, G.W.; Jung, B.H.; Lee, H.W.; Yi, N.J.; Kwon, C.H.D.; Hwang, S.; Suh, K.S.; Joh, J.W.; et al. Increased survival in hepatitis c patients who underwent living donor liver transplant: A case-control study with propensity score matching. Ann. Surg. Treat. Res. 2017, 93, 293–299. [Google Scholar] [CrossRef]
- Jain, A.; Singhal, A.; Kashyap, R.; Safadjou, S.; Ryan, C.K.; Orloff, M.S. Comparative analysis of hepatitis C recurrence and fibrosis progression between deceased-donor and living-donor liver transplantation: 8-year longitudinal follow-up. Transplantation 2011, 92, 453–460. [Google Scholar] [CrossRef]
- Jung, D.H.; Ikegami, T.; Balci, D.; Bhangui, P. Biliary reconstruction and complications in living donor liver transplantation. Int. J. Surg. 2020, 82s, 138–144. [Google Scholar] [CrossRef]
- Reichman, T.W.; Katchman, H.; Tanaka, T.; Greig, P.D.; McGilvray, I.D.; Cattral, M.S.; Renner, E.L.; Selzner, M.; Ghanekar, A.; Levy, G.; et al. Living donor versus deceased donor liver transplantation: A surgeon-matched comparison of recipient morbidity and outcomes. Transpl. Int. 2013, 26, 780–787. [Google Scholar] [CrossRef]
- Freise, C.E.; Gillespie, B.W.; Koffron, A.J.; Lok, A.S.; Pruett, T.L.; Emond, J.C.; Fair, J.H.; Fisher, R.A.; Olthoff, K.M.; Trotter, J.F.; et al. Recipient morbidity after living and deceased donor liver transplantation: Findings from the A2ALL Retrospective Cohort Study. Am. J. Transplant. 2008, 8, 2569–2579. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yan, L.; Tan, Y.; Li, B.; Wen, T.; Yang, J.; Zhao, J. Adult-to-adult right-lobe living donor liver transplantation in recipients with hepatitis B virus-related benign liver disease and high model end-stage liver disease scores. Surg. Today 2013, 43, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Kotsch, K.; Francuski, M.; Reutzel-Selke, A.; Mantouvalou, L.; Klemz, R.; Kuecuek, O.; Jonas, S.; Wesslau, C.; Ulrich, F.; et al. Brain death activates donor organs and is associated with a worse I/R injury after liver transplantation. Am. J. Transplant. 2007, 7, 1584–1593. [Google Scholar] [CrossRef]
- Yankol, Y.; Fernandez, L.A.; Kanmaz, T.; Leverson, G.E.; Mezrich, J.D.; Foley, D.; Mecit, N.; D’Alessandro, A.M.; Acarli, K.; Kalayoglu, M. Results of pediatric living donor compared to deceased donor liver transplantation in the PELD/MELD era: Experience from two centers on two different continents. Pediatr. Transplant. 2016, 20, 72–82. [Google Scholar] [CrossRef]
- Lin, T.S.; Co, J.S.; Chen, C.L.; Ong, A.D. Optimizing biliary outcomes in living donor liver transplantation: Evolution towards standardization in a high-volume center. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 324–327. [Google Scholar] [CrossRef]
- Feng, M.X.; Zhang, J.X.; Wan, P.; Qiu, B.J.; Gu, L.H.; Zhang, J.J.; Xia, Q. Hepatic artery reconstruction in pediatric liver transplantation: Experience from a single group. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 307–310. [Google Scholar] [CrossRef]
- Nadalin, S.; Capobianco, I.; Panaro, F.; Di Francesco, F.; Troisi, R.; Sainz-Barriga, M.; Muiesan, P.; Königsrainer, A.; Testa, G. Living donor liver transplantation in Europe. Hepatobiliary Surg. Nutr. 2016, 5, 159–175. [Google Scholar] [CrossRef]
| Author/Year | Type of Databases (No. of Databases); Last Search | No. of Included Studies | Type of Subjects | Clinical Outcomes | Main Conclusion | AMSTAR2 Final Rating |
|---|---|---|---|---|---|---|
| Grant et al., 2013 [14] | MEDLINE, Embase, PubMed (3); April 2012 | 12 | Only HCC patients | DFS, OS | Decreased DFS after LDLT compared with DDLT for HCC. | Critically low |
| Zhu et al., 2019 [18] | PubMed, Embase, Cochrane Library, Google Scholar, WanFang (5); January 2018 | 29 | Only HCC patients | OS, ITT-OS, Recurrence rate, DFS, Perioperative mortality within 3 months, Complications | LDLT was not inferior to DDLT in consideration of comparable OS, DFS, recurrence rate, mortality within 3 months and postoperative complication rate, but a possible improvement in long-term intention-to-treat survival. | Critically low |
| Cavalcante et al., 2022 [12] | PubMed, Medline (2); June 2021 | 28 | NR | OS, Graft survival | Better patient survival at 1, 3, and 5 years among patients who received LDLT, compared to DDLT, as well as better 1-year graft survival. | Critically low |
| Tang et al., 2020 [15] | PubMed, Embase, Cochrane Library (3); November 2019 | 39 | NR | OS, HCC recurrence rate, CIT, RBC transfusion, DRO, Postoperative Intra-Abdominal Bleeding Rate, Perioperative Mortality, Length of Hospital Stay, Vascular Complication Rate, Biliary Complication Rate, Retransplantation Rate, HCV Recurrence Rate | LDLT was not inferior to DDLT in consideration of RBC transfusion, length of hospital stay, perioperative mortality, retransplantation rate, HCV recurrence rate, and HCC recurrence rate, but it was an improvement in CIT, postoperative intra-abdominal bleeding rate, and OS. | Critically low |
| Liang et al., 2012 [8] | PubMed, MEDLINE, Embase, Cochrane Library (4); NR | 7 | Only HCC patients | OS, RFS, tumor recurrence rates | Patient survival, recurrence, and RFS rates are at least comparable in HCC patients undergoing LDLT and HCC patients undergoing DDLT (especially in those meeting the Milan criteria). | Critically low |
| Zhang et al., 2019 [17] | Cochrane Library, PubMed, Embase (3); October 2017 | 7 | Only HCC patients | Accumulated HCC recurrence rates | There is an overall increased risk for HCC recurrence in LDLT as compared with that of DDLT. | Critically low |
| Barbetta et al., 2021 [6] | PubMed, Embase and Embase Classic, Cochrane Library, Web of Science, Clinicaltrials.gov, Google Scholar (6); March 2018 | 19 | NR | OS, Graft Survival, MELD score at transplant, Time on Waiting List, Hepatic artery thrombosis, Biliary complications, Risk of infection, Length of stay, Rejection | LDLT is associated with improved patient survival, less waiting time, and lower MELD at LT, despite posing a higher risk of biliary complications that did not affect survival posttransplant. | Critically low |
| Wan et al., 2014 [16] | MEDLINE, Embase, Cochrane Library (3); October 2013 | 19 | NR | DRO, Allogeneic RBC Transfusion, Length of the hospital stay, CIT, Biliary complications, Vascular complications, intra-abdominal bleeding rates, perioperative mortality rates, retransplantation rates | LDLT is associated with a higher rate of surgical complications after transplantation. | Low |
| Elkomos et al., 2023 [13] | PubMed, Scopus, Web of Science, Cochrane Library (4); July 2021 | 35 | Only HCC patients | OS, DFS, ITT-OS, Recurrence rates | LDLT provides much better survival benefits to HCC patients, especially in regions that suffer from low deceased organ availability. | Critically low |
| Hu, Anbin et al., 2012 [23] | PubMed, MEDLINE, EMBASE, Cochrane Library (4); NR | 14 | HCV-related diseases | OS, Graft Survival, Acute rejection episodes, HCV Recurrence, Graft loss due to HCV recurrence | LDLT was equivalent to DDLT in terms of patient survival, long-term graft survival, HCV recurrence, and acute rejection rates, with potentially lower short-term patient and graft survival. | Moderate |
| Included Studies | ITEMS | Final Rating | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| Grant et al., 2013 [14] | Y | pY | N | pY | Y | Y | N | Y | pY | N | Y | N | Y | N | Y | N | Critically low |
| Zhu et al., 2019 [18] | Y | Y | N | pY | Y | Y | Y | Y | pY | N | Y | Y | N | Y | Y | Y | Critically low |
| Cavalcante et al., 2022 [12] | N | N | N | pY | Y | N | pY | N | pY | N | Y | Y | N | N | Y | N | Critically low |
| Tang et al., 2020 [15] | N | N | N | pY | Y | Y | Y | Y | pY | N | Y | N | N | Y | N | Y | Critically low |
| Liang et al., 2012 [8] | Y | N | N | pY | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Critically low |
| Zhang et al., 2019 [17] | Y | N | N | pY | Y | N | Y | Y | Y | N | Y | Y | Y | Y | N | Y | Critically low |
| Barbetta et al., 2021 [6] | Y | pY | N | Y | Y | Y | Y | Y | pY | N | Y | N | Y | Y | N | Y | Critically low |
| Wan et al., 2014 [16] | Y | pY | N | pY | Y | N | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Low |
| Elkomos et al., 2023 [13] | Y | pY | Y | pY | Y | Y | Y | Y | pY | N | Y | N | N | Y | Y | Y | Critically low |
| Hu, Anbin et al., 2012 [23] | Y | pY | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; He, Y.-C.; Cai, Y.-S.; Lv, Y.-H.; Liu, C.; Wu, H. Living Donor Liver Transplantation Versus Deceased Donor Liver Transplantation for Hepatocellular Carcinoma and HCV Patients: An Initial Umbrella Review. J. Clin. Med. 2025, 14, 3047. https://doi.org/10.3390/jcm14093047
Yang Y, He Y-C, Cai Y-S, Lv Y-H, Liu C, Wu H. Living Donor Liver Transplantation Versus Deceased Donor Liver Transplantation for Hepatocellular Carcinoma and HCV Patients: An Initial Umbrella Review. Journal of Clinical Medicine. 2025; 14(9):3047. https://doi.org/10.3390/jcm14093047
Chicago/Turabian StyleYang, Ying, Yu-Cheng He, Yun-Shi Cai, Ying-Hao Lv, Chang Liu, and Hong Wu. 2025. "Living Donor Liver Transplantation Versus Deceased Donor Liver Transplantation for Hepatocellular Carcinoma and HCV Patients: An Initial Umbrella Review" Journal of Clinical Medicine 14, no. 9: 3047. https://doi.org/10.3390/jcm14093047
APA StyleYang, Y., He, Y.-C., Cai, Y.-S., Lv, Y.-H., Liu, C., & Wu, H. (2025). Living Donor Liver Transplantation Versus Deceased Donor Liver Transplantation for Hepatocellular Carcinoma and HCV Patients: An Initial Umbrella Review. Journal of Clinical Medicine, 14(9), 3047. https://doi.org/10.3390/jcm14093047






